| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nídia de Sá Caetano | + 5165 word(s) | 5165 | 2021-01-12 03:01:21 |
Video Upload Options
Microalgae are unicellular or simple multicellular photosynthetic microorganisms, which can normally be found in aquatic environments such as freshwater, seawater, or hypersaline lakes. These organisms can be eukaryotic or prokaryotic, the latter being the cyanobacteria, which are commonly referred to as microalgae.
The nature of the cell wall of a given microalgae species can vary, making it easier or harder to access its valuable contents. The rigidity of the cell wall can be provided, for example, by high levels of polysaccharides in the cell wall structure, such as glucose and mannose, present in Chlorella zofingiensis, or by complex sugars composition such as arabinose, galactose, rhamnose, mannose and xylose, as found in Tetraselmis suecia and T. striata. Algaenan or sporopollein is another extremely resistant biopolymer, a non-hydrolyzable biopolymer, composed of long ω-hydroxy fatty acids chains linked by several types of chemical bond, which confer its rigid properties, and that can be found in some species such as Chlorella spp., Nannochloropsis galditana and Scenedesmus spp. Arthorspira spp. cell wals contain peptidoglycan, being less rigid and, consequently, more susceptible to degradation.
Thus, several methods can be applied to breakdown such molecules that, being part of the cell wall, present different level of rigidity and confer them protection against environment factors. The cell wall disruption methods include physical, chemical, enzymatic approaches. In this entry, it will be presented a brief description of these methods.
1. Introduction
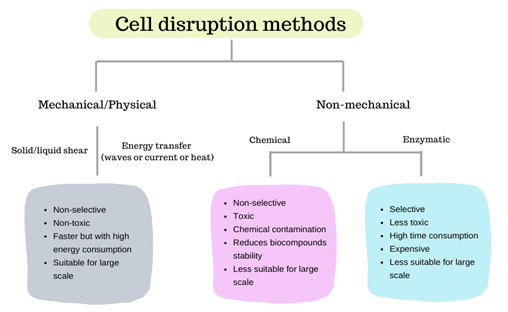
Methods for microalgae cell disruption comprise mechanical, physical or non-mechanical techniques, usually employed to disrupt or disintegrate the cellular membrane, this way increasing the recovery yield of the desired component (e.g., lipids, pigments, proteins) that can be isolated from biomass. Figure 1 shows the general differences between mechanical and non-mechanical cell-disruption methods.
Figure 1. Comparison of different cell-disruption methods.
2. Mechanical and Physical Methods
Mechanical and physical methods may promote cell lysis through solid or liquid-shear forces (e.g., bead milling, high-speed or high-pressure homogenization) or energy transfer through waves (e.g., microwave irradiation, ultrasonication or laser), current (e.g., pulsed electric fields) or heat (e.g., autoclaving, freeze/thaw cycles, thermolysis).
2.1. Bead Milling
The cell disruption principle in bead mill machines is to promote mechanical cell damage by forcing the collision between the cells and the beads. This collision is promoted by a rotating shaft present in the grinding chamber. The diameter and load of the beads are important parameters with a direct influence on the cell disruption effectiveness [1]. The most common materials used in the beads are zirconium (high-density beads) and glass (low-density beads). Zirconium is preferred to process high viscosity media, while glass beads are more suitable for media with low viscosity [2].
In large-scale processes, techniques based on solid/liquid-shear forces are commonly employed due to their high efficiency and scale-up easiness. Furthermore, these methods avoid the contamination of chemical methods and preserve most of the biomolecules’ functionality when compared to chemical and thermal treatments. An optimized mechanical method for Chlorella’s cell wall disruption at industrial scale (i.e., milling chamber volume ≥500 dm3 and/or flow rate >1 m3∙h−1 and/or batch from 1 to 200 m3) has been described in the US patent n° 10465159B2 [3]. The inventors evaluated the effect of specific parameters, such as bead material (glass, zirconium silicate and zirconium oxide), diameter of milling beads (0.3 to 1.7 mm), chamber filling rate (80% to 90%), operational scheme (single or multiple mills in series), peripheral speed of milling disks (8 to 12 m∙s−1, limited to avoid abrasion issues) and cell density (20%, 25.2% and 31.9%) on the specific energy demand and productivity. The configuration recommended by the applicant company in order to combine lower energy consumption with higher productivity is performed by lower diameter-zirconium silicate beads (0.3 to 0.6 mm) at 85–90% chamber filling rate, peripheral speed of milling disks between 11 and 12 m∙s−1 and moderate biomass concentration (25.2%) with several mills in series. It was demonstrated that moderate conditions are preferable to reduce energy consumption to achieve a target degree of milling. Despite high-density beads (based on zirconium) presenting high specific energy, the glass beads (low density) were not efficient, requiring more passes to achieve the same degree of milling, which also increases the specific energy. Therefore, in order to overcome this issue, the inventors combined the use of zirconium silicate, which is less dense than zirconium oxide, with lower diameter to reduce the number of passes required. The same criteria were applied to biomass concentration; higher concentrations lead to higher productivities, but also increase the energy consumption. Additionally, moderate peripheral speed was recommended to avoid excessive abrasion and the filling rate, however, did not present a significant impact on energy consumption among the tested conditions. It is also possible to reduce the specific energy consumption and the process cost by using higher dry cell weight concentrations (0.5–8% w/w) and higher biomass flow rates, but it will also have a negative impact on the cell disruption efficiency [2]. Thus, despite the many advantages of using bead milling, including this being a suitable technique for large-scale production, and the aforementioned optimizations, this process still has high energy consumption [4].
2.2. High-Speed Homogenization
High-speed homogenization (HSH) is a simple and effective method, in which the cells’ disruption is based on hydrodynamic cavitation caused by stirring at high speed (10,000–20,000 rpm) and shear forces at the solid–liquid interphase [2]. Despite presenting some drawbacks such as high energy consumption and protein denaturation, this technique is suitable for industrial scale and requires short processing time. It has been reported operational times of 30 or 60 s at 10,000 or 14,000 rpm for lipid and antioxidant extraction in Nannochloropsis sp., Phaeodactylum tricornutum and Pavlova lutheri [5][6].
González-Delgado and Kafarov [7] compared solvent assisted extraction with HSH and other solvent-based extraction methods for a microalgae biorefinery. The authors tested five microalgae species (Nannochloropsis sp., Guinardia sp., Closterium sp., Amphiprora sp. and Navicula sp.), concluding that despite the higher extraction yields achieved by combining polar and non-polar solvents, the solvent method presents high toxicity and lowest solvent recovery, increasing the process costs.
2.3. High-Pressure Homogenization
The high-pressure homogenization (HPH) method for cell disruption employs high pressure (≈20–120 MPa) to promote turbulence, liquid-shear stress and friction. According to the cell wall properties, parameters such as operating pressure and number of homogenization passes can be optimized to enhance the process efficiency [8]. Additionally, other variables such as dry cell weight concentration, microalgae species and growth conditions, impacts on the specific energy consumption [2].
Bernaerts et al. [9] studied the impact of (ultra) high-pressure homogenization (U)HPH on the rigid cells of Nannochloropsis sp., achieving similar lipid extraction efficiency, using 250 MPa in half of the homogenization passes compared to 100 MPa. However, despite the effective reduction of homogenization passes, the high pressure also heated the sample, resulting in aggregation of the intracellular components released. Besides the reduction of specific energy in (U)HPH by using biomass concentrations up to 25% (w/w), the energy consumption of this technique is still high. However, Elain et al. [10] demonstrated a satisfactory specific energy consumption (0.41 kWh∙kg−1 biomass dry weight) of HPH in a study comparing the performance of this technique in mild conditions (e.g., of room temperature, neutral pH, shorter time, etc.) with conventional thermal treatment (hot water) in cell disruption of Arthrospira platensis, also increasing 2.5-fold the yield of polysaccharides extraction.
Thus, the major drawbacks of HPH comprise the non-selectivity, the formation of undesirable cell debris and the limitation to break harder cell walls. However, despite these disadvantages, HPH is, together with bead milling and HSH, the preferred method for the industrial scale.
2.4. Microwave Irradiation
Microwave irradiation is a simple and scalable method for cell disruption. This method has a well-established optimal operational value for heating (2450 MHz) and the cell walls are disrupted by the electromagnetic effect induced by the microwave irradiance that interacts with polar (e.g., water) and dielectric molecules, also promoting local heating [11]. This method is not suitable when the target component is volatile, but it has been successfully reported as an effective cell disruption technique for lipid extraction. Also, the combination of microwave with solvents, called microwave-assisted extraction (MAE), has been reported as the technique with lower operational costs and extraction time than the conventional techniques, and higher lipid extraction than other non-conventional methods (e.g., ultrasound-assisted extraction) [12].
By comparing different cell disruption methods (autoclaving, bead-beating, microwaves, sonication and osmotic shock), followed by chemical lipid extraction, Lee et al. [13] proved that microwave was the most effective method for cell disruption of three microalgae species (Botryococcus sp., Chlorella vulgaris and Scenedesmus sp.). Also, Viner et al. [14] compared microwave with several cell disruption methods (freeze-drying, ultrasonication, cooling, liquid nitrogen grinding, osmotic shock and switchable osmotic shock) prior to lipid extraction in Scenedesmus sp. using liquid CO2 and methanol. The highest total lipids extraction yield (9.6 wt% of dry algae) was achieved using microwave in the presence of water. Recently, the use of ionic liquids in MAE has been studied as a greener technology to overcome the intrinsic toxicity of the conventional solvents (e.g., chloroform, methanol) [15][16].
2.5. Ultrasonication
The ultrasonication method for cell disruption is based on liquid-shear forces caused by emission of high frequency wave sounds (up to 15–20 kHz). In liquid, these sound waves create gas bubbles or cavities that, after a certain number of cycles, achieve a critical size, collapsing and releasing large amounts of energy. Additionally, acoustic cavitation occurs by increasing local temperature and forming hydroxyl radicals that damage the cell wall [1]. Besides being a scalable technique with low operational cost, it is possible to optimize some parameters (e.g., temperature, cell concentration, acoustic intensity and time) to partially disrupt the cells, resulting in selective release of proteins [8]. Moreover, the promising use of ultrasonication for large-scale treatment of microalgal biomass has been previously pointed out by Adam et al. [17] who suggested that, the large-scale ultrasound extraction reactors used in food and chemical industries, can be easily modified to perform an ultrasound-assisted extraction of microalgae biomolecules in amounts up to 200 kg∙h−1 of biomass dry weight. However, this technique is not very effective for some microalgae species and it is commonly combined with chemical treatments for efficiency improvement and to reduce energy demand [4][8].
2.6. Pulsed Electric Field
A pulsed electric field (PEF) has been described as an alternative method to overcome high energy consumption of classical mechanical methods based on solid/liquid-shear forces. Besides being energetically efficient and scalable, PEF also presents selectivity and fast processing time. However, despite the low operational costs, equipment is expensive and the technique depends on medium conductivity, limiting its use [18]. The disruption mechanism induced by PEF is based on electroporation as a result of transient membrane-permeabilization and electrophoretic movements into the cell caused by charged species [19]. The electroporation can be reversible (0.5–1.5 kV∙cm−1, 0.5–5 kJ∙kg−1), mostly used for genetic engineering or chemotherapy, or irreversible (10–20 kV∙cm−1, 50–200 kJ∙kg−1), being applicable for cell disruption and food processing [20].
Several parameters can influence PEF efficiency such as the electric field strength, pulse (shape, width), frequency, physicochemical parameters (temperature, pH and conductivity), operational time and cell wall properties [21]. Lam et al. [22] tested the use of PEF for protein release from Chlorella vulgaris and Neochloris oleoabundans achieving the maximum of 13% even through use of 10–100 times higher energy than bead milling, which released 45–50% of proteins. On the other hand, Käferböck et al. [23] reported a 90% increase in phycocyanin extraction efficiency from Arthrospira maxima by combining freeze-thawing and PEF, in comparison to bead milling method. However, a recent study comparing PEF, high voltage electrical discharges (HVED) and ultrasonication on aqueous extraction of Nannochloropsis sp., Phaeodactylum tricornutum and Parachlorella kessleri components have demonstrated HVED is most effective for carbohydrates and ultrasonication for proteins and chlorophyll a extraction from these species [24]. These results demonstrate that despite being a promising technology, there are still challenges to overcome for its establishment as a suitable technology for mild or large-scale microalgae biorefinery. However, PEF is a widely employed technology in the food industry that counts on specialized companies that are also involved in projects, approaching the use of PEF to stimulate growth and improve extraction of high-value compounds from microalgae. The German company ELEA Technology, for example, has been running the project iAlgaePro (https://elea-technology.de/project/ialgaepro/) since 2014. In this project the effectiveness of low-intensity PEF treatments to stimulate growth and also enhance the extraction of several compounds (e.g., phycocyanin, vitamins, polyphenols, lipids, among others) were demonstrated. The group reported significant difference between the phycocyanin extraction yield in PEF-treated (66.4 mg mL−1) and non-treated (≤0.2 mg∙mL−1) Spirulina biomass. Thus, this kind of initiative may accelerate the implementation of PEF in microalgae biorefineries.
2.7. Thermal Treatments
Thermal treatments are physical methods that use heat to promote cell disruption, such as thermolysis [25], autoclaving [26] and steam explosion [27]. Despite being simple technologies with low maintenance cost, the physical disruption is frequently associated with low efficiency, high energy consumption, generation of large amounts of undesirable cell debris and applicability limited by thermal resistance of the target product to be extracted. However, as shown in Table 1, steam explosion has many advantages compared to conventional thermal treatments. In this technique the biomass is exposed to high temperatures (160–290 °C), however, to pretreated microalgae biomass it is recommended to use lower temperatures to avoid degradation of the bioproducts, at vapor pressure between 1.03 and 3.45 MPa. The cell disruption occurs when the system is depressurized to return to room conditions [28]. Lorente et al. [27] tested four pretreatments (steam explosion, autoclaving, microwave and ultrasound) in three microalgae species (Nannochloropsis gaditana, Phaeodactylum tricornutum and Chlorella sorokiniana) to enhance lipid extraction using the Bligh and Dyer method. In this study, steam explosion as pretreatment showed the highest lipid yield for all species, especially for N. gaditana and C. sorokiniana. Furthermore, this technique promotes carbohydrates hydrolysis, forming aqueous phase rich in monomeric sugars suitable for subsequent fermentation.
In Table 1 are summarized the main mechanical and physical methods for cell disruption, highlighting the principle of cell disruption and the main advantages and disadvantages of each of them.
Table 1. Main mechanical and physical methods for cell disruption: mechanism of disruption, advantages, disadvantages and remarks.
|
Cell Disruption Method |
Principle of Cell Disruption |
Advantages |
Disadvantages |
Remarks |
Equipment Available |
Specifications |
|
Mechanical deformation by compaction and shear |
· High disruption efficiency · Processes high loads of biomass · Good temperature control · Easily scalable · Equipment commercially available |
· High energy demand · Non-selective procedure · Formation of very fine cell debris |
Suitable for large-scale |
Bead mill for cell disruption—model EDW (ELE® Company) a |
Chamber volume: 5–400 L Flow: 30–200 to > 3000 L h−1 |
|
|
High-speed homogenization [2] |
Cavitation and shear |
· Simple · High disruption efficiency · Short contact times |
· High energy demand · Protein denaturation |
Preferable for large scale (not indicated for mild scale) |
High-speed homogenizer and disperser (Intertech®) b |
Volume: 750–1150 L Flow rate: 650–5200 L min−1 |
|
· Easily scalable · Does not require cell drying · Suitable for processing large volumes |
· Non-selective procedure · Use low dry cell weight concentrations, increasing energy demand and water footprint · Formation of very fine cell debris · Not effective to break hard cell walls · Reduces protein disgestibility · Not recommended for fragile compounds isolation |
· Suitable for emulsification processes · Preferable for large scale (not indicated for mild scale) |
Ariete Series Homogenizers (©GEA Group) c
DeBEE 2000 series (©BEE International) d |
Pressure: 100–1500 bar Flow: 35–80,000 L h−1
Power: 10 hp Pressure: 1333–45,000 bar Flow rate: 0.5–2 L min−1 |
||
|
Increases temperature and molecular energy |
· Easily scalable · Simple · Can be combined with selective extraction (microwave assisted extraction) |
· Not recommended for volatile compounds isolation · Limited to polar solvents |
Not recommended for mild microalgae biorefinery |
MARSTM6 Extraction (©CEM Corporation) e |
Capacity: 55 L, up to 40 vessels Wattage: 2000 W Power density: 36 W L−1 |
|
|
Ultrasonication |
Cavitation and free radical formation |
· Easily scalable · Low operational costs |
· Low cell disruption efficiency for some microalgae species · Heat production |
· Combination of ultrasound with different solvents can improve the effectiveness of cell disruption and reduce the energy demand · Equipment already available for industrial scale |
Industrial ultrasonic devices UIP series (Hielscher Ultrasonics) f |
Power: 0.5–16 kW Frequency: 18 or 20 kHz Flow rate: 0.25–10 m3 h−1 |
|
Irreversible pore formation in cell membrane caused by short electrical pulses (electroporation) |
· High disruption efficiency · Low operational costs · Scalable · Selective · Fast process time |
· Can promote radical formation and undesired reactions, reducing the quality of the product · Depends on the media conductivity · Expensive equipment |
Needs improvements for cell disruption in large-scale |
ELEA PEFPilotTM dual trial system (ELEA Technology) g |
Power: 400 V, 50 Hz; Water and air cooled Dimensions: 1,45 × 1,79 × 1,13 (W × D × H) Capacity: 10 kg per batch, up to 250 L h−1 |
|
|
Exterior heat diffusion through cell membrane to intracellular environment |
· Simple · Low maintenance costs
|
· High energy consumption · Formation of large amount of cell debris · Not indicated for thermal-sensible compounds |
Not indicated for large-scale |
Systec V series (Systec GmbH) h |
Chamber volume: 45/40–166/150 Ltotal/nominal |
|
|
High temperature, vapor pressure and depressurization |
· Low maintenance costs · Relatively low energy consumption · Low corrosion potential · Residual steam can be used to reduce process costs |
· Variable efficiency according to microalgae species |
Very suitable for commercial applications |
Steam generator various models (Garioni Naval) i |
Lab-scale:15–180 kW, 9–170 kgsteam h−1, 7–8 barg Up scales: 300–6000 kgsteam h−1, 3 passes, up to 18 barg; 3000–25,000 kgsteam h−1, 2 passes, up to 21 barg |
Websites: a www.ele-mix.com, b www.intertechglobal.com, c www.gea.com/en/index.jsp, d www.beei.com, e www.cem.com, f https://www.hielscher.com, g www.elea-technology.de, h www.systec-lab.com, i www.garioninaval.com. (W×D×H): Width × Depth × Height.
3. Non-Mechanical Methods
Non-mechanical methods comprise chemical methods that may use acid or alkaline treatments [31][32][33] and detergents [34], osmotic shock [35] or enzymatic treatments [36].
3.1. Chemical Methods
Chemicals such as solvents, acids, alkali, hypochlorites, antibiotics, detergents, among others, can interact with components of the microalgal cell wall causing deformations and promoting cell disruption. Despite being a simple and well-known technique, the use of chemicals raises several environmental and economic concerns, especially for industrial scale. Further, the chemical contamination of the target product limits its application, once the active compound is generally classified as non-food grade [1]. However, the use of surfactants, which can both help harvesting biomass and promote cell disruption, is an interesting option in large scale for species whose harvest is a limiting factor. Surfactants interact with the cell membrane’s phospholipids, causing distortions and consequently, cell disruption, improving release of intracellular components and bioproducts recovery [34]. The most commonly used surfactants are long-chain alkyl groups (C12 to C16) containing quaternary-ammonium cation. These compounds have hydrophobic ends capable of adsorbing or attaching to cell membranes, and once this happens, the quaternary cation makes the cell charge to become less negative, favoring cell aggregation [37]. Lai et al. [38] evaluated this synergistic effect by using cationic surfactants for flocculation and lipid extraction from C. vulgaris. The authors tested three cationic surfactants: dodecyltrimethylammonium bromide (DTAB), myristyltrimethylammonium bromide (MTAB) and hexadecyltrimethylammonium bromide (CTAB), showing that the pretreatment with the surfactant CTAB resulted in the most effective cell disruption, with the highest lipid recovery (nearly 90%) without changing the fatty acid methyl esters (FAME) profile. Moreover, small amounts of CTAB (0.45 mM) were required to improve flocculation and harvesting. Recently, Alhattab et al. [39] tripled the amount of total FAME extracted by 24 hours’ pretreatment with the surfactant CTAB, followed by SFE with supercritical CO2 (sc-CO2) of Chlorella saccharophila biomass. However, they also observed that although the extraction was higher, the FAME composition changed significantly. This possibility of modulating FAME composition may be interesting depending on the desired application, but for biodiesel production, they found that the most suitable composition was obtained with pure sc-CO2. Additionally, in the US patent n° 9994791B1, Zhang et al. [36] described a cell disruption method for microalgae Nannochloropis salina by using sodium dodecylbenzenesulfonate as anionic surfactant associated to pH adjustment and low pressurization, extending this application to other anionic and non-ionic surfactants.
Figure 2 shows the SEM micrograph of the Phaeodactylum tricornutum cells treatead with acid [40].
Figure 2. SEM micrograph of the Phaeodactylum tricornutum cells after chemical treatment (unpublished photo © Monique Branco-Vieira)
3.2. Osmotic Shock
The presence of a high concentration of solute (salt, dextran or polyethylene glycol (PEG)) leads to a decrease of osmotic pressure, causing cell wall damage, increasing its permeability and, consequently, allowing the release of intracellular compounds. In this respect, Rakesh et al. [41] compared the use of autoclaving, microwave, osmotic shock, and pasteurization to Chlorococcum sp. MCC30, Botryococcus sp. MCC31, Botryococcus sp. MCC32, and Chlorella sorokiniana MIC-G5 to facilitate lipid extraction. They found that by applying osmotic shock improved lipid extraction could be achieved for Botryococcus sp. MCC32 (at 15% NaCl) and for C. sorokiniana MIC-G5 (at 5% NaCl). Furthermore, the composition of the extracts varied with the treatment used to facilitate the extraction. Rakesh et al. [41] also found different palmitate (C16:0) contents (25.64% and 34.20%) using osmotic shock (15% NaCl) treatment for Botryococcus sp. MCC32 and microwave (6 min) for Botryococcus sp. MCC31, respectively, while the use of Botryococcus sp. MCC32 as source of oil blends or nutraceuticals was proposed after osmotic shock of 15% NaCl treatment due to its oleic acid and unsaturated fatty acid content (19.95% and 38.17%, respectively). González-González et al. [42] also applied osmotic shock to Chaetoceros muelleri and Dunaliella salina, having achieved a lipid recovery efficiency of 72% and 21% respectively. They also found that the lipid-spent biomass of C. muelleri add one of the highest methane yields reported for microalgae of 484 mL CH4 g VS−1, showing that osmotic shock adds a double positive effect on lipid extraction and biomass quality for anaerobic digestion. López and Morales [43] extracted astaxanthin from Haematococcus pluvialis applying osmotic shock by highly concentrated sacarose solution, or syrup, at high temperatures. They concluded that astaxanthin extracted using osmotic shock remained available for consumption in the syrup. Koyande et al. [44] studied the recovery of whole proteins from Chlorella vulgaris FSP-E using osmotic shock through a liquid biphasic flotation (LBF) system, having concluded that protein recovery of 92.98% with a separation efficiency of 64.91%, partition coefficient of 1.47 and a volume ratio of 9 could be achieved using osmotic shock, whereas without osmotic shock the corresponding values were of only 84.84%, 69.68%, 1.89 and 2.96.
Although simple, the major drawbacks of this technique are that it takes longer than other processes such as autoclaving and microwave irradiation [45], being economically unfeasible at a large scale [46].
3.3. Enzymatic Methods
Enzymatic cell lysis is a high-selective method for cell disruption that requires low energy and operates at mild conditions [66]. The commercial enzymes such as cellulases, proteases, lysozyme and glucanases are vastly employed and commonly used in the immobilized form to increase their lifetime and stability, preventing reduction in catalytic activity [8]. The main drawbacks of using enzymes compared to mechanical or chemical methods are the long process time, low production capacity and the possible product inhibition. In addition, the high cost of the enzymes limits their applications in a microalgae biorefinery [2]. Liang et al. [47] tested the combination of ultrasound with enzymatic lysis (snailase and trypsin) for lipid extraction in three microalgae species, achieving the maximum lipid yield of 49.82% in Chlorella vulgaris, 46.81% in Scenedesmus dimorphus and 11.73% in Nannochloropsis sp. Zhang et al. [36] achieved 86.4% of lipid recovery in Scenedesmus sp. using a mixture of enzymes (cellulase, xylanase and pectinase), also increasing the fatty acid methyl esters (FAME) yield compared to the untreated biomass. However, despite improving the lipid yield from Scenedesmus sp., in the study by Zhang et al., the enzymes were used just as pretreatment followed by an organic solvent extraction, while Liang et al. used a more sustainable method based on enzyme-assisted aqueous extraction. Regarding to recent patents approaching solventless extraction of microalgae biomolecules, Bai et al. [48], US patent n° 10196600B2, described a method to induce self-lysis in microalgae cells (e.g., Chlorella sp., Micractinium sp., Tetraselmis sp., Isochrysis galbana and Dunaliella sp.). The active substance for self-lysis induction was extracted from Bacillus thuringienses ITRI-G1 suspension by vacuum distillation and isolated by high-performance liquid chromatography (HPLC). Once mixed with microalgal cells, the active substance triggers biochemical responses that induce self-lysis. The cell disruption effectiveness was estimated in terms of released-protein content by A280nm (absorbance at 280 nm) measurements. After one hour of use of the active substance the absorbance (A280nm) was four-fold greater (approximately 1.6) when compared to control (approximately 0.4). The results also demonstrated an increase in protein concentration (of almost two-fold) by mixing the active substance without stirring, which represents a desirable economic aspect.
Table 2 shows several cell disruption methods (mechanical, physical and non-mechanical) employed for processing a range of microalgae species and obtaining target bioproducts.
Table 2. Methods employed for microalgal cell disruption and components extraction.
|
Species |
Description a |
Cell Disruption Method |
Target Bioproduct |
Main Results |
|
Arthrospira |
Filamentous cyanobacteria, no heterocystes or alkinetes, helical shape. Cell wall composed by four layer (L-I and III: fibrillar material, L-II: peptidoglycan and L-IV: lipopolysaccharides) [50] |
Milling in a ball jar with porcelain balls at 60 rpm, for 120 min |
Phycocyanin and phenolic compounds |
74.98 mg C-PC g−1/41.60 mg GAE g−1 |
|
Microwave oven 2450 MHz and 1400 W, 2 min |
85.43 mg C-PC g−1/41.90 mg GAE g−1 |
|||
|
Autoclaving 121 °C and 200 kPa, for 30 min |
1.17 mg C-PC g−1/41.55 mg GAE g−1 |
|||
|
Sonication 20% power at 35 kHz, 50% duty cycle for 7 min |
Phycocyanin |
94.89% (Pf: 6.17) |
||
|
Homogenisation, speed 3 for 3 min |
89.51% (Pf: 5.59) |
|||
|
Freeze-thawing, 8 h |
77.10% (Pf: 4.15) |
|||
|
Botryococcus braunii [35] |
Non-filamentous, pyriform shape (7 x 14 µm), colonies can vary from 30 µm to > 2 mm), cell wall composed by polysaccharide with hydrocarbons between [51] |
Ultrasonication 5–60 kHz, for 3–15 min |
Lipids |
28–30% |
|
Bead-beating at 2000–3500 psi, for 15 min |
35–38% |
|||
|
Autoclave 121 °C and 0.15 MPa, 5–90 min |
38–40% |
|||
|
French-press 500–3000 psi |
29–43% |
|||
|
Microwave oven 0–1250 W at 20–200 °C, under 2450 MHz, for 0–25 min |
25–50% |
|||
|
Osmotic Shock 0–2 MNaCl, stirred for 1 min and maintained 48 h |
18–22% |
|||
|
Chlorella vulgaris [47] |
Non-filamentous, spherical format (3–4 μm) and cell wall composed by extracellular polysaccharides, rhamnose, galactose, xylose [39] |
Ultrasound at 600 W for 15 min and enzymatic lysis with snailase and trypsin (37 °C, pH 4.0) |
Lipids |
49.82% |
|
Haematococcus pluvialis [30] |
Non-filamentous. Cell wall mostly composed by cellulose. Under favorable growth conditions can present flagella and a gelatinous thick extracellular matrix. In motile cells, the lost of the flagella result in changes on the extracellular matrix that become amorphous. Under stress conditions the cells can transform into cysts or aplanospores and a secondary wall is formed [52] |
Freezing-thawing in liquid nitrogen, during 5 cycles |
Astaxanthin |
38–95% |
|
Dimethyl sulfoxide and glass beads. Cycles until pellet became colorless (5 or 10 cycles) |
||||
|
PEF (1 kV cm−1, 50 ms, 50 kJ kg−1) + 6 h incubation |
||||
|
Ultrasound at 80% of amplitude in a 450 W ultrasound, 10 times during 10 s (biomass diluted in ethanol) |
||||
|
Thermal treatment at 70 °C for 1 h |
||||
|
Nannochloropsis sp. [47] |
Non-filamentous, round shape (2–4 μm) and cell wall composed by glucose, cellulose, mannans, rhamnose, fucose, galactose and galacturonic acid [39] |
Ultrasound at 600 W for 15 min and enzymatic lysis with snailase and trypsin (37 °C, pH 4.0) |
Lipids |
11.73% |
|
Scenedesmus dimorphus [47] |
Non-filamentous, bean shape (10–12 μm) and cell wall composed by crystalline glycoprotein and algaenan (non-hydrolyzable structure) [39] |
46.81% |
||
|
Cellulase (20 mg g−1), xylanase (14 mg g−1) and pectinase (10 mg g−1) at 45 °C and pH 4.4 and chemical treatment with chloroform:methanol (1:1 v/v) |
13.8 g 100 g−1 (86.4% recovery) |
|||
|
Hydrothermal treatment with water 1:13 (w/v) at 147 °C for 40 min |
Glucose |
14.22 g L−1 (89.32% recovery) |
||
|
Synechocystis sp. [54] |
Non-filamentous cyanobacteria. Although uncommon, it may be surrounded by a thin, colorless, diffluent mucilaginous envelope. Cell wall composed by a peptidoglycan layer and an outer membrane (mostly proteins and lipopolysaccharide) [55] |
Ultrasound at 20–25 kHz for 30 min (cycles of 5 s on/5 s off) |
Proteins |
94.4% cell disruption efficiency 1.88 mg mL−1 protein |
|
Bead milling in glass beads for 10 min with cycles of 30 s vortexing and 30 s cooling on ice |
54.4% cell disruption efficiency 1.09 mg mL−1 protein |
|||
|
Silicon carbide (200–450 mesh) grinding, 3 cycles of 1 min grinding/1 min cooling on ice |
93.3% cell disruption efficiency 1.89 mg mL−1 protein |
|||
|
3 cycles of freezing at −80 °C for 10 min and thawing at 37 °C for 5 min |
43.3% cell disruption efficiency 0.19 mg mL−1 protein |
|||
|
Phaeodactylum tricornutum |
Pleiomorphic diatom with poorly silicified cell walls (up to 10 silica bands), can present different shapes (fusiform, triradiate and cruciform) and the size range from 8–25 μm [39][58] |
5 cycles·min−1 of sonication at 20 kHz for 15 min |
Carotenoids |
81.7 µg g−1 β-carotene; 679.2 µg g−1 zeaxanthin; 5163.4 µg g−1 fucoxanthin |
|
Soaking in ethanol at room temperature for 24 h, Cryogrinding in a ceramic mortar with liquid nitrogen and deionized water, Planetary micro mill, 2 cycles of 4 min at 400 rpm with 1 min of relaxation time, Potter homogenizer with ethanol for 1–5 min, Homogeniser at 18,000 rpm, 10–180 s, 2–4 cycles and 30 s of relaxation time, Sonication, 2–4 pulsed cycles (10 s on/5 s off), 30% power (500 W) and 30 s relation time, Mixer mill stainless steel grinding jars or propylene grinding tubes, bead-beating with ethanol for 1–4 min and 2–4 cycles. |
Metabolites |
Positive effect in cell disruption: bead-beating, planetary micro mill, sonication and mixer mill (both) Negative effect in cell disruption: soaking, cryiogrinding and Potter homogeniser |
||
|
Mixed microalgae feedstock (Ankistrosdesmus sp., Chlamydomonas sp., Chlorella sp., Micromonas sp. and Scenedesmus sp.) [32] |
Ankistrosdesmus: Non-filamentous with mucilaginous envelopes present or absent, commonly find as colonies, fusiform cells (curved, straight or sigmoid), smooth cell wall [59] |
Acid hydrolysis H2SO4 1.5 M at 80–90 °C for 80 min |
Carbohydrates |
10.2 g maltose, 103.1 g glucose and 68.8 g xylose/galactose per Kg of dry biomass |
|
Chlamydomonas, Chlorella and Scenedesmus: Non-filamentous. Chlamydomonas present a complex multilayer cell wall composed by 20–25 proteins and glycoproteins (rich in hydroxyproline) [60] |
||||
|
Micromonas: Flagellate with absent cell wall |
||||
|
Freeze-dried mixed biomass (95% Scenedesmus obliquus, 4% Scenedesmus quadricauda and 1% Nitzschia sp.) [33] |
Nitzschia sp.: diatom that can occur in three cell types: normal (fusiform, straight or curved, no longer than 35µ), oval (8µ long and 3–4µ broad) or triradiate (arms varying from 6 to10µ) [61] |
Enzymatic lysis with cellulase (from Tricoderma reesei), β-glucosidase (from Aspergillus niger), pH 4.9, 50 °C and 300 rpm for 48 h
|
Carbohydrates (sugars) and byproducts (alcohols and organic acids) |
Total sugars: 9.84 g per 100 g of dry biomass Total byproducts: 1.09 g L−1 |
|
Freeze-dried mixed biomass (61% Aphanothece sp. and 39% Scenedesmus obliquus) [33] |
Aphanothece sp.: cells can occur in many shapes (oval, ellipsoidal, straight or slightly curved) with absent of mucilaginous envelope |
Total sugars: 0.02 g per 100 g of dry biomass Total byproducts: 7.38 g L−1 |
a Complementary data available on: http://algaebase.org. PEF: pulsed electric field. C-PC: C-phycocyanin. GAE: gallic acid equivalents. PUFA: polyunsaturated fatty acids. Pf: purity factor.
Larrosa et al. [26] improved phycocyanin extraction in Arthrospira platensis using microwave irradiation from 85.43 mg∙g−1 to 74.98 mg∙g−1 by milling and 1.17 mg g−1 by autoclaving. The expressive low content of phycocyanin obtained by autoclaving is probably due to protein denaturation caused by the operational conditions. In addition, Chia et al. [49] showed a variation in phycocyanin recovery ranging from 77.10% to 94.89% and purity from 4.15 to 6.17 in A. platensis by changing the cell-disruption method (e.g., freeze-thawing, microwave, homogenization and sonication). Zhou et al. [54] also tested different cell disruption methods in Synechocystis sp. for protein release, achieving the highest cell disruption with ultrasound (94.4%) followed by silicon carbide grinding (93.3%), bead-milling (54.4%) and freeze-thawing (43.3%). As shown in Table 2, the efficiency of the cell-disruption method can significantly change according to the microalgae species and the properties of the target product. In this sense, the use of two cell-disruption methods is a way to enhance cell disruption efficiency and, consequently, the recovery of the target biocompound [62].
4. Conclusion
Several methods are available to disrupt the cell walls and make easily available its content. There is not an universal method that should be used to break the cell wall, and frequently a combination of methods should be preferred, The choice depends not only on the specific microalga species but also on the final objective or the target products. The user must take into consideration important aspects as contamination of the materials to be recovered, cost of equipment, operational costs, among others. Moreover, it is important to emphasize that besides the extraction efficiency and quality of bioproducts, the chosen cell-disruption method can also directly influence the subsequent purification steps.
References
- Meng Wang; Shibao Chen; Wenguang Zhou; Wenqiao Yuan; Duo Wang; Algal cell lysis by bacteria: A review and comparison to conventional methods. Algal Research 2020, 46, 101794, 10.1016/j.algal.2020.101794.
- E. Günerken; E. D'hondt; M.H.M. Eppink; Linsey Garcia-Gonzalez; Kathy Elst; René H. Wijffels; Cell disruption for microalgae biorefineries. Biotechnology Advances 2015, 33, 243-260, 10.1016/j.biotechadv.2015.01.008.
- US Patent 10465159B2
- D’Hondt, E.D.; Martín-Juárez, J.; Kasperoviciene, J.; Koreiviene, J.; Sulcius, S.; Elst, K.; Bastiaens, L.. Microalgae-Based Biofuels and Bioproducts–From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C.; Muñoz, R., Eds.; Woodhead Publishing Series in Energy: Duxford, UK, 2017; pp. 133-154.
- Rajesh Kumar Balasubramanian; Thi Thai Yen Doan; Jeffrey Philip Obbard; Factors affecting cellular lipid extraction from marine microalgae. Chemical Engineering Journal 2013, 215-216, 929-936, 10.1016/j.cej.2012.11.063.
- A. Catarina Guedes; Helena M. Amaro; Maria S. Gião; F. Xavier Malcata; Optimization of ABTS radical cation assay specifically for determination of antioxidant capacity of intracellular extracts of microalgae and cyanobacteria. Food Chemistry 2013, 138, 638-643, 10.1016/j.foodchem.2012.09.106.
- González-Delgado, A.D.; Kafarov, V.; Microalgae based biorefinery: Evaluation of oil extraction methods in terms of efficiency, costs, toxicity and energy in lab-scale. Rev. Ion 2013, 26, 29-37.
- Tatiane Aparecida Gomes; Cristina Maria Zanette; Michele Rigon Spier; An overview of cell disruption methods for intracellular biomolecules recovery. Preparative Biochemistry & Biotechnology 2020, 50, 635-654, 10.1080/10826068.2020.1728696.
- Tom M.M. Bernaerts; Lore Gheysen; Imogen Foubert; Marc E. Hendrickx; Ann M. Van Loey; Evaluating microalgal cell disruption upon ultra high pressure homogenization. Algal Research 2019, 42, 101616, 10.1016/j.algal.2019.101616.
- Anne Elain; C. Nkounkou; M. Le Fellic; K. Donnart; Green extraction of polysaccharides from Arthrospira platensis using high pressure homogenization. Journal of Applied Phycology 2020, 32, 1719-1727, 10.1007/s10811-020-02127-y.
- Agata Piasecka; Izabela Krzemińska; Jerzy Tys; Physical Methods of Microalgal Biomass Pretreatment. International Agrophysics 2014, 28, 341-348, 10.2478/intag-2014-0024.
- Nour Zghaibi; Rozita Omar; Siti Mazlina Mustapa Kamal; Dayang Radiah Awang Biak; Razif Harun; Microwave-Assisted Brine Extraction for Enhancement of the Quantity and Quality of Lipid Production from Microalgae Nannochloropsis sp.. Molecules 2019, 24, 3581, 10.3390/molecules24193581.
- Jae-Yon Lee; Chan Yoo; So-Young Jun; Chi-Yong Ahn; Hee-Mock Oh; Comparison of several methods for effective lipid extraction from microalgae. Bioresource Technology 2010, 101, S75-S77, 10.1016/j.biortech.2009.03.058.
- Kelsey Viner; Pascale Champagne; Philip G. Jessop; Comparison of cell disruption techniques prior to lipid extraction from Scenedesmus sp. slurries for biodiesel production using liquid CO2. Green Chemistry 2018, 20, 4330-4338, 10.1039/c8gc01695j.
- Yunchang Fan; Zeyu Niu; Chen Xu; Lei Yang; Feiyang Chen; He Zhang; Biocompatible protic ionic liquids-based microwave-assisted liquid-solid extraction of astaxanthin from Haematococcus pluvialis. Industrial Crops and Products 2019, 141, 111809, 10.1016/j.indcrop.2019.111809.
- Sooridarsan Krishnan; Noraini Abd Ghani; Noor Fathanah Aminuddin; Khurrum Shehzad Quraishi; Ninna Sakina Azman; Giancarlo Cravotto; Jean-Marc Leveque; Microwave-assisted lipid extraction from Chlorella vulgaris in water with 0.5%–2.5% of imidazolium based ionic liquid as additive. Renewable Energy 2020, 149, 244-252, 10.1016/j.renene.2019.12.063.
- Fanny Adam; Maryline Abert Vian; Gilles Peltier; Farid Chemat; “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresource Technology 2012, 114, 457-465, 10.1016/j.biortech.2012.02.096.
- Juan M. Martínez; Carlota Delso; Ignacio Álvarez; J. Raso; Pulsed electric field‐assisted extraction of valuable compounds from microorganisms. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 530-552, 10.1111/1541-4337.12512.
- Tomás Lafarga; Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Food Reviews International 2019, 36, 559-583, 10.1080/87559129.2019.1655572.
- Toepfl, S.; Heinz, V.; Knorr, D.. Pulsed Electric Fields Technology for the Food Industry; Raso, J.; Heinz, V., Eds.; Food Engineering Series; Springer: Boston, USA, 2006; pp. 197-221.
- Rai Naveed Arshad; Zulkurnain Abdul-Malek; Abdullah Munir; Zolkafle Buntat; Mohd Hafizi Ahmad; Yanti M.M. Jusoh; Alaa El-Din Bekhit; Ume Roobab; Muhammad Faisal Manzoor; Rana Muhammad Aadil; et al. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends in Food Science & Technology 2020, 104, 1-13, 10.1016/j.tifs.2020.07.008.
- G.P. 't Lam; P.R. Postma; D.A. Fernandes; R.A.H. Timmermans; M.H. Vermuë; M.J. Barbosa; M.H.M. Eppink; R.H. Wijffels; G. Olivieri; Pulsed Electric Field for protein release of the microalgae Chlorella vulgaris and Neochloris oleoabundans. Algal Research 2017, 24, 181-187, 10.1016/j.algal.2017.03.024.
- Anna Käferböck; Sergiy Smetana; Ronald De Vos; Christoph Schwarz; Stefan Toepfl; Oleksii Parniakov; Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Algal Research 2020, 48, 101914, 10.1016/j.algal.2020.101914.
- Rui Zhang; Nikolai Lebovka; Luc Marchal; Eugène Vorobiev; Nabil Grimi; Pulsed electric energy and ultrasonication assisted green solvent extraction of bio-molecules from different microalgal species. Innovative Food Science & Emerging Technologies 2020, 62, 102358, 10.1016/j.ifset.2020.102358.
- Jonathan R. McMillan; Ian Watson; Mehmood Ali; Weaam Jaafar; Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Applied Energy 2013, 103, 128-134, 10.1016/j.apenergy.2012.09.020.
- Ana P. Q. Larrosa; Álisson S. Camara; J.M. Moura; Luiz A.A. Pinto; Spirulina sp. biomass dried/disrupted by different methods and their application in biofilms production. Food Science and Biotechnology 2018, 27, 1659-1665, 10.1007/s10068-018-0397-y.
- Esther Lorente; Xavier Farriol; Joan Salvado; Steam explosion as a fractionation step in biofuel production from microalgae. Fuel Processing Technology 2015, 131, 93-98, 10.1016/j.fuproc.2014.11.009.
- Mariam Al Hattab Abdel Ghaly; Microalgae Oil Extraction Pre-treatment Methods: Critical Review and Comparative Analysis. Journal of Fundamentals of Renewable Energy and Applications 2015, 5, 172, 10.4172/2090-4541.1000172.
- Rahul Vijay Kapoore; Thomas O. Butler; Jagroop Pandhal; Seetharaman Vaidyanathan; Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18, 10.3390/biology7010018.
- Juan Manuel Martínez; Zivan Gojkovic; Lorenza Ferro; Marcos Maza; Ignacio Álvarez; Javier Raso; Christiane Funk; Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresource Technology 2019, 289, 121694, 10.1016/j.biortech.2019.121694.
- Reena Singh; Ashutosh Kumar; Yogesh Chandra Sharma; Biodiesel synthesis from microalgae (Anabaena PCC 7120) by using barium titanium oxide (Ba2TiO4) solid base catalyst.. Bioresource Technology 2019, 287, 121357, 10.1016/j.biortech.2019.121357.
- Yessica A. Castro; Joshua T. Ellis; Charles D. Miller; Ronald C. Sims; Optimization of wastewater microalgae saccharification using dilute acid hydrolysis for acetone, butanol, and ethanol fermentation. Applied Energy 2015, 140, 14-19, 10.1016/j.apenergy.2014.11.045.
- Judit Martín Juárez; Ana Lorenzo Hernando; Raúl Muñoz Torre; Saúl Blanco Lanza; Silvia Bolado Rodríguez; Saccharification of microalgae biomass obtained from wastewater treatment by enzymatic hydrolysis. Effect of alkaline-peroxide pretreatment. Bioresource Technology 2016, 218, 265-271, 10.1016/j.biortech.2016.06.087.
- Wen-Can Huang; Jong-Duk Kim; Cationic surfactant-based method for simultaneous harvesting and cell disruption of a microalgal biomass. Bioresource Technology 2013, 149, 579-581, 10.1016/j.biortech.2013.09.095.
- Geun Ho Gim; Si Wouk Kim; Optimization of Cell Disruption and Transesterification of Lipids from Botryococcus braunii LB572. Biotechnology and Bioprocess Engineering 2018, 23, 550-556, 10.1007/s12257-018-0277-6.
- Yi Zhang; Xiaoying Kong; Zhongming Wang; Yongming Sun; Shunni Zhu; Lianhua Li; Pengmei Lv; Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp.. Renewable Energy 2018, 125, 1049-1057, 10.1016/j.renene.2018.01.078.
- Yun Zhou; YenJung Sean Lai; Everett Eustance; Bruce E. Rittmann; PromotingSynechocystissp. PCC 6803 Harvesting by Cationic Surfactants: Alkyl-Chain Length and Dose Control for the Release of Extracellular Polymeric Substances and Biomass Aggregation. ACS Sustainable Chemistry & Engineering 2018, 7, 2127-2133, 10.1021/acssuschemeng.8b04776.
- YenJung Sean Lai; Yun Zhou; Rebecca Martarella; Zhaocheng Wang; Bruce E. Rittmann; Synergistic Integration of C12–C16 Cationic Surfactants for Flocculation and Lipid Extraction from Chlorella Biomass. ACS Sustainable Chemistry & Engineering 2016, 5, 752-757, 10.1021/acssuschemeng.6b02095.
- Mariam Alhattab; Azadeh Kermanshahi-Pour; Marianne Su-Ling Brooks; Dispersed air flotation of Chlorella saccharophila and subsequent extraction of lipids – Effect of supercritical CO2 extraction parameters and surfactant pretreatment. Biomass and Bioenergy 2019, 127, 105297, 10.1016/j.biombioe.2019.105297.
- M. Branco-Vieira; Sergio San Martin; Cristian Agurto; Marcos A.V. Freitas; Teresa M. Mata; António A. Martins; N.S. Caetano; Phaeodactylum tricornutum derived biosilica purification for energy applications. Energy Procedia 2018, 153, 279-283, 10.1016/j.egypro.2018.10.020.
- Suchitra Rakesh; Dolly Wattal Dhar; Radha Prasanna; Anil K. Saxena; Supradip Saha; Madhulika Shukla; Khushbu Sharma; Cell disruption methods for improving lipid extraction efficiency in unicellular microalgae. Engineering in Life Sciences 2015, 15, 443-447, 10.1002/elsc.201400222.
- Lina María González-González; Sergi Astals; Steven Pratt; Paul D. Jensen; Peer M. Schenk; Impact of osmotic shock pre-treatment on microalgae lipid extraction and subsequent methane production. Bioresource Technology Reports 2019, 7, 100214, 10.1016/j.biteb.2019.100214.
- Brenda Hernandez Lopez; Juan Manuel Vera Morales; Extraction of antioxidants from Haematococcus pluvialis by osmotic shock with sucrose, for use in the preparation of syrup for jams. 2018 XIV International Engineering Congress (CONIIN) 2018, -, 1-5, 10.1109/coniin.2018.8489795.
- Apurav Krishna Koyande; Vera Tanzil; Haridharan Murraly Dharan; Manivarman Subramaniam; Ryann Noel Robert; Phei Li Lau; Ianatul Khoiroh; Pau Loke Show; Integration of osmotic shock assisted liquid biphasic system for protein extraction from microalgae Chlorella vulgaris. Biochemical Engineering Journal 2020, 157, 107532, 10.1016/j.bej.2020.107532.
- Young Mok Heo; Hanbyul Lee; Changsu Lee; Juwon Kang; Joon-Woo Ahn; Young Min Lee; Kyu-Young Kang; Yoon-E Choi; Jae-Jin Kim; An integrative process for obtaining lipids and glucose from Chlorella vulgaris biomass with a single treatment of cell disruption. Algal Research 2017, 27, 286-294, 10.1016/j.algal.2017.09.022.
- Paula Mercer; Roberto E. Armenta; Developments in oil extraction from microalgae. European Journal of Lipid Science and Technology 2011, 113, 539-547, 10.1002/ejlt.201000455.
- Kehong Liang; Qinghua Zhang; Wei Cong; Enzyme-Assisted Aqueous Extraction of Lipid from Microalgae. Journal of Agricultural and Food Chemistry 2012, 60, 11771-11776, 10.1021/jf302836v.
- US Patent 10196600B2
- Shir Reen Chia; Kit Wayne Chew; Pau Loke Show; Ao Xia; Shih-Hsin Ho; Jun Wei Lim; Spirulina platensis based biorefinery for the production of value-added products for food and pharmaceutical applications. Bioresource Technology 2019, 289, 121727, 10.1016/j.biortech.2019.121727.
- van Eykelenburg, C. Spirulina platensis: Morphology and Ultrastructure. Ph.D. Thesis, Delft University, Delft, The Nether-lands, 1980. Available online: https://repository.tudelft.nl/islandora/object/uuid%3A744c0474-507a-42c7-b29b-4139f7cb4f22 (accessed on 20 September 2020)
- Gouveia, J.D.G. The Effects of Light Quality on the Morphology and Hydrocarbon Production of Botryococcus braunii. Master’s thesis, University of Exeter, UK, Sep 2010. Available online: https://core.ac.uk/download/pdf/12826395.pdf (accessed on 20 September 2020)
- Sheng-Bing Wang; Qiang Hu; Milton Sommerfeld; Feng Chen; Cell wall proteomics of the green algaHaematococcus pluvialis (Chlorophyceae). PROTEOMICS 2004, 4, 692-708, 10.1002/pmic.200300634.
- Tao Yuan; Xu Jingliang; Shiyuan Xiao; Ying Guo; Weizheng Zhou; Jingliang Xu; Zhenhong Yuan; Microalgae pretreatment with liquid hot water to enhance enzymatic hydrolysis efficiency. Bioresource Technology 2016, 220, 530-536, 10.1016/j.biortech.2016.08.117.
- Jie Zhou; Fuliang Zhang; Hengkai Meng; Guanhui Bao; Yanping Zhang; Yin Li; Development of a silicon carbide disruption method enables efficient extraction of proteins from cyanobacterium Synechocystis sp. PCC 6803. Process Biochemistry 2014, 49, 2199-2202, 10.1016/j.procbio.2014.09.012.
- D. Woitzik; J. Weckesser; U. J. Jurgens; Isolation and Characterization of Cell Wall Components of the Unicellular Cyanobacterium Synechococcus sp. PCC 6307. Microbiology 1988, 134, 619-627, 10.1099/00221287-134-3-619.
- Andrea Gille; Rebecca Hollenbach; Andreas Trautmann; Clemens Posten; Karlis Briviba; Effect of sonication on bioaccessibility and cellular uptake of carotenoids from preparations of photoautotrophic Phaeodactylum tricornutum. Food Research International 2019, 118, 40-48, 10.1016/j.foodres.2017.12.040.
- Benoit Serive; Raymond Kaas; Jean-Baptiste Berard; Virginie Pasquet; Laurent Picot; Jean-Paul Cadoret; Selection and optimisation of a method for efficient metabolites extraction from microalgae. Bioresource Technology 2012, 124, 311-320, 10.1016/j.biortech.2012.07.105.
- Alessandra De Martino; Agnès Meichenin; Juan Shi; Kehou Pan; Chris Bowler; Genetic and phenotypic characterization ofPhaeodactylum tricornutum(Bacillariophyceae) accessions1. Journal of Phycology 2007, 43, 992-1009, 10.1111/j.1529-8817.2007.00384.x.
- Geraldo José Peixoto Ramos; Carlos Eduardo De Mattos Bicudo; Aristóteles Góes Neto; Carlos Wallace Do Nascimento Moura; Monoraphidium and Ankistrodesmus (Chlorophyceae, Chlorophyta) from Pantanal dos Marimbus, Chapada Diamantina, Bahia State, Brazil. Hoehnea 2012, 39, 421-434, 10.1590/s2236-89062012000300006.
- S H Imam; W J Snell; The Chlamydomonas cell wall degrading enzyme, lysin, acts on two substrates within the framework of the wall.. The Journal of Cell Biology 1988, 106, 2211-2221, 10.1083/jcb.106.6.2211.
- Douglas P. Wilson; The Triradiate and other Forms of Nitzschia Closterium (Ehrenberg) Wm. Smith, Forma Minutissima of Allen and Nelson. Journal of the Marine Biological Association of the United Kingdom 1946, 26, 235-270, 10.1017/s002531540001211x.
- Alok Patel; Fabio Mikes; Leonidas Matsakas; An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Micro-Organisms. Molecules 2018, 23, 1562, 10.3390/molecules23071562.