| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aleksandra Rybak | + 3130 word(s) | 3130 | 2021-01-22 02:22:57 |
Video Upload Options
The manuscript covers the issues related to the characteristics, application, and some methods of rare earth elements (REEs) recovery from coal fly ashes. REEs are elements with growing demand and a very wide range of application, especially when it comes to modern technologies. The development of the REE recovery technology would involve solving several problems related to REE speciation, optimization of factors controlling their extractivity and selection of the REE separation method from obtained extraction solutions with a very extreme pH and complicated composition. The paper presented advantages and disadvantages of usually used methods of REE separation from coal fly ashes, like physical and acid–base leaching. It was also presented alternative REE recovery techniques in the form of membrane and biological methods and based on ion liquids (ILs) or chelating agents. The directions of further modifications, which will allow the efficient REE recovery were presented. The aim of this article was to propose specific solutions based on the creation of appropriate multistage method of REE recovery. It will be a combination of magnetic and size separation, acid–base leaching (including roasting in justified cases), removal of matrix elements with ILs (Al, Si, and Fe), and finally REE membrane separation, allowing one to obtain the appropriate process efficiency.
1. Introduction
The rare earth elements (REEs) group consists of seventeen chemical elements, in-cluding fifteen lanthanides and yttrium and scandium (occur in the same ore deposits and have similar chemical properties). All these elements exist in the form of trace quantities in natural materials, which are unevenly distributed around the world (estimated average concentration in the Earth’s crust ranging from around 130 to 240 µg/g). They could be categorized in many ways, among others as critical (Nd, Y, Eu, Dy, Tb, and Er), uncritical (Pr, Sm, La, and Gd), and excessive (Tm, Ce, Ho, Lu, and Yb) or as light rare earth elements (LREEs) and heavy rare earth elements (HREEs). LREEs are in the form of bastnasite ((Ce, La)CO3(F,OH)) and monazite ((Ce,La,Nd,Th)PO4), while HREEs are usually present in the form of apatite, cheralite, eudialyte, loparite, phosphorites, rare-earth-bearing (ion adsorption) clays, secondary monazite, and xenotime. The chemical and physical properties of rare earth elements are due to the peculiar nature of their electron configuration. These elements differ from one another by the number of inner-core electrons in the 4f subshell, from lanthanum to lutetium. Lanthanides have a similar electronic structure of the type 1s22s22p63s23p63d104s24p64d104fn5d16s2 or 4fn+16s2. The 4f electrons have lower energies and are a part of the ion core, lying inside, surrounded by outer valence electrons. Therefore, they do not participate directly in the formation of bonds with other elements and the formation of chemical compounds. Hence their chemical similarity, mutual presence in various minerals, and difficulties with their separation. The outer or valence electrons of the 14 lanthanides and lanthanum are the same, 5d16s2 or 6s2, for the scandium 3d14s2, and for yttrium 4d15s2. However, there are some differences in the chemical properties of the lanthanides due to the lanthanide contraction or the mixing of the 4f electrons with valence electrons. The phenomenon called lanthanide contraction is caused by an increase in nuclear charge that is not completely screened by the additional 4f electron as it moves from one lanthanide to the next. Therefore, electrostatic attraction of electrons by the nucleus increases, and, thus, the atomic and ionic radii decrease. This is an important characteristic property of lanthanides that results in two features. Firstly, there are only small differences in their chemical properties as their atomic number increases, because of the similar ionic radii and valence states. Secondly, there are major differences in REE atomic spectra and magnetic properties. This contraction is also responsible for the decreased basicity from lanthanum to lutetium and is the basis of various separation techniques [1,10].
Namely, the normal valence state is mainly the trivalent oxidation state, Ln(III). However, cerium could occur in the tetravalent oxidation state, Ce(IV), and europium and ytterbium occur in the divalent oxidation states, Eu(II) and Yb(II). Cerium, praseodymium, and terbium lose electron 4f when they become ionic, and their radii decrease. On the other hand, samarium, europium, and ytterbium gain the 4f electron during the transition to the ionic state and their radii increase. This can be used to separate Ce, Eu, and Yb from the rest of the trivalent ions. The electronic structure also influences their melting points, which increase with increasing atomic number (from 798 °C for cerium to 1663 °C for lutetium), while the melting points of scandium and yttrium are comparable to the last representatives of the trivalent lanthanides. In turn, the low melting points of europium and ytterbium result from their divalence. The boiling points of rare earth elements also differ. This is due to the difference in electronic states when they are solid or gaseous. Namely, the boiling points of Ce, Pr, Y, and Lu are the highest, while those of Eu and Yb are the lowest. This is because Ce, Pr, Y, and Lu have three outer electrons in both states, while Eu and Yb only have two outer electrons. The remaining lanthanides are trivalent solids, but their gaseous forms have two outer electrons. The electrical resistivity of rare earth elements varies from 29 to 134 μΩ·cm. Most trivalent REEs have resistance in the range 57–90 μΩ·cm. The lowest resistance 29 μΩ·cm relates to the divalent Yb, while the highest to Gd and Tb, which result from the magnetic contribution to the electrical resistivity near the magnetic ordering temperature of a material. In turn, the magnetic properties of rare earth elements depend on the number of unpaired 4f electrons. Metals that do not have unpaired electrons such as Sc, Y, La, Lu, and Yb are weakly magnetic. The remaining lanthanides from Ce to Tm are strongly magnetic because they have unpaired 4f electrons. Most of them have at least two magnetic structures. Where Gd, in which all the 4f spins are aligned in one direction, is ferromagnetic. The remaining REEs, in turn, have 4f spins that align antiparallel to each other, which is why they are antiferromagnetic [10].
As for the chemical reactivity of rare earth elements, it depends largely on their type and the difference between light and heavy lanthanides. For example, light lanthanides oxidize much faster than heavy lanthanides (gadolinium to lutetium), scandium, and yttrium. This difference is partly due to the variability of the resulting oxide product. For example, light lanthanides (from lanthanum to neodymium) form the hexagonal A-type REE2O3 structure; middle lanthanides (from samarium to gadolinium) form a monoclinic B-type REE2O3 phase; while heavy lanthanides, scandium, and yttrium form a cubic C-type REE2O3 modification. Metallic europium, which has the body-centered cubic (bcc) crystal structure, oxidizes the most rapidly of all rare earth elements, and in humid air forms hydrate oxide Eu(OH)2.H2O, which is an unusual reaction product because all other rare earth metals form oxides. REEs react vigorously with all acids except hydrofluoric acid (HF), releasing H2 gas and forming the corresponding rare earth anionic compound. Rare earth metals also react readily with hydrogen gas to form REEH2 and, under high hydration conditions, an REEH3 phase—except Sc, which does not form a trihydrate. They are also the most electropositive elements, forming an ionic bond in solids, so they are extremely reactive with hydrogen and electronegative elements such as halogens, oxygen, nitrogen, and sulphur, creating, stable hydrides, halides, oxides, nitrides, and sulphides. This property is used extensively for the removal of impurities from high-purity atmospheres or for the preparation of hydrogen storage compounds used in rechargeable secondary batteries. Just like other reactive metals, REE physical properties are strongly influenced by the amount of interstitial impurities (oxygen, nitrogen, carbon, and hydrogen) present in their metal lattice structure [4,10]. Currently, REE become more and more important because of their unique chemical, physical, catalytic, luminescent, and magnetic features and of course many industrial applications (electronics, clean energy technologies, defense, optics, automotive, agriculture, medicine, energy, etc.) [11]. For instance, neodymium is applied in super magnets for disk drives, cerium in autocatalysts and all in flat-panel TVs production and smart batteries for electric vehicles [1]. Due to their application all modern gadgets can be more efficient, smaller, faster, and lighter. Therefore, the demand for REE will continue to increase in the near future [6].
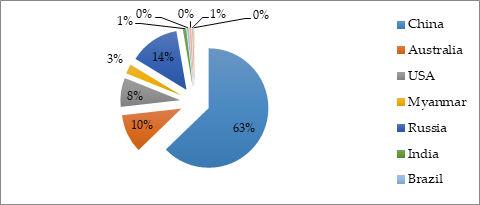
In the twentieth century, REE were mined mainly in California and Australia [12]. Currently, the main REEs producers are China (63%), Russia (14%), Australia (10%), USA, Myanmar, Brazil, and India (Figure 1) [1]. Except that also Kazakhstan, Kyrgyzstan, Tajikistan, Uzbekistan, and Turkmenistan show some REE mineral occurrence [13,14]. However, the largest REE exporter is China. Its monopoly forces other countries to look for other alternative sources and developing REE recovery techniques. Except that the conventional REE mining techniques, which include ore deposits, are energy-intensive and generate significant volumes of toxic wastes and acidic wastewater [3]. Therefore, new sources of REE should be sought. It turned out that coal fly ashes could be one of their sources.
Figure 1. Rare earth element (REE) production in the world in 2020.
2. Characterization and Recovery Methods of REE from Coal Fly Ashes
2.1. Physical Separation Methods for REE Recovery
In traditional processes for REE obtaining from REE ores are used physical separation methods, like magnetic, size and density separation, electrostatic separation, and flotation. However, their application in REEs recovery from fly ashes was rather limited. The flotation process is effective for the particle size range from 10 to 100 µm. So, the process of fly ashes flotation for REE minerals below 5 µm had lower efficiency (20–30% of REE recovery). The conventional flotation with the usage of fatty acids and octanohydroxamic acid (OHA) was not efficient [41–43]. That is why the novel ultrafine particle concentrator known as a hydrophobichydrophilic separator (HHS) was introduced. Further modification of rare earth mineral concentration process consisted in the application of the primary and secondary hydrophobizing agent (potassium octylhydroxamate (KOHX) and sorbitan monooleate (SMO), respectively). Honaker et al. achieved in that way, the enrichment ratio 53:1 [38].
In turn, Dai et al. used the magnetic separation method. Using this method, they have separated fly ash in three fractions: magnetic, MCQ (mullite + corundum + quartz), and glass phases. They have also stated that depending on the type of REEs they were enriched in various phases. Namely heavy REE were more enriched in the glass phase, when light REE were more enriched in the magnetic and MCQ phases [41,44–46].
The content of REE in fly ash depended also on its granulation and it was found that their concentration increased with the decrease of particle size [17,39,47]. Considering, the influence of these parameters on REE concentration, Blissett et al. used a multistage REE enrichment consisting of froth flotation, magnetic separation, and hydrocyclone separation, getting enrichment of REE from 419 to 529 ppm [43]. If it is going about density separation it was found that REE concentration was not the highest in the highest density fractions as it was expected. That indicates again that REE are trapped in other minerals and are not effectively released using physical methods [38,39,41].
2.2. Acid and Alkaline Leaching Methods
According to many authors the REEs in coal fly ashes are mainly in the form of fine minerals dispersed in the aluminosilicate glass phase (strong correlation between Al oxide and REE content). Analysis of mineralogical composition of coal fly ashes has shown that they consist of over 70% of amorphous glass and almost 30% of mineral phases, like quartz, iron oxides, and mullite [33,48]. While their dissolution in 4% HF has shown that about 90% of REEs is associated with amorphous glass. This means that aggressive extraction methods will be required [11]. As the main target will be the aluminosilicate phase, the methods used for Al recovery (acid leaching, sintering with alkali reagents followed by acid leaching, and pyrometallurgical methods) may be applicable to REE extraction [49–52]. The recovering process of REEs from coal fly ashes consists of a few steps, firstly the REE should be leached into solution and secondly extracted from the solution with appropriate reagents. This first step is very often the limiting one, because of low leaching efficiency. Usually as a first step of REE extracting from fly ash acid leaching was used. The next method was alkaline leaching and the method combined acid and alkaline leaching, which was introduced to reduce the reagents usage and maximize the REE extraction efficiency. So far, an extensive research was done on characterization of fly ash composition, but very little research has been done on effective REE extraction methods from coal fly ash (no commercialized technologies until now) [19].
2.3. REE Recovery Methods from Obtained Leachates
As it was mentioned above during the leaching of elements from coal fly ash were used strong acids like for instance HCl, HNO3, H2SO4, or alkalines, like NaOH, which seems to be quite efficient in some cases. However, such leachates are characterized by extreme pH and complex composition (major elements, like Si, Ca, Fe, Al, Na, and Mg, which have much higher concentrations than REEs) [69]. To solve that problem some conventional, but also innovative techniques were proposed. From chemical methods of REE recovery were used adsorption on special adsorbents (double layered hydroxide, activated carbon, or alginic acid) [70–74], ion exchange [35,68], and chemical precipitation [69]. However, these chemical methods have some limitations, like high energy consumption and application of huge amounts of chemical reagents, which is associated with high environmental and operational costs. To solve this problem, membrane techniques, biological methods, ionic liquids, and chelating reagents were proposed for REE separation from leaching solutions. Most effective techniques for the recovery of REE from leachate solutions use their presence in the form of trivalent ions REE3+, which form, for example, characteristic complexes with complexing or chelating agents, react with carrier molecules or are used as template ions in creating ion imprinted polymers (IIPs). Unlike the rest of the matrix ions in the separated solution.
3. Summary and Proposition of Procedure for the Recovery of REEs from Coal Fly Ash
The authors have presented advantages and disadvantages of methods usually used for REE recovery from coal fly ashes, like physical, acid–alkaline leaching, and techniques for REE separation from obtained leachates. Unfortunately, the application of physical methods of REE recovery (flotation, magnetic separation, or based on size, density, and electrostatic properties) was ineffective, especially for fly ashes [38,39,41]. Of course, to create the most efficient method of REE recovery should be solved several problems, connected with the speciation of REE, composition of fly ash (especially existence of Ca, Mg, and Fe compounds), and the optimization of factors controlling the extractivity of REE. A solution to these problems could be found by comparing and combining results obtained from a few different techniques focusing on REE speciation (ICP-MS, S/TEM-EDX, XRD, EPMA, μXRF, μXANES, SHRIMP-RG, etc.) and their extractivity (sequential acid extraction and leaching) [5].
The next group are methods based on acid and base leaching. In their case, the main factors deciding about the process efficiency are connected with physicochemical characteristics of the ashes, leaching of other metals (in much higher concentrations) along with REE, and the influence of the extract composition on further preparation of the REE concentrate. Unfortunately, they are also characterized by a high consumption of reagents and energy [11,59–61]. Therefore, numerous modifications have been introduced, such as roasting with various reagents, selection of optimal parameters, and removal of Fe oxides from the ashes, to enhance REE recovery. It was found that the efficiency of acid leaching method was largely dependent on the temperature (optimal increased by approximately 80 °C), type of acid, and physicochemical composition of the ash (higher CaO content caused greater REE leachability) [19,53]. The combination with physical methods such as flotation, magnetic, and size separation, allowed one to increase the recovery (92–108%) [38]. Another modification was alkaline roasting of ashes with compounds such as Na2O2 and NaOH [11]. In turn, the alkaline leaching was much less used than acid leaching, maybe because of lower process efficiency (49–86%) [19]. To increase the REE recovery it was combined with HCl leaching (recovery 88%) and the preliminary stage of magnetic separation and sieving (recovery over 100%) [57]. Unfortunately, conventional methods, such as adsorption, ion exchange, and precipitation, used to recover REE from the obtained extracts have many limitations [70–73]. Therefore, alternative methods were introduced, such as membrane techniques, biological methods, and methods based on chelating compounds and ionic liquids. The main problems that must be faced in the case of REE recovery is the high complexity of the obtained solutions, extreme pH, and high concentrations of the remaining ash components, extracted along with the REE. It was stated that all applied membranes allowed one to obtain REE enrichment above 90%, especially in the case of techniques based on NF and ELM and HFLM (almost 100%) [77–81]. To increase the efficiency of this process and extend the life of the membrane were proposed some modifications, such as combination with an additional MF stage, complexation with EDTA or DTPA (increase of REE recovery and removal of Fe, Al, and Si) or introduction of IIPs (separation of individual REEs from mixture) [69,77,82,83]. The second group are biological methods based on bioleaching with the help of microorganisms. It was stated, that REE recovery depended mainly on the source of these elements (higher from of ashes and monazite, over 63%) and on the type of applied microorganisms (the highest with Candida bombicola and Aspergillus ficuum—over 74%) [84,93]. However, these processes are less efficient than other techniques, but are environmentally friendly. The next method is based on the use of ionic liquids. It was stated that the use of appropriately selected ionic liquids and multistage extraction allows for enrichment of REE above 95% [101,102]. While, the use of popular chelating compounds, such as EDTA, EDDS, and HIDS, made it possible to obtain small REE recovery (18% of Ce) [105]. Another solution may be the use of tailored complexing compounds, such as H3Tri-NOx, which allows for obtaining much higher REE enrichment (until 94%) [106].
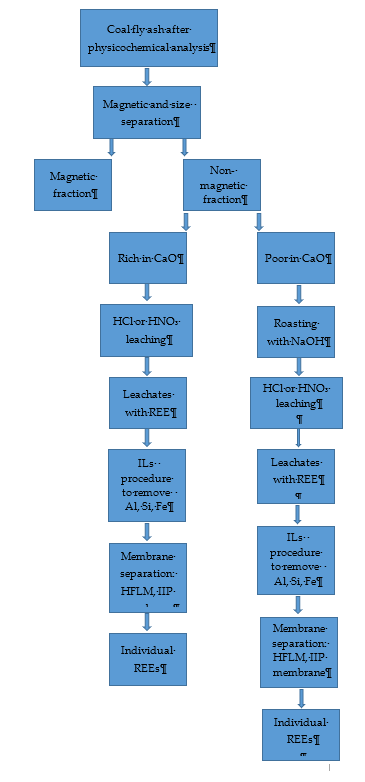
So, it can be seen, that work on developing an efficient and ecological method of REE recovery from fly ash should be continued. One of the solutions may be the introduction of efficient technology of REE leaching from waste after coal combustion and for separating individual REEs from the complex extracts with extreme pH and REE low concentrations, compared to other matrix components. For this purpose, membrane techniques or techniques based on ionic liquids can be used. Considering the above-mentioned data, it should be stated that the most effective method of REE recovery from ashes (Figure 2) would be to combine physical methods, like magnetic and size separation with acid leaching under appropriate conditions, eventually combined with roasting with NaOH. Then removal of matrix elements from leachates using ILs, and finally REE separation using membrane methods based on HFLM and IIPs membranes (recovery above 90%).
Figure 2. Scheme of the proposed procedure for the recovery of REEs from coal fly ash.