
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ljubomir Vitkov | + 3443 word(s) | 3443 | 2021-01-19 14:56:07 | | | |
| 2 | Peter Tang | Meta information modification | 3443 | 2021-01-27 04:40:43 | | |
Video Upload Options
Periodontitis is a general term for diseases characterised by inflammatory destruction of tooth-supporting tissues, gradual destruction of the marginal periodontal ligament and resorption of alveolar bone. Early-onset periodontitis is due to disturbed neutrophil extracellular trap (NET) formation and clearance. Indeed, mutations that inactivate the cysteine proteases cathepsin C result in deficient NET formation and concomitant early-onset periodontitis. In contrast, exaggerated NET formation due to polymorphonuclear neutrophil (PMN) hyper-responsiveness drives the pathology of late-onset periodontitis by damaging and ulcerating the gingival epithelium and retarding epithelial healing. Periodontitis progression ends with almost complete loss of the periodontal ligament and subsequent tooth exfoliation.
1. Introduction
Neutrophil extracellular traps (NETs) are evolutionary conserved innate immunity structures produced by activated polymorphonuclear neutrophils (PMNs) mainly as a response to pathogen challenge. NETs have a backbone of DNA and are decorated with histones and neutrophil protease, as well as other bactericidal agents, such as lactoferrin, cathepsins and myeloperoxidase (MPO) [1][2][3]. NET formation can be stimulated by activation of neutrophils via receptors for (I) chemokines, (II) cytokines, (III) immune complexes, (IV) pathogen-associated molecular patterns (PAMPs), (V) damage-associated molecular patterns (DAMPs), (VI) C3a or 5a, and (VII) complement C3 and C4 and their derivatives [4]. Many of the mechanisms for NET formation initiated by engagement of the aforementioned receptors are linked to the NADPH oxidase (NOX) machinery, but NOX-independent processes have also been described [5][6][7]. Upon activation, the azurophilic granular proteins NE and MPO translocate to the nucleus to promote nuclear and chromatin decondensation [8]. Histone hypercitrullination by peptidylarginine deiminase 4 (PADI4) also contributes [9]. However, NE- and PADI4-independent pathways have also been reported [10][11].
Depending on the context, NETs are known to exert either pro- or anti-inflammatory effects. NETs induce the inflammasome [12], type I interferons and pro-inflammatory cytokines; damage the endothelium [13]; and can occlude ducts in various organs, thereby promoting organ damage [14][15][16]. Furthermore, disbalanced NET formation carries the risk of developing periodontal pathologies.
In areas of low neutrophil densities (e.g., in blood or during early stages of inflammation), individual neutrophils form NETs and trap pathogens in an inflammatory manner, releasing their load of proinflammatory mediators (e.g., cytokines, chemokines). Consequently, further neutrophils are attracted, and the density of neutrophils increases. In areas of high cell densities, the NET-forming neutrophils clump together and form felted aggregates (aggNETs). The latter initially trap and finally degrade several inflammatory mediators. The NET structures exert anti-inflammatory effects leading to downregulation of inflammatory responses by promoting the trapping and cleavage of pro-inflammatory mediators through NET-bound proteases [17][18][19]. This process initiates the resolution of inflammation.
Periodontal inflammation starts as gingival inflammation, which is completely reversible. Periodontitis is either early onset (earlier denoted aggressive) or late-onset (earlier denoted chronic). Despite the common morphological destruction of the periodontium, these disease entities differ in their inflammatory response. The early onset periodontitis affects both human dentitions and is a consequence of genetic defects [20] mostly concerning PMNs. Impaired neutrophil extracellular trap (NET) formation, resulting from e.g., mutations that inactivate cathepsin C [21] or neutrophils elastase (NE) [22], are accompanied by severe forms of periodontitis. In contrast, late-onset periodontitis predominantly emerges in individuals with an age above 35 years [23]. It is characterised by a hyper-responsiveness of the PMN [24][25][26][27][28]. Late-onset periodontitis is a heritable [29] inflammatory age-related disease of tooth supporting tissues [30], characterised by resorption of periodontal ligament and alveolar bone. With the resorption of periodontal ligament, the marginal gingival sulcus deepens, and the so-called periodontal pocket forms, a deep narrow space enclosed between the gingival epithelium and the dental root. The denudate root is frequently covered with subgingival concrements and/or dental plaque and is emerged in gingival crevicular fluid, a blood plasma transudate [31] rich in activated neutrophils and NETs [32][33] (Figure 1). Late-onset periodontitis progression takes place through recurrent bursts of destruction followed by varying periods of stagnation. This temporally and spatially haphazard mode of disease progression has been named the random burst model [34][35][36]. Periodontitis progression ends with tooth exfoliation [37].
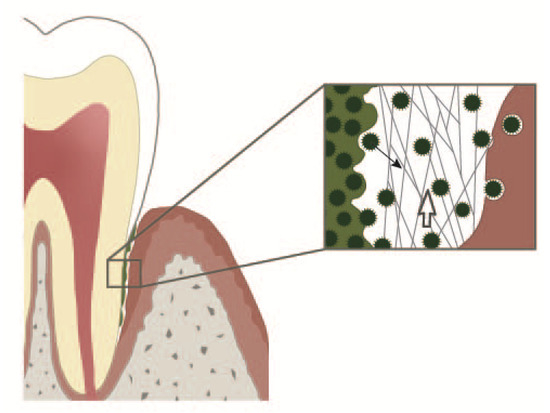
Figure 1. Periodontal crevice. On the left: subgingival plaque. On the right: an epithelial cell with an adherent bacterium and bacteria in different stages of internalisation. In the middle: periodontal crevice. Neutrophil extracellular trap (NETs) build a 3D web work protecting the gingiva from bacteria dispersing out of the subgingival plaque. NETs together with the entrapped bacteria are continuously pushed into the oral cavity by the crevicular exudate outflow. Open white arrow: direction of the crevicular exudate outflow. Solid black arrow: a bacterium dispersing from the subgingival plaque.
Currently, the prevailing opinion is that inflammatory bone resorption is the main driving force of alveolar bone destruction in late-onset periodontitis [38][39][40][41]. Osteoclast differentiation and activation are promoted by the binding of receptor activator of nuclear factor kappa-Β ligand (RANKL) (expressed by osteoblasts and activated T-cells and B-cells) to RANK on osteoclast precursors; osteoprotegerin acts to block the RANKL–RANK interaction and restrains osteoclastogenesis. It has been assumed that Th17 cells, activated by oral pathogens, induce bone destruction via production of RANKL and subsequent osteoclast proliferation. Following the hypothesis of the crucial IL17 role in late-onset periodontitis [40][41] and considering the fact that the implant–gingiva attachment is not tighter than the tooth–gingiva one, dental implants in patients with late-onset periodontitis should also be prone to inflammatory osteolysis to the same extent, but this is not the case. Dental implants in patients with late-onset periodontitis have the same or only slightly reduced surviving rate as compared to patients without late-onset periodontitis [42]. The lack of positive effects of bisphosphonates on late-onset periodontitis in humans [43] indicates that the inflammatory osteolysis [38][39][40][41] cannot be the main reason for late-onset periodontitis. All of these findings cast doubt on the idea that Th17-induced osteolysis be the primary driving force of bone resorption in late-onset periodontitis.
Many epidemiological data demonstrate that both cells and molecules of the innate and adaptive immune response are adversely impacted by aging, yielding a reasonable tenet that the increased periodontitis noted in aging populations is reflective of the age-associated immune dysregulation [44][45]. Thus, an increased basal level of NF-κB activation has been reported in dendritic cells (DCs) from aged subjects [46]. The epithelial cells in the mucosa secrete factors, such as retinoic acid and TGF-β, which act upon DCs to induce tolerance to prevent response against harmless antigens and commensal microbiome [47][48][49]. DCs from aged subjects display impaired response to retinoic acid and are deficient in inducing T regulatory cells for tolerance [50]. The basal level of activation of DCs from aged subjects leads to low-level secretion of pro-inflammatory cytokines, which activates the epithelium even in the absence of infection [51]. Thus, PMN hyper-responsiveness in late-onset periodontitis PMN [24][25][26][27][28] and loss of tolerance [52], i.e., an exaggerated immune response, appears reasonable as a main cause of late-onset periodontitis [53][54][55][56][57].
2. NET Formation and Aggregation in Late-Onset Periodontitis
Canonical NETs, adequate to bacterial challenge, are a main protector of periodontal tissues, and insufficient NETs cause severe early-onset periodontitis [56]. However, exaggerated NET formation may be responsible for tissue damages in late-onset periodontitis. The exaggerated PMN response in late-onset periodontitis may have various causes: one of them is the hyper-responsiveness of PMNs in late-onset periodontitis [24][25][26][28]. Additionally, altering the mucosal PMNs due to the mucosal transmigration takes place, particularly characterised by delayed PMN apoptosis [58][59], as evident by transcriptome changes in oral PMNs [60]. NETs form exclusively on the surface of the crevicular (pocket) epithelium [32]. Deep pockets have a resting volume of up to 1.5 µL and also gingival crevicular fluid flow rates of up to 44 µL/h [34], i.e., the gingival crevicular fluid has a very short dwelling time of nearly 2 min within the crevice. However, the time of PMN adhesion to the epithelial surface may prolong the PMN dwelling time within the crevice. Nevertheless, this time is too short, as no PMNs accumulate on the crevicular surface in late-onset periodontitis [61]. However, the canonical NET formation requires at least 2 h [2]. The only type of NET formation in a few minutes is the bicarbonate-induced non-canonical NET formation [15].
Cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in a number of epithelia in humans, including the gingival epithelium. Moreover, CFTR expression in gingival epithelial from periodontitis biopsies is strongly elevated and even present in the gingival connective tissue [62]. Degradation of HCO3− by crevicular carbonic anhydrases (CAs) causes alkalinisation. Indeed, CA-1 is regularly located in PMNs [63], some epithelia [64] and both healthy [65] and CPD-affected gingiva [66]. In particular, elevated CA-1 of gingival crevicular fluid in late-onset periodontitis [65][66][67][68] suggests a connection to elevated pH of gingival crevicular fluid. The pH of gingival crevicular fluid varies in late-onset periodontitis from 6.9 up to 8.7 due to the high bicarbonate concentration [69][70], but direct measurement of pH within the gingival pocket has yielded a somewhat lower range of 6.35–8.10 [71]. Gingivitis does not appear to be associated with major changes in crevicular pH, and the mean crevicular pH is near 7.0, but in about 7% of periodontitis individuals, there is at least one site with a pH between 7.5 and 7.8 [65]. Bicarbonates above 37 mM and pH above 7.55 cause extremely quick NET formation, namely non-canonical NETs [72], as in the case of pancreatitis [15]. Thus, a bicarbonate non-canonical NET formation may be expected in the alkaline gingival crevicular fluid of late-onset periodontitis. The elevated gingival CFTR expression in late-onset periodontitis [62] is an important requirement for the exaggerated NET formation, as it enables the bicarbonate enrichment and alkalosis of gingival crevicular fluid. Cystic fibrosis (CF) patients have mutated CFTR and completely lack the ability to secrete HCO3−. However, data concerning the late-onset periodontitis in CF patients are inconclusive [73] due to fact that the life expectancy of CF patients is below 50 years, and this period is too short for the development of late-onset periodontitis. However, gingivitis, the precursor of late-onset periodontitis, is significantly lower in adult CF patients as compared to healthy controls [73][74], and the periodontal risk is at a low level [75]. Particularly, aggregated NETs (aggNETs) [18] have also been reported [32] in purulent gingival crevicular fluid, but the final stage of bicarbonate-induced NETosis [72] has never been reported, probably due to the short dwelling of PMNs within the gingival periodontal crevice.
Exaggerated NETs and, hence, oversupply of proteases in the crevice are major tissue-damaging factors in late-onset periodontitis.
3. Periodontal Damage by PMNs, NETs and NET Degradation Products
In periodontal tissues and crevices, both serine and metalloproteases are almost exclusively delivered by PMNs [76][77][78]. The NETs may dissolve the basal lamina [79] and damage or even kill the epithelial cells [80] causing epithelial ulceration. Indeed, increased gingival crevicular fluid levels of laminin-332 [81][82][83], PMN proteases [76][77][78] and the epithelial ulceration in late-onset periodontitis [84][85] have been reported. Epithelial ulceration may be assisted by periodontal pathogens [86] and enables an unimpeded influx of NET-derived proteases into the periodontal ligament. Patients with late-onset periodontitis are regarded as being immunised with periodontal pathogens [87]. Immunisation against bacterial components and the subsequent formation of immune complexes induces PMN infiltration, NET formation [88][89] and thus precipitates periodontitis [87][90]. Smoking further aggravates late-onset periodontitis [91][92][93][94]. Cigarette smoke fosters NET formation [95] and PMN-derived proteases, which are responsible for the connective tissue breakdown [96]. In both mentioned cases of external damaging factors, PMNs and NETs are key mediators of the periodontal tissue injury.
Regardless of how destruction of the junctional epithelium and the underlying periodontal ligament takes place, it inevitably results in long junctional epithelium formation [87] (Figure 1). After destruction of marginal periodontal ligament, keratinocyte proliferation provides the seal of the connective tissue wound, namely, the long junctional epithelium, which replaces the destroyed connective tissues. As a result, the marginal periodontal ligament is gradually destroyed with each exacerbation [34][35][36] up to exfoliation of the affected tooth.
Alveolar bone resorption follows the loss of periodontal ligament, as demonstrated in cases post extraction [97][98] and in animal models [99][100][101]. Thus, bone remodelling rather than inflammatory osteolysis appears to be responsible for the alveolar bone resorption in late-onset periodontitis.
4. Crevicular Occlusion by NETs
The periodontal crevice, i.e., the space between dental root and the crevicular gingiva, is filled with gingival crevicular fluid. The length of the crevice increases with deepening of the periodontal pocket and can even rich the root apex. Gingival crevicular fluid is continuously produced by the gingival epithelium and drained into the oral cavity. Thus, the crevice may be viewed as a duct, which evacuates the gingival crevicular fluid along with bacteria, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) from the crevicular bottom into the oral cavity (Figure 1). The quantity of gingival crevicular fluid and its density, i.e., content of NETs, depend on whether remission or exacerbation of late-onset periodontitis takes place. In most heavy cases of exacerbation, purulent exudate is produced [102]. This exudate is rich in aggNETs and extremely viscous [32]. The extracellular DNA excess of aggNETs cause increased viscosity [103][104] of gingival crevicular fluid and, as a result, obstruction of or even possibly occlusion of gingival crevicular fluid evacuation out of the crevice. Such a mechanism of a duct occlusion by bicarbonate-induced NETs has been reported in pancreatitis [15][72]. Marginal occlusion of the pocket leads to gingival crevicular fluid retention, abscess formation in the pocket and its extension into the surrounding periodontal tissues [105][106][107]. In a few cases, pH of saliva in the quiescent state is 7.5 or somewhat higher, but does not reach 8.0 [108]. However, as a reaction to oesophageal charges, salivary pH rises up to 8.02 (a 3.3-fold increase), and the salivary bicarbonate concentration also rises, showing a 3.7-fold increase [109][110]. Thus, in certain cases, high salivary pH might critically accelerate NET formation in the marginal periodontal crevice and contribute to its occlusion. Furthermore, salivary sialyl LewisX induces NETs within 15 min, thus possibly contributing to the occlusion of the marginal periodontal crevice [111]. Generally, two modes of periodontal abscess development in periodontitis patients have been reported: (i) the unprompted one in untreated periodontitis and (ii) after periodontitis treatment [106]. The unprompted periodontal abscess might be a result of occlusion by bicarbonate NETs, whereas after periodontitis treatment, an additional mechanism may also come into question. Surgical wounds in marginal areas of the periodontal pocket heal by long junctional epithelium formation [87] in three days, even in the absence of stromal healing [112]. This ability is due to the high activation of the oral epithelium showing upregulation of genes linked to epithelial and immune cell migration, as well as increased proliferation, as demonstrated by analysis of the transcriptome of the oral epithelium [112]. The capability of the gingival epithelium to "reattach" to the root surface by long junctional epithelium formation is routinely used in the modified Widman flap surgical technique [113], where, however, the whole crevicular epithelium is removed. Healing the marginal areas of the periodontal pocket comprises the epithelial attachment to the root surface and may lead to occlusion of deeper parts of the periodontal pocket (Figure 2). The contaminated pocket epithelium below the epithelium reattachment continues to produce gingival crevicular fluid, which cannot be eliminated by draining. The marginal crevicular occlusion may further increase the PMN transmigration into the crevice and ultimately results into periodontal abscess formation.
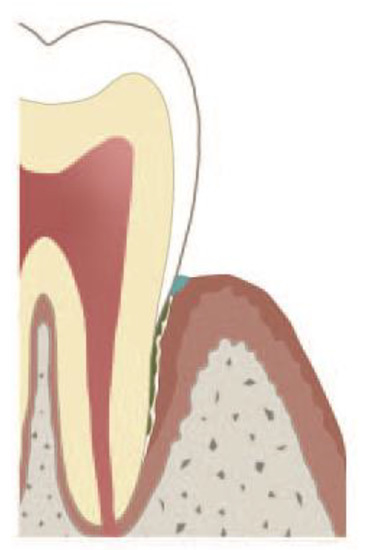
Figure 2. Occlusion of marginal pocket either by aggregated NETs (aggNETs), or by attachment of the marginal epithelium to the root. The underlying pocket epithelium continues to secrete gingival crevicular fluid, and polymorphonuclear neutrophils (PMNs) continue to transmigrate. The exact nature of the plug (depicted blue) has not been clarified yet [87][113].
Independent of how the crevice occludes, the pocket epithelium below the place of occlusion sustains PMN transmigration and NET formation, and this retention yields a periodontal abscess.
5. NETs in Gingival Ulceration Healing
The pocket epithelium is characterised by the tendency to reveal micro-ulcerations [84]. Epithelium ulcerations increase the chance for invasion of microorganisms and the penetration of their PAMPs into the soft connective tissue, thereby aggravating the course of periodontitis. However, transitory bacteraemia is not pathognomonic for periodontitis. Mechanically caused gingival micro-ulcerations result in transitory bacteraemia lasting minutes [114][115][116]Similarly, the skin, which has a considerably larger surface, undergoes frequently multitudes of micro-traumas such as insect bites or minor skin scratches, which also results in transitory bacteraemia [117]. A main task of NETs is to limit the bacterial spreading [1]. However, in late-onset periodontitis [118][119] and in experimental periodontitis [40], i.e., in a heavily contaminated pocket epithelium, crevicular NETs are overwhelmed and appear unable to completely prevent periodontal pathogen dissemination to inner organs, a finding demonstrating the limitation of NETs to prevent bacterial spreading in a heavily contaminated environment. However, exaggerated NET formation suppresses wound healing [120]. Three findings indicate the suppressive effect of exaggerated NET formation in late-onset periodontitis: (i) increased NET degradation has been observed in late-onset periodontitis as a consequence of periodontal therapy [121]; (ii) the correlation between diabetes and late-onset periodontitis [122][123] appears to be a consequence of increased NET formation in diabetes [120]; (iii) cigarette smoke, a factor aggravating the course of periodontitis, fosters NET overproduction [95].
Either suppression of exaggerated NET formation or NET lysis by DNase can ameliorate the negative effects of NETs on wound healing [120]. The healing of gingival ulcerations in late-onset periodontitis by attenuation of NET formation, e.g. through NET lysis, opens a new perspective to amplify the late-onset periodontitis therapy.
6. NET-Driven Periodontal Inflammation
PMN hyper-responsiveness [24][25][26][27][28], delayed PMN apoptosis [124], immune-senescence [46][50][125] and PAMP overload, i.e., dental plaque accumulation, dominate the immune response in late-onset periodontitis [53][54][55][56][57]. Once late-onset periodontitis occurs, peripheral PMN hyper-responsiveness is evident, which does not disappear even after successful periodontal therapy [126]. The PMN hyper-responsiveness results in exaggerated NET formation and even in epithelial damages including ulceration [120] and, thus, sustains and aggravates periodontal inflammations. The oral epithelium healing mechanism cannot compensate the damages due to the exaggerated NET formation and is incapable of providing epithelial repair. As consequence, heavy inflammatory infiltration of the connective tissue and periodontal ligament damaging take place. The healing of damaged periodontal ligaments occurs via replacement with the junctional epithelium [127]. Due to the high proliferation rate of oral epithelial cells, after just a few days, the large part of the periodontal wound would inevitably be delimitated by the epithelium [128], even in the absence of stromal healing [112]. This phenomenon is denoted either apical migration of the junctional epithelium, or long junctional epithelium formation. This healing through repair reduces the periodontal ligament surface and subsequently the load-bearing capacity of the affected tooth. This results in periodontium overburdening, which causes trauma-induced inflammation. Thus, even when the gingival inflammation is in a state of remission [34][35][36], moderate bacteria challenges or mechanical overloading may inflame the inflammation anew.
The PMN hyper-responsiveness is considered the main pathogenic factor in localised aggressive periodontitis, a subtype of late-onset periodontitis [126], and is also responsible for overproduction of NETs. Exaggerated NETs cause epithelial cytotoxicity [126][129] and liberate proteases, damaging even the basal lamina [79]. This clearly indicates the destructive role of exaggerated NET formation in periodontitis. Clinically, the topical application of prednisolone alone [130] into the periodontal pocket in cases of periodontitis exacerbation is sufficient for a temporary resolution of periodontitis inflammation, or at least for its attenuation. Similarly, topical application of resolvin E1 in an animal model caused resolution of inflammation via PMN depriming [130]. These findings clearly indicate that the bacterial challenge in periodontitis plays a secondary role.
The exaggerated immune response in late-onset periodontitis, in particular, the exaggerated NET formation, appears to be responsible for tissue damages, impairs tissue regeneration and causes non-resolution of inflammation.
7. Role of NETs in the Resolution of Gingival Inflammation
Periodontal NETs have been reported on for over a decade [32], but their role in the resolution of gingival and periodontal inflammation remains largely unexplored due to technical difficulties in the examination of periodontal NETs in both humans and animal models, as well as the lack of interest on the side of periodontal societies. NET indispensability for periodontal health is nevertheless evident in patients with certain gene defects that are inevitably concomitant with both deficient NET formation and early-onset periodontitis [57]. The early-onset periodontitis is resolved only with the teeth loss [21][22]. These findings indicate the crucial role of canonical NETs in the resolution of gingival inflammation.
The inner mucous layer of the mucosal epithelia, where goblet cells are located, shields and aggregates bacterial pathogens [131]. Gingival epithelia lack the mucus layer, and its task has been suggested to be taken over by NETs [57]. The role of NETs in maintaining periodontal health and resolution of periodontal inflammation is comprehensible through analogy to other mucosal surface-lacking goblet cells, e.g., the cornea. Corneal NETs render a "dead zone" for bacteria and prevent tissue invasion by bacteria [132]. Indeed, the NET ability to block off and aggregate bacteria and nano-particles is beyond doubt [133][134].
Late-onset periodontitis progression is characterised by recurrent bursts of destruction, followed by varying periods of stagnation, the so-called random burst model [34][35][36]. The mechanism of this "spontaneous" switch from exacerbated inflammation to resolution of inflammation remains obscure. However, aggNETs, which are common in purulent periodontitis [32], are able to resolve inflammation through the degradation of inflammatory cytokines and mediators [18], as well as by detoxifying the extracellular histones [19]. Thus, aggNETs might be a crucial factor for the resolution of exacerbated periodontal inflammation.
Further in vivo and clinical examinations on crevicular aggNETs are needed to clarify their role in the pathology of periodontitis.
References
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535, doi:10.1126/science.1092385.
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241, doi:10.1083/jcb.200606027.
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639, doi:10.1371/journal.ppat.1000639.
- Kaplan, M.J.; Radic, M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J. Immunol. 2012, 189, 2689–2695, doi:10.4049/jimmunol.1201719.
- Röhm, M.; Grimm, M.J.; D’Auria, A.C.; Almyroudis, N.G.; Segal, B.H.; Urban, C.F. NADPH Oxidase Promotes Neutrophil Extracellular Trap Formation in Pulmonary Aspergillosis. Infect. Immun. 2014, 82, 1766–1777, doi:10.1128/iai.00096-14.
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822, doi:10.1073/pnas.1414055112.
- Pieterse, E.; Rother, N.; Yanginlar, C.; Gerretsen, J.; Boeltz, S.; Muñoz, L.E.; Herrmann, M.; Pickkers, P.; Hilbrands, L.B.; Van Der Vlag, J. Cleaved N-terminal histone tails distinguish between NADPH oxidase (NOX)-dependent and NOX-independent pathways of neutrophil extracellular trap formation. Ann. Rheum. Dis. 2018, 77, 1790–1798, doi:10.1136/annrheumdis-2018-213223.
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691, doi:10.1083/jcb.201006052.
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–62.
- Martinod, K.; Witsch, T.; Farley, K.; Gallant, M.; Remold-O’Donnell, E.; Wagner, D.D. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J. Thromb. Haemost. 2016, 14, 551–558, doi:10.1111/jth.13239.
- Díaz-Godínez, C.; Fonseca, Z.; Néquiz, M.; Laclette, J.P.; Rosales, C.; Carrero, J.C. Entamoeba histolytica Trophozoites Induce a Rapid Non-classical NETosis Mechanism Independent of NOX2-Derived Reactive Oxygen Species and PAD4 Activity. Front. Cell. Infect. Microbiol. 2018, 8, 184, doi:10.3389/fcimb.2018.00184.
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil Extracellular Trap–Associated Protein Activation of the NLRP3 Inflammasome Is Enhanced in Lupus Macrophages. J. Immunol. 2013, 190, 1217–1226, doi:10.4049/jimmunol.1202388.
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–53.
- Muñoz, L.E.; Boeltz, S.; Bilyy, R.; Schauer, C.; Mahajan, A.; Widulin, N.; Grüneboom, A.; Herrmann, I.; Boada, E.; Rauh, M.; et al. Neutrophil Extracellular Traps Initiate Gallstone Formation. Immunity 2019, 51, 443–450.e4, doi:10.1016/j.immuni.2019.07.002.
- Leppkes, M.; Maueröder, C.; Hirth, S.; Nowecki, S.; Günther, C.; Billmeier, U.; Paulus, S.; Biermann, M.; Muñoz, L.E.; Hoffmann, M.; et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat. Commun. 2016, 7, 10973, doi:10.1038/ncomms10973.
- Schapher, M.; Koch, M.; Weidner, D.; Scholz, M.; Wirtz, S.; Mahajan, A.; Herrmann, I.; Singh, J.; Knopf, J.; Leppkes, M.; et al. Neutrophil Extracellular Traps Promote the Development and Growth of Human Salivary Stones. Cells 2020, 9, 2139, doi:10.3390/cells9092139.
- Hahn, J.; Schauer, C.; Czegley, C.; Kling, L.; Petru, L.; Schmid, B.; Weidner, D.; Reinwald, C.; Biermann, M.H.C.; Blunder, S.; et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2018, 33, 1401–1414, doi:10.1096/fj.201800752r.
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhofer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517.
- Knopf, J.; Leppkes, M.; Schett, G.; Herrmann, M.; Muñoz, L.E. Aggregated NETs Sequester and Detoxify Extracellular Histones. Front. Immunol. 2019, 10, 2176, doi:10.3389/fimmu.2019.02176.
- Vieira, A.R.; Albandar, J.M. Role of genetic factors in the pathogenesis of aggressive periodontitis. Periodontol. 2000 2014, 65, 92–106, doi:10.1111/prd.12021.
- Wani, A.A.; Devkar, N.; Patole, M.S.; Shouche, Y. Description of Two New Cathepsin C Gene Mutations in Patients With Papillon-Lefèvre Syndrome. J. Periodontol. 2006, 77, 233–237, doi:10.1902/jop.2006.050124.
- Ye, Y.; Carlsson, G.; Wondimu, B.; Fahlén, A.; Karlsson-Sjöberg, J.; Andersson, M.; Engstrand, L.; Yucel-Lindberg, T.; Modéer, T.; Pütsep, K. Mutations in the ELANE Gene are Associated with Development of Periodontitis in Patients with Severe Congenital Neutropenia. J. Clin. Immunol. 2011, 31, 936–945, doi:10.1007/s10875-011-9572-0.
- Fine, D.H.; Patil, A.G.; Loos, B.G. Classification and diagnosis of aggressive periodontitis. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S95–S111, doi:10.1111/jcpe.12942.
- Fredriksson, M.I.; Gustafsson, A.K.; Bergström, K.G.; Åsman, B.E. Constitutionally Hyperreactive Neutrophils in Periodontitis. J. Periodontol. 2003, 74, 219–224.
- Gustafsson, A.; Ito, H.; Asman, B.; Bergström, K. Hyper-reactive mononuclear cells and neutrophils in chronic periodontitis. J. Clin. Periodontol. 2006, 33, 126–129, doi:10.1111/j.1600-051x.2005.00883.x.
- Johnstone, A.M.; Koh, A.; Goldberg, M.B.; Glogauer, M. A Hyperactive Neutrophil Phenotype in Patients With Refractory Periodontitis. J. Periodontol. 2007, 78, 1788–1794, doi:10.1902/jop.2007.070107.
- Ling, M.R.; Chapple, I.L.C.; Matthews, J.B. Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun. 2015, 21, 714–725, doi:10.1177/1753425915589387.
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Cooper, P.R.; Chapple, I.L.C. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis: Neutrophil hyperactivity and reactivity in chronic periodontitis. Clin. Exp. Immunol. 2006, 147, 255–264.
- Schaefer, A.S. Genetics of periodontitis: Discovery, biology, and clinical impact. Periodontol. 2000 2018, 78, 162–173, doi:10.1111/prd.12232.
- Persson, G.R. Periodontal complications with age. Periodontol. 2000 2018, 78, 185–194, doi:10.1111/prd.12227.
- Goodson, J.M. Gingival crevice fluid flow. Periodontol. 2000 2003, 31, 43–54, doi:10.1034/j.1600-0757.2003.03104.x.
- Vitkov, L.; Klappacher, M.; Hannig, M.; Krautgartner, W.D. Extracellular neutrophil traps in periodontitis. J. Periodontal Res. 2009, 44, 664–672, doi:10.1111/j.1600-0765.2008.01175.x.
- Vitkov, L.; Klappacher, M.; Hannig, M.; Krautgartner, W.D. Neutrophil Fate in Gingival Crevicular Fluid. Ultrastruct. Pathol. 2010, 34, 25–30, doi:10.3109/01913120903419989.
- Goodson, J.M.; Tanner, A.C.R.; Haffajee, A.D.; Sornberger, G.C.; Socransky, S.S. Patterns of progression and regression of advanced destructive periodontal disease. J. Clin. Periodontol. 1982, 9, 472–481, doi:10.1111/j.1600-051x.1982.tb02108.x.
- Haffajee, A.D.; Socransky, S.S.; Goodson, J.M. Comparison of different data analyses for detecting changes in attachment level. J. Clin. Periodontol. 1983, 10, 298–310, doi:10.1111/j.1600-051x.1983.tb01278.x.
- Socransky, S.S.; Haffajee, A.D.; Goodson, J.M.; Lindhe, J. New concepts of destructive periodontal disease. J. Clin. Periodontol. 1984, 11, 21–32, doi:10.1111/j.1600-051x.1984.tb01305.x.
- Loos, B.G.; Van Dyke, T.E. The role of inflammation and genetics in periodontal disease. Periodontol. 2000 2020, 83, 26–39, doi:10.1111/prd.12297.
- Hajishengallis, G.; Korostoff, J.M. Revisiting the Page & Schroeder model: The good, the bad and the unknowns in the periodontal host response 40 years later. Periodontol. 2000 2017, 75, 116–151, doi:10.1111/prd.12181.
- Sima, C.; Viniegra, A.; Glogauer, M. Macrophage immunomodulation in chronic osteolytic diseases—The case of periodontitis. J. Leukoc. Biol. 2019, 105, 473–487, doi:10.1002/jlb.1ru0818-310r.
- Tsukasaki, M.; Komatsu, N.; Nagashima, K.; Nitta, T.; Pluemsakunthai, W.; Shukunami, C.; Iwakura, Y.; Nakashima, T.; Okamoto, K.; Takayanagi, H. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 2018, 9, 701, doi:10.1038/s41467-018-03147-6.
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642, doi:10.1038/s41577-019-0178-8.
- Smith, M.M.; Knight, E.T.; Al‐Harthi, L.; Leichter, J.W. Chronic periodontitis and implant dentistry. Periodontol. 2000 2017, 74, 63–73, doi:10.1111/prd.12190.
- Preshaw, P.M. Host modulation therapy with anti-inflammatory agents. Periodontol. 2000 2017, 76, 131–149, doi:10.1111/prd.12148.
- Ebersole, J.L.; Dawson, D.A., 3rd; Huja, P.E.; Pandruvada, S.; Basu, A.; Nguyen, L.; Zhang, Y.; Gonzalez, O.A. Age and Periodontal Health—Immunological View. Curr. Oral. Health Rep. 2018, 5, 229–241.
- Persson, G.R. Dental geriatrics and periodontitis. Periodontol. 2000 2017, 74, 102–115, doi:10.1111/prd.12192.
- Agrawal, A.; Tay, J.; Ton, S.; Agrawal, S.; Gupta, S. Increased Reactivity of Dendritic Cells from Aged Subjects to Self-Antigen, the Human DNA. J. Immunol. 2009, 182, 1138–1145, doi:10.4049/jimmunol.182.2.1138.
- Bakdash, G.; Vogelpoel, L.T.C.; Van Capel, T.M.M.; Kapsenberg, M.L.; De Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2014, 8, 265–278, doi:10.1038/mi.2014.64.
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–64.
- Del Rio, M.-L.; Bernhardt, G.; Rodriguez-Barbosa, J.-I.; Förster, R. Development and functional specialization of CD103+dendritic cells. Immunol. Rev. 2010, 234, 268–281, doi:10.1111/j.0105-2896.2009.00874.x.
- Agrawal, S.; Ganguly, S.; Tran, A.; Sundaram, P.; Agrawal, A. Retinoic acid treated human dendritic cells induce T regulatory cells via the expression of CD141 and GARP which is impaired with age. Aging 2016, 8, 1223–1235, doi:10.18632/aging.100973.
- Prakash, S.; Agrawal, S.S.; Vahed, H.; Ngyuen, M.; BenMohamad, L.; Gupta, S.; Agrawal, A. Dendritic cells from aged subjects contribute to chronic airway inflammation by activating bronchial epithelial cells under steady state. Mucosal Immunol. 2014, 7, 1386–1394, doi:10.1038/mi.2014.28.
- Agrawal, A.; Agrawal, S.; Gupta, S. Role of Dendritic Cells in Inflammation and Loss of Tolerance in the Elderly. Front. Immunol. 2017, 8, 896, doi:10.3389/fimmu.2017.00896.
- Graves, D.T.; Oates, T.; Garlet, G.P. Review of osteoimmunology and the host response in endodontic and periodontal lesions. J. Oral Microbiol. 2011, 3, 5304, doi:10.3402/jom.v3i0.5304.
- Hajishengallis, G.; Moutsopoulos, N.M.; Hajishengallis, E.; Chavakis, T. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin. Immunol. 2016, 28, 146–158, doi:10.1016/j.smim.2016.02.002.
- Hasturk, H.; Kantarci, A.; Van Dyke, T.E. Paradigm Shift in the Pharmacological Management of Periodontal Diseases. In Frontiers of Oral Biology; Kinane, D.F., Mombelli, A., Eds.; KARGER: Basel, Switzerland, 2011; Volume 15, pp. 160–176.
- Vitkov, L.; Hannig, M.; Minnich, B.; Herrmann, M. Periodontal sources of citrullinated antigens and TLR agonists related to RA. Autoimmunity 2018, 51, 304–309, doi:10.1080/08916934.2018.1527907.
- Vitkov, L.; Hartl, D.; Minnich, B.; Hannig, M. Janus-Faced Neutrophil Extracellular Traps in Periodontitis. Front. Immunol. 2017, 8, 1404, doi:10.3389/fimmu.2017.01404.
- Ina, K.; Kusugami, K.; Hosokawa, T.; Imada, A.; Shimizu, T.; Yamaguchi, T.; Ohsuga, M.; Kyokane, K.; Sakai, T.; Nishio, Y.; et al. Increased mucosal production of granulocyte colony‐stimulating factor is related to a delay in neutrophil apoptosis in Inflammatory Bowel disease. J. Gastroenterol. Hepatol. 1999, 14, 46–53, doi:10.1046/j.1440-1746.1999.01807.x.
- Le’Negrate, G.; Rostagno, P.; Auberger, P.; Rossi, B.; Hofman, P. Downregulation of caspases and Fas ligand expression, and increased lifespan of neutrophils after transmigration across intestinal epithelium. Cell Death Differ. 2003, 10, 153–162, doi:10.1038/sj.cdd.4401110.
- Lakschevitz, F.S.; Aboodi, G.M.; Glogauer, M. Oral Neutrophil Transcriptome Changes Result in a Pro-Survival Phenotype in Periodontal Diseases. PLoS ONE 2013, 8, e68983, doi:10.1371/journal.pone.0068983.
- Vitkov, L.; Krautgartner, W.D.; Hannig, M. Surface Morphology of Pocket Epithelium. Ultrastruct. Pathol. 2005, 29, 121–127, doi:10.1080/01913120590916832.
- Ajonuma, L.C.; Lu, Q.; Cheung, B.P.K.; Leung, W.K.; Samaranayake, L.P.; Jin, L. Expression and localization of cystic fibrosis transmembrane conductance regulator in human gingiva. Cell Biol. Int. 2010, 34, 147–152.
- Campbell, A.R.; Andress, D.L.; Swenson, E.R. Identification and characterization of human neutrophil carbonic anhydrase. J. Leukoc. Biol. 1994, 55, 343–348, doi:10.1002/jlb.55.3.343.
- Sowden, J.; Leigh, S.; Talbot, I.; Delhanty, J.; Edwards, Y. Expression from the proximal promoter of the carbonic anhydrase 1 gene as a marker for differentiation in colon epithelia. Differentiation 1993, 53, 67–74, doi:10.1111/j.1432-0436.1993.tb00647.x.
- Yaprak, E.; Kasap, M.; Akpınar, G.; Kayaaltı-Yüksek, S.; Sinanoğlu, A.; Guzel, N.; Kocasarac, H.D. The prominent proteins expressed in healthy gingiva: A pilot exploratory tissue proteomics study. Odontology 2018, 106, 19–28, doi:10.1007/s10266-017-0302-9.
- Monari, E.; Cuoghi, A.; Bellei, E.; Bergamini, S.; Lucchi, A.; Tomasi, A.; Cortellini, P.; Zaffe, D.; Bertoldi, C. Analysis of protein expression in periodontal pocket tissue: A preliminary study. Proteome Sci. 2015, 13, 1–11, doi:10.1186/s12953-015-0089-y.
- Baliban, R.C.; Sakellari, D.; Li, Z.; DiMaggio, P.A.; Garcia, B.A.; Floudas, C.A. Novel protein identification methods for biomarker discovery via a proteomic analysis of periodontally healthy and diseased gingival crevicular fluid samples. J. Clin. Periodontol. 2012, 39, 203–212, doi:10.1111/j.1600-051x.2011.01805.x.
- Tsuchida, S.; Satoh, M.; Takiwaki, M.; Nomura, F. Current Status of Proteomic Technologies for Discovering and Identifying Gingival Crevicular Fluid Biomarkers for Periodontal Disease. Int. J. Mol. Sci. 2018, 20, 86, doi:10.3390/ijms20010086.
- Bickel, M.; Cimasoni, G. The pH of human crevicular fluid measured by a new microanalytical technique. J. Periodontal Res. 1985, 20, 35–40, doi:10.1111/j.1600-0765.1985.tb00408.x.
- Bickel, M.; Munoz, J.L.; Giovannini, P. Acid-base properties of human gingival crevicular fluid. J. Dent. Res. 1985, 64, 1218–1220.
- Eggert, F.; Drewell, L.; Bigelow, J.; Speck, J.; Goldner, M. The pH of gingival crevices and periodontal pockets in children, teenagers and adults. Arch. Oral Biol. 1991, 36, 233–238, doi:10.1016/0003-9969(91)90091-8.
- Daniel, C.; Leppkes, M.; Munoz, L.E.; Schley, G.; Schett, G.; Herrmann, M. Extracellular DNA traps in inflammation, injury and healing. Nat. Rev. Nephrol. 2019, 15, 559–575, doi:10.1038/s41581-019-0163-2.
- Pawlaczyk-Kamieńska, T.; Borysewicz-Lewicka, M.; Śniatała, R.; Batura-Gabryel, H.; Cofta, S. Dental and periodontal manifestations in patients with cystic fibrosis—A systematic review. J. Cyst. Fibros. 2019, 18, 762–771, doi:10.1016/j.jcf.2018.11.007.
- Duruel, O.; Berker, E.; Özşin-Özler, C.; Gharibzadeh-Hızal, M.; Gürpınar, Ö.; Eryılmaz-Polat, S.; Ataman-Duruel, E.T.; Tan, Ç.; Karabulut, E.; Tekçiçek, M.; et al. Levels of pro- and anti-inflammatory cytokines in cystic fibrosis patients with or without gingivitis. Cytokine 2020, 127, 154987, doi:10.1016/j.cyto.2020.154987.
- Pawlaczyk-Kamieńska, T.; Borysewicz-Lewicka, M.; Śniatała, R. Periodontal condition and periodontal risk assessment in adult patients with cystic fibrosis. Ann. Agric. Environ. Med. 2020, 27, 235–239, doi:10.26444/aaem/106087.
- Mäntylä, P.; Stenman, M.; Kinane, D.F.; Tikanoja, S.; Luoto, H.; Salo, T.; Sorsa, T. Gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis. J. Periodontal Res. 2003, 38, 436–439, doi:10.1034/j.1600-0765.2003.00677.x.
- Figueredo, C.M.; Fischer, R.; Gustafsson, A. Aberrant Neutrophil Reactions in Periodontitis. J. Periodontol. 2005, 76, 951–955, doi:10.1902/jop.2005.76.6.951.
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol. 2000 2015, 70, 142–163, doi:10.1111/prd.12101.
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361, eaao4227, doi:10.1126/science.aao4227.
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil Extracellular Traps Directly Induce Epithelial and Endothelial Cell Death: A Predominant Role of Histones. PLoS ONE 2012, 7, e32366, doi:10.1371/journal.pone.0032366.
- Figueredo, C.M.; Gustafsson, A. Increased amounts of laminin in GCF from untreated patients with periodontitis. J. Clin. Periodontol. 2000, 27, 313–318, doi:10.1034/j.1600-051x.2000.027005313.x.
- Emingil, G.; Atilla, G.; Sorsa, T.; Savolainen, P.; Baylas, H. Effectiveness of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid laminin-5 gamma2 chain levels in chronic periodontitis. J. Periodontol. 2004, 75, 1387–96.
- Emingil, G.; Kuula, H.; Pirilä, E.; Atilla, G.; Sorsa, T. Gingival crevicular fluid laminin-5 gamma2-chain levels in periodontal disease. J. Clin. Periodontol. 2006, 33, 462–468, doi:10.1111/j.1600-051x.2006.00933.x.
- Epidemiology, Etiology, and Prevention of Periodontal Diseases: Report of a WHO Scientific Group; WHO: Geneva, Switzerland, 1978; Volume 621, pp. 1–60.
- Bosshardt, D.D. The periodontal pocket: Pathogenesis, histopathology and consequences. Periodontology 2000 2018, 76, 43–50, doi:10.1111/prd.12153.
- Hiyoshi, T.; Domon, H.; Maekawa, T.; Nagai, K.; Tamura, H.; Takahashi, N.; Yonezawa, D.; Miyoshi, T.; Yoshida, A.; Tabeta, K.; et al. Aggregatibacter actinomycetemcomitans induces detachment and death of human gingival epithelial cells and fibroblasts via elastase release following leukotoxin-dependent neutrophil lysis. Microbiol. Immunol. 2019, 63, 100–110.
- Noguchi, S.; Ukai, T.; Kuramoto, A.; Yoshinaga, Y.; Nakamura, H.; Takamori, Y.; Yamashita, Y.; Hara, Y. The histopathological comparison on the destruction of the periodontal tissue between normal junctional epithelium and long junctional epithelium. J. Periodontal Res. 2016, 52, 74–82, doi:10.1111/jre.12370.
- Aleyd, E.; Al, M.; Tuk, C.W.; van der Laken, C.J.; van Egmond, M. IgA Complexes in Plasma and Synovial Fluid of Patients with Rheumatoid Arthritis Induce Neutrophil Extracellular Traps via FcalphaRI. J. Immunol. 2016, 197, 4552–4559.
- Behnen, M.; Leschczyk, C.; Moller, S.; Batel, T.; Klinger, M.; Solbach, W.; Laskay, T. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcgammaRIIIB and Mac-1. J. Immunol. 2014, 193, 1954–65.
- Yoshinaga, Y.; Ukai, T.; Kaneko, T.; Nakatsu, S.; Shiraishi, C.; Kuramoto, A.; Oshino, K.; Ichimura, I.; Hara, Y. Topical application of lipopolysaccharide into gingival sulcus promotes periodontal destruction in rats immunized with lipopolysaccharide. J. Periodontal Res. 2012, 47, 674–680, doi:10.1111/j.1600-0765.2012.01486.x.
- Bergstrom, J. Cigarette smoking as risk factor in chronic periodontal disease. Community Dent. Oral Epidemiol. 1989, 17, 245–247, doi:10.1111/j.1600-0528.1989.tb00626.x.
- Do, L.G.; Slade, G.D.; Roberts-Thomson, K.F.; Sanders, A.E. Smoking-attributable periodontal disease in the Australian adult population. J. Clin. Periodontol. 2008, 35, 398–404, doi:10.1111/j.1600-051x.2008.01223.x.
- Haber, J.; Wattles, J.; Crowley, M.; Mandell, R.; Joshipura, K.; Kent, R.L. Evidence for Cigarette Smoking as a Major Risk Factor for Periodontitis. J. Periodontol. 1993, 64, 16–23, doi:10.1902/jop.1993.64.1.16.
- Yanagisawa, T.; Ueno, M.; Shinada, K.; Ohara, S.; Wright, F.A.C.; Kawaguchi, Y. Relationship of smoking and smoking cessation with oral health status in Japanese men. J. Periodontal Res. 2010, 45, 277–283, doi:10.1111/j.1600-0765.2009.01233.x.
- Qiu, S.-L.; Zhang, H.; Tang, Q.-Y.; Bai, J.; He, Z.; Zhang, J.-Q.; Li, M.-H.; Deng, J.-M.; Liu, G.-N.; Zhong, X. Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. Thorax 2017, 72, 1084–1093, doi:10.1136/thoraxjnl-2016-209887.
- Dhami, R.; Gilks, B.; Xie, C.; Zay, K.; Wright, J.L.; Churg, A. Acute Cigarette Smoke–Induced Connective Tissue Breakdown Is Mediated by Neutrophils and Prevented by α 1-Antitrypsin. Am. J. Respir. Cell Mol. Biol. 2000, 22, 244–252, doi:10.1165/ajrcmb.22.2.3809.
- Atwood, D.A. Bone Loss of Edentulous Alveolar Ridges. J. Periodontol. 1979, 50, 11–21, doi:10.1902/jop.1979.50.4s.11.
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218, doi:10.1111/j.1600-051x.2005.00642.x.
- Akizuki, T.; Oda, S.; Komaki, M.; Tsuchioka, H.; Kawakatsu, N.; Kikuchi, A.; Yamato, M.; Okano, T.; Ishikawa, I. Application of periodontal ligament cell sheet for periodontal regeneration: A pilot study in beagle dogs. J. Periodontal Res. 2005, 40, 245–251, doi:10.1111/j.1600-0765.2005.00799.x.
- Flores, M.G.; Yashiro, R.; Washio, K.; Yamato, M.; Okano, T.; Ishikawa, I. Periodontal ligament cell sheet promotes periodontal regeneration in athymic rats. J. Clin. Periodontol. 2008, 35, 1066–1072, doi:10.1111/j.1600-051x.2008.01326.x.
- Tsumanuma, Y.; Iwata, T.; Kinoshita, A.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Yamato, M.; Okano, T.; Izumi, Y. Allogeneic Transplantation of Periodontal Ligament-Derived Multipotent Mesenchymal Stromal Cell Sheets in Canine Critical-Size Supra-Alveolar Periodontal Defect Model. BioResearch Open Access 2016, 5, 22–36, doi:10.1089/biores.2015.0043.
- Armitage, G.C. Clinical evaluation of periodontal diseases. Periodontol. 2000 1995, 7, 39–53, doi:10.1111/j.1600-0757.1995.tb00035.x.
- Picot, R.; Das, I.; Reid, L. Pus, deoxyribonucleic acid, and sputum viscosity. Thorax 1978, 33, 235–242, doi:10.1136/thx.33.2.235.
- Shak, S.; Capon, D.J.; Hellmiss, R.; Marsters, S.A.; Baker, C.L. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc. Natl. Acad. Sci. USA 1990, 87, 9188–9192, doi:10.1073/pnas.87.23.9188.
- Kareha, M.J.; Rosenberg, E.S.; DeHaven, H. Therapeutic considerations in the management of a periodontal abscess with an intrabony defect. J. Clin. Periodontol. 1981, 8, 375–386, doi:10.1111/j.1600-051x.1981.tb00887.x.
- Herrera, D.; Alonso, M.S.; De Arriba, L.; Cruz, I.S.; Serrano, C.; Sanz, M. Acute periodontal lesions. Periodontol. 2000 2014, 65, 149–177, doi:10.1111/prd.12022.
- Newman, M.G.; Sims, T.N. The Predominant Cultivable Microbiota of the Periodontal Abscess. J. Periodontol. 1979, 50, 350–354, doi:10.1902/jop.1979.50.7.350.
- Prodan, A.; Brand, H.S.; Ligtenberg, A.J.M.; Imangaliyev, S.; Tsivtsivadze, E.; Van Der Weijden, F.; Crielaard, W.; Keijser, B.; Veerman, E.C.I. Interindividual variation, correlations, and sex-related differences in the salivary biochemistry of young healthy adults. Eur. J. Oral Sci. 2015, 123, 149–157, doi:10.1111/eos.12182.
- Yandrapu, H.; Marcinkiewicz, M.; Sarosiek, I.; Sarosiek, J.; Poplawski, C.; Han, K.; Goldin, G.; Zbroch, T.; Namiot, Z. Role of Saliva in Esophageal Defense: Implications in Patients With Nonerosive Reflux Disease. Am. J. Med. Sci. 2015, 349, 385–391, doi:10.1097/maj.0000000000000443.
- Skoczylas, T.; Yandrapu, H.; Poplawski, C.; Asadi, M.; Wallner, G.; Sarosiek, J. Salivary Bicarbonate as a Major Factor in the Prevention of Upper Esophageal Mucosal Injury in Gastroesophageal Reflux Disease. Dig. Dis. Sci. 2014, 59, 2411–2416, doi:10.1007/s10620-014-3099-1.
- Mohanty, T.; Sjögren, J.; Kahn, F.; Abu-Humaidan, A.H.A.; Fisker, N.; Assing, K.; Mörgelin, M.; Bengtsson, A.A.; Borregaard, N.; Sørensen, O.E. A novel mechanism for NETosis provides antimicrobial defense at the oral mucosa. Blood 2015, 126, 2128–2137, doi:10.1182/blood-2015-04-641142.
- Iglesias-Bartolome, R.; Uchiyama, A.; Molinolo, A.A.; Abusleme, L.; Brooks, S.R.; Callejas-Valera, J.L.; Edwards, D.; Doci, C.; Asselin-Labat, M.-L.; Onaitis, M.W.; et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci. Transl. Med. 2018, 10, eaap8798, doi:10.1126/scitranslmed.aap8798.
- Deas, D.E.; Moritz, A.J.; Sagun, R.S.; Gruwell, S.F.; Powell, C.A. Scaling and root planing vs. conservative surgery in the treatment of chronic periodontitis. Periodontol. 2000 2016, 71, 128–139, doi:10.1111/prd.12114.
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–25.
- Crasta, K.; Daly, C.G.; Mitchell, D.; Curtis, B.; Stewart, D.; Heitz-Mayfield, L.J.A. Bacteraemia due to dental flossing. J. Clin. Periodontol. 2009, 36, 323–332, doi:10.1111/j.1600-051x.2008.01372.x.
- Tomã¡s, I.; Diz, P.; Tobías, A.; Scully, C.; Donos, N. Periodontal health status and bacteraemia from daily oral activities: Systematic review/meta-analysis. J. Clin. Periodontol. 2011, 39, 213–228, doi:10.1111/j.1600-051x.2011.01784.x.
- Yazdi, H.; Moradi, A.; Herbort, M. The effect of gentamicin in irrigating solutions on articular infection prophylaxis during arthroscopic ACL reconstruction. Arch. Orthop. Trauma Surg. 2014, 134, 257–261, doi:10.1007/s00402-013-1910-7.
- Kozarov, E.V.; Dorn, B.R.; Shelburne, C.E.; Dunn, W.A., Jr.; Progulske-Fox, A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arter. Thromb. Vasc. Biol. 2005, 25, e17-8.
- Blanc, V.; O’Valle, F.; Pozo, E.; Puertas, A.; León, R.; Mesa, F. Oral bacteria in placental tissues: Increased molecular detection in pregnant periodontitis patients. Oral Dis. 2015, 21, 905–912, doi:10.1111/odi.12364.
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819, doi:10.1038/nm.3887.
- Moonen, C.G.; Buurma, K.G.; Faruque, M.R.; Balta, M.G.; Liefferink, E.; Bizzarro, S.; Nicu, E.A.; Loos, B.G. Periodontal therapy increases neutrophil extracellular trap degradation. Innate Immun. 2019, 26, 331–340, doi:10.1177/1753425919889392.
- Polak, D.; Sanui, T.; Nishimura, F.; Shapira, L. Diabetes as a risk factor for periodontal disease—Plausible mechanisms. Periodontol. 2000 2020, 83, 46–58, doi:10.1111/prd.12298.
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: Association studies. Periodontol. 2000 2020, 83, 40–45, doi:10.1111/prd.12270.
- Gamonal, J.; Sanz, M.; O’Connor, A.; Acevedo, A.; Suarez, I.; Sanz, A.; Martínez, B.; Silva, A. Delayed neutrophil apoptosis in chronic periodontitis patients. J. Clin. Periodontol. 2003, 30, 616–623, doi:10.1034/j.1600-051x.2003.00350.x.
- Agrawal, A.; Agrawal, S.; Cao, J.-N.; Su, H.; Osann, K.; Gupta, S. Altered Innate Immune Functioning of Dendritic Cells in Elderly Humans: A Role of Phosphoinositide 3-Kinase-Signaling Pathway. J. Immunol. 2007, 178, 6912–6922, doi:10.4049/jimmunol.178.11.6912.
- Matthews, J.; Wright, H.; Roberts, A.; Ling-Mountford, N.; Cooper, P.; Chapple, I. Neutrophil Hyper-responsiveness in Periodontitis. J. Dent. Res. 2007, 86, 718–722, doi:10.1177/154405910708600806.
- Listgarten, M.A.; Rosenberg, S.; Lerner, S. Progressive Replacement of Epithelial Attachment by a Connective Tissue Junction after Experimental Periodontal Surgery in Rats. J. Periodontol. 1982, 53, 659–670, doi:10.1902/jop.1982.53.11.659.
- Susin, C.; Wikesjö, U.M.E. Regenerative periodontal therapy: 30 years of lessons learned and unlearned. Periodontol. 2000 2013, 62, 232–242, doi:10.1111/prd.12003.
- Silvestre-Roig, C.; Braster, Q.; Wichapong, K.; Lee, E.Y.; Teulon, J.M.; Berrebeh, N.; Winter, J.; Adrover, J.M.; Santos, G.S.; Froese, A.; et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nat. Cell Biol. 2019, 569, 236–240, doi:10.1038/s41586-019-1167-6.
- Jager, J.; Obst, K.; Lohan, S.B.; Viktorov, J.; Staufenbiel, S.; Renz, H.; Unbehauen, M.; Haag, R.; Hedtrich, S.; Teutloff, C.; et al. Characterization of hyperbranched core-multishell nanocarriers as an innovative drug delivery system for the application at the oral mucosa. J. Periodontal Res. 2017, 53, 57–65, doi:10.1111/jre.12487.
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; Van Der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20, doi:10.1111/imr.12182.
- Thanabalasuriar, A.; Scott, B.N.V.; Peiseler, M.; Willson, M.E.; Zeng, Z.; Warrener, P.; Keller, A.E.; Surewaard, B.G.J.; Dozier, E.A.; Korhonen, J.T.; et al. Neutrophil Extracellular Traps Confine Pseudomonas aeruginosa Ocular Biofilms and Restrict Brain Invasion. Cell Host Microbe 2019, 25, 526–536.e4.
- Bilyy, R.; Fedorov, V.; Vovk, V.; Leppkes, M.; Dumych, T.; Chopyak, V.; Schett, G.; Herrmann, M. Neutrophil Extracellular Traps Form a Barrier between Necrotic and Viable Areas in Acute Abdominal Inflammation. Front. Immunol. 2016, 7, 7, doi:10.3389/fimmu.2016.00424.
- Muñoz, L.E.; Bilyy, R.O.; Biermann, M.H.C.; Kienhöfer, D.; Maueröder, C.; Hahn, J.; Brauner, J.M.; Weidner, D.; Chen, J.; Scharin-Mehlmann, M.; et al. Nanoparticles size-dependently initiate self-limiting NETosis-driven inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, E5856–E5865, doi:10.1073/pnas.1602230113.




