
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rosanna Chianese | + 3736 word(s) | 3736 | 2021-01-21 09:49:40 | | | |
| 2 | Lily Guo | Meta information modification | 3736 | 2021-02-01 08:04:09 | | | | |
| 3 | Lily Guo | Meta information modification | 3736 | 2021-02-01 08:41:58 | | |
Video Upload Options
Besides ATP production, mitochondria are key organelles in several cellular functions, such as steroid hormone biosynthesis, calcium homoeostasis, intrinsic apoptotic pathway, and the generation of reactive oxygen species (ROS). Despite the loss of the majority of the cytoplasm occurring during spermiogenesis, mammalian sperm preserves a number of mitochondria that rearrange in a tubular structure at the level of the sperm flagellum midpiece. Although sperm mitochondria are destroyed inside the zygote, the integrity and the functionality of these organelles seem to be critical for fertilization and embryo development.
1. Mitochondria: A Central Role in Sperm Physiology
Mitochondria are classically known for being eukaryotic cell powerhouses due to their ability to produce ATP via oxidative phosphorylation[1]. They are highly dynamic organelles, able to adapt their shape to the physiological needs of the cell, suggesting their participation in numerous other physiological functions beyond ATP production.
In this regard, they create transient contacts with endoplasmic reticulum membranes and lysosomes, essential for autophagy, mitochondrial motility and fission, lipid and calcium (Ca2+) fluxes,[2][3] as well as glucose homeostasis and mitochondrial DNA (mtDNA) replication[4]. Mitochondrial Ca2+ uptake regulates cytosolic Ca2+ homeostasis, thus influencing extracellular Ca2+ entry[5][6]. Mitochondrial electron transfer chain also promotes reactive oxygen species (ROS) generation, besides its involvement in ATP synthesis. These molecules participate in both signalling pathways and in oxidative stress, if unbalanced produced[7]. Mitochondrial contribution to steroid hormone biosynthesis—by catalyzing the conversion of cholesterol to pregnenolone—has also been investigated[8].
The number, shape and structure of the mitochondria dramatically change during mammalian spermatogenesis, with secondary spermatocytes and spermatids that have more condensed mitochondria[9][10]. Despite the loss of the majority of the cytoplasm during spermatid differentiation, a number of mitochondria still remain in spermatozoa (SPZ), rearranging in tubular structures at the level of the midpiece of the flagellum[7]. During sperm maturation, mitochondria become more polarized in rodent species after epididymal maturation or wrapped in humans after capacitation [11].
Mitochondria’s role as energy provider is surely fundamental for sperm motility. In fact, defects in sperm mitochondrial ultrastructure are associated with decreased sperm motility and asthenozoospermia [12][13]. However, both metabolic pathways—glycolysis and mitochondrial oxidative phosphorylation—may sustain sperm motility, and several glycolytic enzymes are distributed in the sperm tail [14], thus suggesting a great versatility of SPZ in their metabolism by using glycolysis exclusively, mitochondrial oxidative phosphorylation or both as dual sources of energy according to the availability of substrates in the female genital tracts[15][16].
An important prerequisite to produce ATP is the maintenance of a positively charged membrane potential [17]. The treatment of human sperm with an oxidative uncoupler reduces mitochondrial membrane potential, impairing sperm motility and fertility [18]. Accordingly, low mitochondrial membrane potential and high ROS production have been detected in SPZ from infertile patients [19].
MtDNA is another candidate aspect strongly correlated with sperm physiology and quality. MtDNA has a loosely packaged structure and, therefore, it is more easily damaged by ROS than the nuclear genome[20]. Point mutations, rearrangement and/or decreased content of mtDNA are all features correlated with sperm dysfunctions and infertility[17][21]. Conversely, a low mtDNA copy number has been suggested as an indicator of good-quality sperm[22]; thus, its manipulation may be a powerful therapeutic strategy to decrease aging-associated mtDNA mutations [23]. Interestingly, even if still controversial, DNA methylation in mtDNA has been found to be associated with both transcriptional regulation and mtDNA copy number[24]. Such an epigenetic process takes part to the largely unexplored field of the mitochondrial epigenetics, together with the presence of non-coding RNAs inside the mitochondria.
Coding and non-coding RNAs have been widely analyzed as epigenetic regulators involved in the modulation of sperm functions[25][26][27][28][29]. In this regard, microRNAs (miRNAs), encoded by the nuclear or mitochondrial genome, have a dual role through the regulation of the nuclear genome, encoding mitochondria-related proteins, or translocating into the mitochondria in order to regulate mitochondrial genome expression[30]. MiRNAs might modulate sperm functions through mitochondria-dependent pathways; their aberrant expression in sperm of aging males has been correlated with poor semen quality caused by the suppression of the mitochondrial function and the reduction of ATP production[31]. Other small RNAs encoded by the mtDNA, overall known as mitosRNAs, have been recently discovered[32]. Interestingly, different isoforms of miRNAs derived from mtDNA have been found in oocytes, SPZ, and zygotes, with SPZ that show a predominance of the mito-miRNA isoform named paramiR, partially corresponding to the 5′ region of the canonical miRNA. Among mitosRNAs, mito-piRNAs are the most predominant mitosRNA population in the mitochondria of mouse germ cells. Both piRNAs and their associated proteins play a key role in mitochondrial homeostasis and nuclear communication[32]. MtDNA also encodes a set of long non-coding RNAs (lncRNAs)[33]. Intense crosstalk exists between mitochondria and nucleus; it is mediated by lncRNAs of nuclear origin, through molecular trafficking that is still an exciting issue to investigate. Once imported into the mitochondria, lncRNAs regulate mtDNA replication, RNA processing, hormone signalling, mitochondria-mediated apoptosis, and mitochondrial bioenergetics[34]. Mitochondria-encoded lncRNAs (mt-lncRNAs) have a different structure in comparison with the nuclear lncRNAs; they are chimeric, deriving from more than one gene with the merging of their transcripts as a post-transcriptional product via trans-splicing reactions[35]. A typical chimeric mt-lncRNA has been localized in the nucleus of mouse sperm, suggesting the export of mitochondrial material towards the nucleus. Conversely, limited evidence exists about circular RNAs (circRNAs) of mitochondrial origin (mt-circRNAs). This recently discovered class of non-coding RNAs plays critical roles in key physiological functions, working as microRNA (miRNA) sponges, protein scaffolds, and translation templates. Evidence in testis and SPZ correlates them with germ cell progression and sperm quality[36]. Gao et al.[37] found three mt-circRNAs by studying circRNAs expression in cattle testis, but their functions were not explored. CircRNAs whose host genes are derived from the mitochondrial genome have also been discovered in human testis [38] and SPZ [28], but, as in cattle, their potential role has not been thorough.
Although the characterization of the mitoRNA landscape in mouse male germ cells, gametes, and zygotes opens the door to novel mechanisms of regulation in mitochondria, much effort is required to unravel the biological functions of these RNAs in germ cell functions and how these molecules may coordinate signalling pathways between nucleus and mitochondria[39].
In the scenario of the mitochondria involvement in sperm physiology, proteomic studies have tried to identify dysfunctional mitochondrial proteins responsible for infertility[40][41]. Interestingly, a large percentage of these proteins—especially engaged in cell metabolism and energy production, protein folding/degradation, vesicle trafficking and cytoskeleton organization—are deregulated in low motile SPZ[40][42] As the endoplasmic reticulum, mitochondria need dedicated protein-folding machinery in order to control the amount of unfolded or misfolded proteins produced under stress conditions[43][44]. Such machinery appears deregulated in the case of male infertility [45].
Another intriguing aspect concerns the fate of sperm-derived mitochondria during fertilization, since in most mammals, the sperm tail is also incorporated along with the sperm genome into the oocyte[46]. However, the selective elimination of paternal mitochondria from the zygote may be the result of a developmental pressure promoting the strictly maternal inheritance of mitochondria. Such a transmission is known as maternal inheritance [47][48] or cleverly nicknamed the paradigm of Mitochondrial Eve by Lewin (1987)[49]. The mechanism in support of the maternal inheritance of mitochondria includes an early modification of sperm mitochondria, already during spermatogenesis, through a pre-labelling with ubiquitin[50]. Into the zygote, ubiquitin-labelled sperm mitochondria are selectively recognized by the proteasome-dependent proteolytic machinery and then eliminated by lysosomes (Figure 1A) [51]. Actually, a cascade of events may be involved, with autophagy—referred to as “sperm mitophagy”—as an intermediate mechanism between ubiquitination and lysosome degradation [52] and the combined action of multiple proteins working, at least in higher mammals[48][53]. However, by using transgenic mouse strains, mitophagy has been excluded as the involved pathway in sperm mitochondrial degradation; rather, the elimination of sperm mtDNA in most motile SPZ before fertilization has been suggested as a passive casual event, at least in mice, that leaves in cells just vacuolar mitochondria—deprived of mtDNA—in order to supply the amount of energy necessary for fertilization (Figure 1B)[54]. Such evidence does not exclude that—in rare cases—some cells and tissues could inherit paternal mtDNA, known as the Mitochondrial Adam mechanism, with an uneven distribution of mitochondria (Figure 1C). However, since human eggs contain more than 100.000 copies of mtDNA in comparison with sperm that just contains 100 copies, a possible dilution effect has also been hypothesized. Interestingly, in cases of diseases caused by mtDNA mutations, the coexistence of normal and mutant mtDNA molecules in a single cell—a situation called heteroplasmy[55]—not only contributes to the disease severity, but it could not be explained just by maternal inheritance, thus suggesting that paternal mtDNA could be passed to the offspring[56].
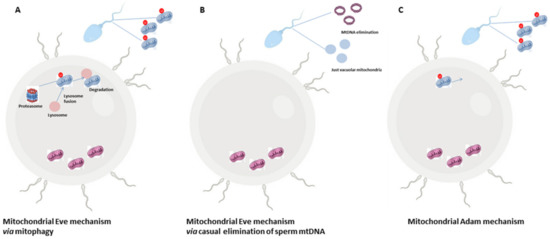
Figure 1. The Mitochondrial Eve or Adam mechanism. Most literature hypothesizes that the zygote exclusively inherits maternal mitochondria so that paternal ones that enter into the zygote are eliminated by the involvement of the proteasome-autophagy-lysosome pathway (A). A second hypothesis that still sustains maternal inheritance expects a precocious elimination of paternal mtDNA from motile SPZ and a change in mitochondria morphology creating vacuolar organelles in order to provide energy for sperm motility (B). A third hypothesis does not exclude the paternal inheritance of mitochondria with an uneven distribution of these organelles inside the embryo (C).
Although the central dogma of maternal inheritance of mtDNA still remains, the potential impact of paternal mtDNA on embryo development cannot be ignored[54][57][58].
2. Mitochondria: Key Producers of ROS. A Focus
ROS generation requires the activation of the mitochondrial electron transport chain and mainly takes place on the inner mitochondrial membrane during the process of oxidative phosphorylation [59]. This essential cellular process involves five big protein complexes that, in succession, transfer electrons donated from nicotine adenine dinucleotide (NADH) to O2. Meanwhile, mitochondrial membrane potential is created through an active pumping of positively charged protons (H+) from the mitochondrial matrix into the intermembrane space; in this way, when protons re-enter in the mitochondrial matrix through the enzymatic complex V, there is the generation of a proton-motive force that allows it to generate ATP[60]. Under stress conditions or by accident, the electron transfer along the mitochondrial electron transport chain may not be perfect, with the leakage of electrons and the partial reduction of oxygen to form superoxide anion (O2−) as a consequence. Such an anion can be thrown towards the mitochondrial matrix from complex I and towards both the intermembrane space and mitochondrial matrix from complex III [61]. Subsequently, two dismutases (SOD enzymes) quickly dismutate the superoxide anion to hydrogen peroxide (H2O2) in the mitochondrial intermembrane space. Afterwards, H2O2 is fully reduced to water by glutathione peroxidase (GPX). However, both O2−. and H2O2, generated in this process, are considered as mitochondrial ROS. In addition, O2−. can undergo a radical-radical reaction with nitric oxide (NO) to form peroxynitrite (ONOO2.−). While O2−. is not considered a good candidate as a signalling transduction molecule because it has electrophilic properties and short half-life and can hardly pass through the mitochondrial outer membrane, H2O2 is electrophobic and more stable; thus, its concentration inside the mitochondria is 100 times greater than that of O2−.[62].
Mitochondrial ROS are highly reactive and toxic molecules so that mammalian cells need a number of antioxidant enzyme systems to scavenge them. Usually, after SOD action, H2O2 is quickly reduced to water by two other enzymes, catalase (CAT) and GPX. All these mitochondrial antioxidant enzymes are encoded by the nuclear genome and need to be imported into the mitochondria after their synthesis in the cytoplasm. The action of the antioxidant enzymes is surely corroborated by several natural antioxidants such as vitamin E, whose effectiveness is, however, limited since it is not able to accumulate within mitochondria. The development of synthetic mitochondrial ROS scavengers able to easily pass through all biological membranes has been a useful instrument for addressing this issue[63].
In mitochondria, ROS generation is strictly regulated by several factors. First of all, the mitochondrial membrane potential: a higher, more polarized potential has been widely associated with greater mitochondrial ROS generation[61], and the metabolic state of mitochondria—measured in terms of ATP synthesis—modulates the endogenous production of ROS. Also converging in such a direction are sirtuins, NAD+-dependent deacetylases able to counteract the overproduction of ROS via epigenetic modifications[64]. They are finely localized among the nucleus, the cytosol, and the mitochondria, and are activated by resveratrol, an antioxidant polyphenol compound isolated from grape skins. Among the seven members of the sirtuin family, a prominent role is played by Sirt1, whose activity is deeply impaired by oxidative stress, suggesting a crosstalk between Sirt1 function and ROS signalling [65]. Furthermore, the potential ability of Sirt1 to counteract oxidative stress has also been investigated in the testis as a consequence of exposure to environmental contaminants[66].
What is clear is that, once thought as merely the by-products of cellular metabolism, nowadays mitochondrial ROS are deeply investigated as important signalling molecules. High ROS levels signal in cells, especially by promoting the oxidation of protein targets, thus triggering apoptosis/autophagy pathways and causing cell death as the final consequence [67].
3. Mitochondrial ROS and Sperm Quality
As previously described, aerobic cells physiologically produce ROS, such as hydroxyl radicals (•OH), O2−., H2O2, NO, and so on, as obligatory metabolic products. Antioxidant systems—including enzymes such as superoxide dismutase (SOD), CAT, glutathione peroxidases (GPXs), thioredoxins (TRXs), and peroxiredoxins (PRDXs)—are charged with keeping ROS at low levels in cells[68].
The testis has developed a sophisticated array of enzymatic antioxidant systems[69][70], but also it relies on small non-enzymatic factors that work as free radical scavengers, such as: zinc—a core constituent of SOD, able to counteract lipid peroxidation [71]; vitamin C—especially produced by Sertoli cells and pachytene spermatocytes, whose deficiency leads to oxidative stress in testis[72]; and the pineal hormone melatonin, able to readily cross the blood-testes barrier to protect the germinal epithelium against oxidative stress.
An exacerbated production of ROS levels—known as oxidative stress—due to an overproduction of ROS and/or a dysregulation of the antioxidant scavenging system, becomes harmful in cells[73].
Given the complicated and dynamic sequence of events occurring during spermatogenesis (mitosis, meiosis and cell differentiation), with control systems required at both central and peripheral levels[74][75], a copious amount of ROS is physiologically generated by germ cells as by-products of their metabolism[76]. However, a moderate quantity of ROS is also convenient for regular functions, such as cell signalling, homeostasis, sperm capacitation, and sperm-egg interaction[77][78][79]. In particular, sperm capacitation is benefited by ROS mediation in cAMP generation, sperm plasma membrane cholesterol efflux, and tyrosine phosphatase activity inhibition. Conversely, the accumulation of ROS in the testis induces morphological alterations in the seminiferous epithelium and cytoplasmic vacuolizations in both germinal and Sertoli cells[80] and apoptosis[81].
Multiple levels of the structural organization of sperm cells may be threatened by ROS: genome, epigenome, proteome, lipidome. All these aspects will be discussed in the following paragraphs.
3.1. Impact of Mitochondrial ROS on Sperm Genome and Epigenome
Among germ cells, SPZ are highly susceptible cells to oxidative insults; in fact, ROS-mediated damage to both the structural and functional integrity of SPZ is one of the major contributors to male infertility. The outcome of a pregnancy, as well as the health trajectories of the offspring, are negatively impacted by damaged or defective SPZ[82][83].
It is well known that during spermiogenesis, spermatids drastically change the folding of their genome, replacing histones with transition proteins first and protamines later[36][84]. Alternatively, transition proteins do not displace histones, but rather drive the recruitment and processing of protamines, which are themselves responsible for histone eviction, thus suggesting a cooperation between transition proteins and protamines, instead of a consequential activity [85]. However, although the majority of the sperm genome is bound to protamines, a small percentage (~5–10%) of DNA is still organized in nucleosomes by residual histones, intriguingly containing telomeres and promoters of genes involved in early embryonic development [86]. This genomic compartment is particularly vulnerable to oxidative stress[83] Moreover, in mice, the sperm nucleus shows a regionalized sensitiveness to oxidative DNA alterations, with peripheral and basal nuclear regions—this last one localized close to the midpiece—that are more sensitive[87]. Since there is non-random localization of chromosomes into the sperm nucleus and the notion of chromosomal territories [88], it is logical to find some autosomes, such as Chr19, Chr18 and Chr17, highly vulnerable to oxidative damage[89]. Conversely, sex chromosomes appear to be particularly well-protected [90].
Oxidative DNA damage in SPZ includes DNA fragmentation by single-strand and double-strand breaks, the introduction of abasic sites, such as O6-methylguanine, or oxidated bases, such as the 8-hydroxy-2′-deoxyguanosine (8-OHdG)—one of the main products of DNA oxidation, purine, pyrimidine and deoxyribose modifications, DNA-protein cross-linking with gene transcription arrested or inducted, as a consequence[91]. These effects are certainly compounded by the physical architecture of SPZ; since they suffer from the lack of essential cytoplasmic enzymes or a fully functional DNA repair system, the inability of the transcriptional activation of genes encoding the involved antioxidant enzymes, and the protection of their nuclear DNA by the entering of nucleases. What is alarming is that SPZ with damaged DNA are still able to fertilize, with dangerous implications for the embryo. Increased oxidative DNA damage in SPZ has a strong impact on next generations; it has been correlated with childhood cancers[92], brain disorders such as autism and schizophrenia[93], and so on.
Oxidative stress also affects epigenetic marks[94]. The presence of DNA base adducts, such as the 8-OH-dG, in CpG islands alters the interaction between DNA and DNA methyltransferases, preventing the adjacent cytosine methylation and leading to a global hypomethylation which is associated with Sertoli cell-only syndrome, testis cancer, and hypospermatogenesis in humans[95][96]. After fertilization, conventional methylcytosine (mC) undergoes oxidation in 5-hydroxymethylcytosine (5HmC) via the action of the Ten-Eleven Translocation (TET) enzymes. This chemical modification is the starting point for active demethylation of paternal chromatin[97]. Post-testicular oxidative alterations of SPZ may generate an excessive production of 5HmC that changes the kinetics of paternal DNA demethylation influencing the embryo development. The oxidation can also affect DNA methyltransferase activity itself, thus decreasing DNA methylation[98].
Paternal histones and protamines are also targets of oxidative stress, as will be explained below, with potential hazardous effects on the embryo development and the health of future generations. As a part of the epigenetic signature of sperm cells, the non-coding RNA payload is gaining attention. Interestingly, along the epididymis, sperm non-coding RNA profile dynamically changes as a consequence of the epididymal epithelial cell secretion, via epididymosomes and/or in stress conditions [99]. A useful animal model to shed light on the effect of the oxidative stress on sperm non-coding RNA payload is the GPX5 knockout mouse, whose epididymal epithelium has a decreased piRNA content[100].
3.2. Impact of ROS on Sperm Lipids and Proteins
Beyond the genome and epigenome, numerous other macromolecules carried by SPZ are in the crosshairs of oxidative stress. These are lipids and proteins.
Sperm fragility to ROS is, in fact, aggravated by a very peculiar lipid composition of its plasma membrane that—in comparison to all the other differentiated cells—is richer in polyunsaturated fatty acids (PUFAs,[101]), a class of particularly vulnerable lipids whose peroxidation affects membrane fluidity and permeability, important properties for both flagellar movements and fusion with the vitelline membrane of the oocyte[102]. As a key target of ROS, the sperm plasma membrane can stimulate a downstream signal cascade, damaging both nuclear and mitochondrial genomes.
The involved organelles are, therefore, mitochondria: they are both source and targets of ROS. Antioxidant system dysregulation alters mitochondria membrane potential with higher and lower production of free radicals and ATP, respectively[103], which in turn can trigger lipid peroxidation[82]. In germ cells, mitochondria dysfunction implies meiotic arrest, whereas in SPZ this means disorganization of the axonemal apparatus required for sperm motility and asthenozoospermia as a consequence[104]. Sperm motility is also damaged by thiol oxidation of the α-tubulin protein, a structural component of the sperm flagellum that impairs microtubule polymerization[105]. In this regard, the first observation that—under high oxygen tension conditions—human SPZ lose their motility dates back to 1943, with studies by MacLeod et al.[106]. Another important aspect linked to sperm mitochondria is their genome, not compacted by protamines and thus more vulnerable to oxidative attacks[89]. Considering that the most ascertained hypothesis describes that paternal mitochondria are quickly destroyed after fertilization to make way for the maternal mitochondria, oxidative damage to mtDNA may not be relevant for embryo development. However, as previously described, some evidence does not exclude a potential paternal inheritance of mitochondria. In that case, a damaged paternal mtDNA may be involved in several pathological processes inside the embryo or future generations.
Together with lipids, sperm proteins, especially localized in the nucleus, can be affected by ROS through carbonylation and redox thiol modification[107]. Sperm nuclear proteins that contain thiols are especially protamines whose oxidation completely alters chromatin folding and function. Although protamine change is not expected to be damaging to the embryo considering their quick removal after fertilization, it is plausible that protamine carbonylation affects protein–protein cross-linking and the global nucleus architecture[108]. More dangerous for the embryo is the oxidation of paternal histones that still remain after fertilization, creating unsuspected problems in the developing embryos. In this regard, oxidative stress increases histone methylation, correlated with double-stranded breaks and poor sperm quality. Together with methylation, histone acetylation is also impaired by oxidative stress[109]. Chromatin remodelling is unavoidably compromised.
Several other protein modifications can be promoted by ROS in SPZ. S-nitrosylation generally affects enzymes involved in ATP production and ion channels; tyrosine (Tyr) nitration alters sperm protein function leading to physiological or pathological effects, depending on the protein target and the level of ROS generated. Enzymes involved in glycolysis and Krebs cycle are especially impaired by ROS through a Tyr nitration modification[110]. As a direct consequence, ATP production is severely diminished and sperm motility impaired. Concerning sperm motility and beyond thiol oxidation, α-tubulin may also be modified by Tyr nitration, thus to interfere with the appropriate microtubule polymerization in the sperm flagellum. Sperm capacitation is also associated with Tyr nitration. All these redox modifications of sperm proteins are mechanisms by which ROS control cell signalling, stimulating or inhibiting the activity of proteins involved in a large variety of processes linked to sperm physiology[110].
It is clear that sperm cells are both vulnerable to ROS and good producers of ROS, especially at the onset of capacitation. Under stress conditions and as a result of membranous lipid peroxidation, SPZ generate cytotoxic lipid aldehydes such as malondialdehyde (MDA) and, above all, 4-hydroxynonenal (4-HNE;[111]. These molecules, in turn, stress ROS production, interfering with mitochondria activity and stimulating inflammation.
References
- Hill, B.G.; Benavides, G.A.; Lancaster, J.R.; Jr.; Ballinger, S.; Dell’Italia, L.; Jianhua, Z.; Darley-Usmar, V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012, 393, 1485–1512.
- Naon, D.; Scorrano, L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim. Biophys. Acta 2014, 1843, 2184–2194.
- Wong, Y.; Ysselstein, D.; Krainc, D. Mitochondria–lysosome contacts regulate mitochondrial fission via RAB7 GTP hydroly-sis. Nature 2018, 554, 382–386.
- Rieusset, J. The role of endoplasmic reticulum-mitochondria contact sites in the control of glucose homeostasis: An update. Cell Death Dis. 2018, 9, 388.
- Giorgi, C.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Poletti, F.; Rimessi, A.; et al. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion 2012, 12, 77–85.
- Paupe, V.; Prudent, J. New insights into the role of mitochondrial calcium homeostasis in cell migration. Biochem. Biophys. Res. Commun. 2018, 500, 75–86.
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174.
- Ramalho-Santos, J.; Amaral, S. Mitochondria and mammalian reproduction. Mol. Cell. Endocrinol. 2013, 379, 74–84.
- Ramalho-Santos, J.; Varum, S.; Amaral, S.; Mota, P.C.; Sousa, A.P.; Amaral, A. Mitochondrial functionality in reproduction: From gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Update 2009, 15, 553–572.
- Da Silva, A.F.; Mariotti, F.R.; Máximo, V.; Campello, S. Mitochondria dynamism: Of shape, transport and cell migration. Cell. Mol. Life Sci. 2014, 71, 2313–2324.
- Vorup-Jensen, T.; Hjort, T.; Abraham-Peskir, J.V.; Guttmann, P.; Jensenius, J.C.; Uggerhoj, E.; Medenwaldt, R. X-ray micros-copy of human spermatozoa shows change of mitochondrial morphology after capacitation. Hum. Reprod. 1999, 14, 880–884.
- Pelliccione, F.; Micillo, A.; Cordeschi, G.; D’Angeli, A.; Necozione, S.; Gandini, L.; Lenzi, A.; Francavilla, F.; Francavilla, S. Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertil. Steril. 2011, 95, 641–646.
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124.
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R.S. Differences in ATP Generation via Glycolysis and Oxidative Phos-phorylation and Relationships with Sperm Motility in Mouse Species. J. Biol. Chem. 2015, 290, 20613–20626.
- Du Plessis, S.S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230–235.
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochon-drial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363.
- Luo, S.M.; Schatten, H.; Sun, Q.Y. Sperm mitochondria in reproduction: Good or bad and where do they go? J. Genet. Genom. 2013, 40, 549–556.
- Agnihotri, S.K.; Agrawal, A.K.; Hakim, B.A.; Vishwakarma, A.L.; Narender, T.; Sachan, R.; Sachdev, M. Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 953–960.
- Wang, X.; Sharma, R.K.; Gupta, A.; George, V.; Thomas, A.J.; Falcone, T.; Agarwal, A. Alterations in mitochondria mem-brane potential and oxidative stress in infertile men: A prospective observational study. Fertil. Steril. 2003, 80, 844–850.
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519.
- Rosati, A.J.; Whitcomb, B.W.; Brandon, N.; Buck Louis, G.M.; Mumford, S.L.; Schisterman, E.F.; Pilsner, J.R. Sperm mito-chondrial DNA biomarkers and couple fecundity. Hum. Reprod. 2020, 35, 2619–2625.
- May-Panloup, P.; Chrétien, M.F.; Savagner, F.; Vasseur, C.; Jean, M.; Malthièry, Y.; Reynier, P. Increased sperm mitochon-drial DNA content in male infertility. Hum. Reprod. 2003, 18, 550–556.
- Jiang, M.; Kauppila, T.E.S.; Motori, E.; Atanassov, I.; Folz-Donahue, K.; Bonekamp, N.A.; Albarran-Gutierrez, S.; Stewart, J.B.; Larsson, N.G. Increased Total mtDNA Copy Number Cures Male Infertility Despite Unaltered mtDNA Mutation Load. Cell Metab. 2017, 26, 429–436.
- Iacobazzi, V.; Castegna, A.; Infantino, V.; Andria, G. Mitochondrial DNA methylation as a next-generation biomarker and diagnostic tool. Mol Genet Metab 2013, 110, 25–34.
- Lambard, S.; Galeraud-Denis, I.; Martin, G.; Levy, R.; Chocat, A.; Carreau, S. Analysis and significance of mRNA in human ejaculated sperm from normozoospermic donors: Relationship to sperm motility and capacitation. Mol. Hum. Reprod. 2004, 10, 535–541.
- Wang, X.; Yang, C.; Guo, F.; Zhang, Y.; Ju, Z.; Jiang, Q.; Zhao, X.; Liu, Y.; Zhao, H.; Wang, J.; et al. Integrated analysis of mRNAs and long noncoding RNAs in the semen from Holstein bulls with high and low sperm motility. Sci. Rep. 2019, 9, 2092.
- Ragusa, M.; Barbagallo, D.; Chioccarelli, T.; Manfrevola, F.; Cobellis, G.; Di Pietro, C.; Brex, D.; Battaglia, R.; Fasano, S.; Ferraro, B.; et al. CircNAPEPLD is expressed in human and murine spermatozoa and physically interacts with oocyte miRNAs. RNA Biol. 2019, 16, 1237–1248.
- Chioccarelli, T.; Manfrevola, F.; Ferraro, B.; Sellitto, C.; Cobellis, G.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Chianese, R. Expression Patterns of Circular RNAs in High Quality and Poor Quality Human Spermatozoa. Front. Endocrinol. 2019, 10, 435.
- Manfrevola, F.; Chioccarelli, T.; Cobellis, G.; Fasano, S.; Ferraro, B.; Sellitto, C.; Marella, G.; Pierantoni, R.; Chianese, R. CircRNA Role and circRNA-Dependent Network (ceRNET) in Asthenozoospermia. Front. Endocrinol. 2020, 11, 395.
- Zhang, S.; Liu, C.; Zhang, X. Mitochondrial Damage Mediated by miR-1 Overexpression in Cancer Stem Cells. Mol. Ther. Nucleic Acids 2019, 18, 938–953.
- Ma, J.; Chen, Q.; Wang, S.; Ma, R.; Jing, J.; Yang, Y.; Feng, Y.; Zou, Z.; Zhang, Y.; Ge, X.; et al. Mitochondria-related miR-574 reduces sperm ATP by targeting ND5 in aging males. Aging 2020, 12, 8321–8338.
- Larriba, E.; Rial, E.; del Mazo, J. The landscape of mitochondrial small non-coding RNAs in the PGCs of male mice, sper-matogonia, gametes and in zygotes. BMC Genom. 2018, 19, 634.
- Mercer, T.R.; Neph, S.; Dinger, M.E.; Crawford, J.; Smith, M.A.; Shearwood, A.M.; Haugen, E.; Bracken, C.P.; Rackham, O.; Stamatoyannopoulos, J.A.; et al. The human mitochondrial transcriptome. Cell 2011, 146, 645–658.
- Dong, Y.; Yoshitomi, T.; Hu, J.F.; Cui, J. Long noncoding RNAs coordinate functions between mitochondria and the nucleus. Epigenetics Chromatin 2017, 10, 41.
- Villegas, J.; Zárraga, A.M.; Muller, I.; Montecinos, L.; Werner, E.; Brito, M.; Meneses, A.M.; Burzio, L.O. A novel chimeric mitochondrial RNA localized in the nucleus of mouse sperm. DNA Cell Biol. 2000, 19, 579–588.
- Chioccarelli, T.; Pierantoni, R.; Manfrevola, F.; Porreca, V.; Fasano, S.; Chianese, R.; Cobellis, G. Histone post-translational modifications and circRNAs in mouse and human spermatozoa: Potential epigenetic marks to assess human sperm quality. J. Clin. Med. 2020, 9, 640.
- Gao, Y.; Wu, M.; Fan, Y.; Li, S.; Lai, Z.; Huang, Y.; Lan, X.; Lei, C.; Chen, H.; Dang, R. Identification and characterization of circular RNAs in Qinchuan cattle testis. R. Soc. Open Sci. 2018, 5, 180413.
- Dong, W.W.; Li, H.M.; Qing, X.R.; Huang, D.H.; Li, H.G. Identification and characterization of human testis derived circular RNAs and their existence in seminal plasma. Sci. Rep .2016, 6, 39080.
- Cavalcante, G.C.; Magalhães, L.; Ribeiro-dos-Santos, Â.; Vidal, A.F. Mitochondrial epigenetics: Non-coding RNAs as a novel layer of complexity. Int. J. Mol. Sci. 2020, 21, 1838.
- Amaral, A.; Paiva, C.; Attardo Parrinello, C.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Identification of pro-teins involved in human sperm motility using high-throughput differential proteomics. J. Proteome Res. 2014, 13, 5670–5684.
- Nowicka-Bauer, K.;, Lepczynski, A.; Ozgo, M.; Kamieniczna, M.; Fraczek, M.; Stanski, L.; Olszewska, M.; Malcher, A.; Skrzypczak, W.; Kurpisz, M.K. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated as-thenozoospermia. J. Physiol. Pharmacol. 2018, 69, 403–417.
- Agarwal, A.; Sharma, R.; Samanta, L.; Durairajanayagam, D.; Sabanegh, E. Proteomic signatures of infertile men with clini-cal varicocele and their validation studies reveal mitochondrial dysfunction leading to infertility. Asian J. Androl. 2016, 18, 282–291.
- Saito, A.; Imaizumi, K. Unfolded protein response-dependent communication and contact among endoplasmic reticulum, mitochondria, and plasma membrane. Int. J. Mol. Sci. 2018, 19, 3215.
- Pellegrino, M.W.; Nargund, A.M.; Haynes, C.M. Signaling the mitochondrial unfolded protein response. Biochim. Biophys. Acta-Mol. Cell Res. 2013, 1833, 410–416.
- Santiago, J.; Santos, M.A.S.; Fardilha, M.; Silva, J.V. Stress response pathways in the male germ cells and gametes. Mol. Hum. Reprod. 2020, 26, 1–13.
- Ramalho-Santos, J. A sperm’s tail: The importance of getting it right. Hum. Reprod. 2011, 26, 2590–2591.
- Ankel-Simons, F.; Cummins, J.M. Misconceptions about mitochondria and mammalian fertilization: Implications for theo-ries on human evolution. Proc. Natl. Acad. Sci. USA 1996, 93, 13859–13863.
- Sutovsky, P.; Song, W.H. Post-fertilisation sperm mitophagy: The tale of Mitochondrial Eve and Steve. Reprod. Fertil. Dev. 2017, 30, 56–63.
- Lewin, R. The unmasking of mitochondrial Eve. Science 1987, 238, 24–26.
- Sutovsky, P.; Moreno, R.D.; Ramalho-Santos, J.; Dominko, T.; Simerly, C.; Schatten, G. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol. Reprod. 2000, 63, 582–590.
- Thompson, W.E.; Ramalho-Santos, J.; Sutovsky, P. Ubiquitination of prohibitin in mammalian sperm mitochondria: Possi-ble roles in the regulation of mitochondrial inheritance and sperm quality control. Biol. Reprod. 2003, 69, 254–260.
- Al Rawi, S.; Louvet-Vallée, S.; Djeddi, A.; Sachse, M.; Culetto, E.; Hajjar, C.; Boyd, L.; Legouis, R.; Galy, V. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science 2011, 334, 1144–1147.
- Song, W.H.; Yi, Y.J.; Sutovsky, M.; Meyers, S.; Sutovsky, P. Autophagy and ubiquitin-proteasome system contribute to sperm mitophagy after mammalian fertilization. Proc. Natl. Acad. Sci. USA 2016, 113, E5261–E5270.
- Luo, S.M.; Ge, Z.J.; Wang, Z.W.; Jiang, Z.Z.; Wang, Z.B.; Ouyang, Y.C.; Hou, Y.; Schatten, H.; Sun, Q.Y. Unique insights into maternal mitochondrial inheritance in mice. Proc. Natl. Acad. Sci. USA 2013b, 110, 13038–13043.
- Holt, I.J.; Harding, A.E.; Morgan-Hughes, J.A. Deletions of muscle mitochondrial DNA in patients with mitochondrial my-opathies. Nature 1988, 331, 717–719.
- Luo, S.; Valencia, C.A.; Zhang, J.; Lee, N.C.; Slone, J.; Gui, B.; Wang, X.; Li, Z.; Dell, S.; Brown, J.; et al. Biparental Inheritance of Mitochondrial DNA in Humans. Proc. Natl. Acad. Sci. USA 2018, 115, 13039–13044.
- Schwartz, M.; Vissing, J. Paternal inheritance of mitochondrial DNA. N. Engl. J. Med. 2002, 347, 576–580.
- McWilliams, T.G.; Suomalainen, A. Mitochondrial DNA can be inherited from fathers, not just mothers. Nature 2019, 565, 296–297.
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13.
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19.
- Madamanchi, N.R.; Runge, M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007, 100, 460–473.
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230.
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656.
- Sauve, A.A. Sirtuin chemical mechanisms. Biochim. Biophys. Acta 2010, 1804, 1591–1603.
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859.
- Chianese, R.; Viggiano, A.; Urbanek, K.; Cappetta, D.; Troisi, J.; Scafuro, M.; Guida, M.; Esposito, G.; Ciuffreda, L.P.; Rossi, F.; et al. Chronic exposure to low dose of bisphenol A impacts on the first round of spermatogenesis via SIRT1 modulation. Sci. Rep. 2018, 8, 2961.
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15.
- Halliwell, B.; Gutteridge, J. Antioxidant defences: Endogenous and diet derived. InFree Radicals in Biology and Medicine; Hal-liwell, B., Gutteridge, J., Eds.; Oxford University Press: New York, NY, USA, 2007; pp. 79–186.
- Maiorino, M.; Bosello, V.; Ursini, F.; Foresta, C.; Garolla, A.; Scapin, M.; Sztajer, H.; Flohe, L. Genetic variations of gpx-4 and male infertility in humans. Biol. Reprod. 2003, 68, 1134–1141.
- Ischi, T.; Matsuki, S.; Iuchi, Y.; Okada, F.; Toyosaki, S.; Tomita, Y.; Ikeda, Y.; Fujii, J. Accelerated impairment of spermato-genic cells in SOD1-knockout mice under heat stress. Free Radic. Res. 2005, 39, 695–705.
- Khan, S.; Khan, M.A.; Bhatnagar, D.; Yadav, P.; Sarkar, S. Zinc protection against lipid peroxidation from cadmium. Indian J. Exp. Biol. 1991, 29, 823–825.
- Angulo, C.; Maldonado, R.; Pulgar, E.; Mancilla, H.; Córdova, A.; Villarroel, F.; Castro, M.A.; Concha, I.I. Vitamin C and oxidative stress in the seminiferous epithelium. Biol. Res. 2011, 44, 169–180.
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658.
- Meccariello, R.; Chianese, R.; Chioccarelli, T.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Cobellis, G. Intra-testicular signals regulate germ cell progression and production of qualitatively mature spermatozoa in vertebrates. Front. Endocrinol. 2014, 5, 69.
- Rudolph, L.M.; Bentley, G.E.; Calandra, R.S.; Paredes, A.H.; Tesone, M.; Wu, T.J.; Micevych, P.E. Peripheral and Central Mechanisms Involved in the Hormonal Control of Male and Female Reproduction. J. Neuroendocrinol. 2016, 28, 10.
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of reactive oxygen species in the spermatogenesis regula-tion. Front. Endocrinol. 2014, 5, 56.
- O’Flaher, C.; Beorlegui, N.; Beconi, M.T. Participation of superoxide anion in the capacitation of cryopreserved bovine sperm. Int. J. Androl. 2003, 26, 109–114.
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990.
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111.
- Migliaccio, V.; Sica, R.; Scudiero, R.; Simoniello, P.; Putti, R.; Lionetti, L. Physiological Adaptation to Simultaneous Chronic Exposure to High-Fat Diet and Dichlorodipheniletylhene (DDE) in Wistar Rat Testis. Cells 2019, 8, 443.
- Dong, J.; Wang, Z.; Zou, P.; Zhang, G.; Dong, X.; Ling, X.; Zhang, X.; Liu, J.; Ye, D.; Cao, J.; Ao, L. Induction of DNA damage and G2 cell cycle arrest by diepoxybutane through the activation of the Chk1-dependent pathway in mouse germ cells. Chem. Res. Toxicol. 2015, 28, 518–531.
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006, 250, 66–69.
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485.
- Steger, K.; Pauls, K.; Klonisch, T.; Franke, F.E. and Bergmann, M. Expression of protamine 1 and 2 mRNA during human spermiogenesis. Mol. Hum. Reprod. 2000, 6, 219–225.
- Barral, S.; Morozumi, Y.; Tanaka, H.; Montellier, E.; Govin, J.; de Dieuleveult, M.; Charbonnier, G.; Couté, Y.; Puthier, D.; Buchou, T.; et al. Histone Variant H2A.L.2 Guides Transition Protein-Dependent Protamine Assembly in Male Germ Cells. Mol. Cell 2017, 66, 89–101.
- Erkek, S.; Hisano, M.; Liang, C.Y.; Gill, M.; Murr, R.; Dieker, J.; Schübeler, D.; Vlag, J.; Van Der Stadler, M.B.; Peters, A.H.F.M. Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa. Nat. Struct. Mol. Biol. 2013, 20, 868–875.
- Noblanc, A.; Damon-Soubeyrand, C.; Karrich, B.; Henry-Berger, J.; Cadet, R.; Saez, F.; Guiton, R.; Janny, L.; Pons-Rejraji, H.; Alvarez, J.G.; et al. DNA oxidative damage in mammalian spermatozoa: Where and why is the male nucleus affected? Free Radic. Biol. Med. 2013, 65, 719–723.
- Zalensky, A.; Zalenskaya, I. Organization of chromosomes in spermatozoa: An additional layer of epigenetic information? Biochem. Soc. Trans. 2007, 35, 609–611.
- Kocer, A.; Henry-Berger, J.; Noblanc, A.; Champroux, A.; Pogorelcnik, R.; Guiton, R.; Janny, L.; Pons-Rejraji, H.; Saez, F.; Johnson, G.D.; et al. Oxidative DNA damage in mouse sperm chromosomes: Size matters. Free Radic. Biol. Med. 2015, 89, 993–1002.
- Aitken, R.J. Not every sperm is sacred; a perspective on male infertility. Mol. Hum. Reprod. 2018, 24, 287–298.
- Bauer, N.C.; Corbett, A.H.; Doetsch, P.W. The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res. 2015, 43, 10083–10101.
- Lee, K.M.; Ward, M.H.; Han, S.; Ahn, H.S.; Kang, H.J.; Choi, H.S.; Shin, H.Y.; Koo, H.H.; Seo, J.J.; Choi, J.E.; et al. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk. Res. 2009, 33, 250–258.
- Smith, T.B.; De Iuliis, G.N.; Lord, T.; Aitken, R.J. The senescence-accelerated mouse prone 8 as a model for oxidative stress and impaired DNA repair in the male germ line. Reproduction 2013, 146, 253–262.
- Sharma, P.; Ghanghas, P.; Kaushal, N.; Kaur, J.; Kaur, P. Epigenetics and oxidative stress: A twin-edged sword in spermato-genesis. Andrologia 2019, 51, e13432.
- Franco, R.; Schoneveld, O.; Georgakilas, A.G.; Panayiotidis, M.I. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008, 266, 6–11.
- Urdinguio, R.G.; Bayón, G.F.; Dmitrijeva, M.; Toraño, E.G.; Bravo, C.; Fraga, M.F.; Bassas, L.; Larriba, S.; Fernández, A.F. Ab-errant DNA methylation patterns of spermatozoa in men with unexplained infertility. Hum. Reprod. 2015, 30, 1014–1028.
- Ménézo, Y.; Entezami, F.; Lichtblau, I.; Belloc, S.; Cohen, M.; Dale, B. Oxidative stress and fertility: Incorrect assumptions and ineffective solutions. Zygote 2014, 22, 80–90.
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97.
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contrib-ute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400.
- Chu, C.; Henry-Berger, J.; Ru, Y.; Kocer, A.; Champroux, A.; Li, Z.T.; He, M.; Xie, S.; Ma, W.; Ni, M.; et al. Knockout of gluta-thione peroxidase 5 down-regulates the piRNAs in the caput epididymis of aged mice. Asian J. Androl. 2020, 22, 590–601.
- Wathes, D.C.; Abayasekara, D.R.; Aitken, R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007, 77, 190–201.
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38.
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen spe-cies in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207.
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact sperma-tozoa and on sperm axonemes. J. Androl. 1992, 13, 368–378.
- Clark, H.M.; Hagedorn, T.D.; Landino, L.M. Hypothiocyanous acid oxidation of tubulin cysteines inhibits microtubule polymerization. Arch. Biochem. Biophys. 2014, 541, 67–73.
- MacLeod, J. The role of oxygen in the metabolism and motility of human spermatozoa. Am. J. Physiol. 1943, 138, 512–518.
- Lone, S.A.; Mohanty, T.K.; Baithalu, R.K.; Yadav, H.P. Sperm protein carbonylation. Andrologia 2019, 51, e13233.
- Tirmarche, S.; Kimura, S.; Dubruille, R.; Horard, B.; Loppin, B. Unlocking sperm chromatin at fertilization requires a dedi-cated egg thioredoxin in Drosophila. Nat. Commun. 2016, 7, 13539.
- Montjean, D.; Ravel, C.; Benkhalifa, M.; Cohen-Bacrie, P.; Berthaut, I.; Bashamboo, A.; & McElreavey, K. Methylation chang-es in mature sperm deoxyribonucleic acid from oligozoospermic men: Assessment of genetic variants and assisted repro-ductive technology outcome. Fertil. Steril. 2013, 100, 1241–1247.
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585.
- Moazamian, R.; Polhemus, A.; Connaughton, H.; Fraser, B.; Whiting, S.; Gharagozloo, P.; Aitken, R.J. Oxidative stress and human spermatozoa: Diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol. Hum. Reprod. 2015, 21, 502–515.




