
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jicheng Zhan | + 4379 word(s) | 4379 | 2020-12-07 14:32:01 | | | |
| 2 | Dean Liu | -2430 word(s) | 1949 | 2021-01-25 07:13:09 | | |
Video Upload Options
Proanthocyanidins are essential secondary plant metabolites that contribute to the nutritional value and sensory quality of many fruits and the related processed products. The accumulation of proanthocyanidins is associated with the resistance of plants against a broad spectrum of abiotic and biotic stress conditions. Many specific regulators were involved in the proanthocyanidins biosynthetic network in response to various environmental conditions.
1. Introduction
Horticultural crops, including vegetables and fruits, are a crucial food source for human nutrition as they contain carbohydrates, proteins, vitamins, organic acids, and minerals. Proanthocyanidins (PAs), also known as condensed tannins, are among the most abundant polyphenols in plants. They are naturally present in woody plants and herbaceous species. PAs are present in the leaves, fruits, seeds, roots, and other parts of plants[1], and play essential roles in the growth and development of leaves and fruits, as well as, the modulation of seed dormancy and germination[2][3][4][5][6][7]. In addition, PAs also influence mouthfeel by enhancing the astringency of fruits and in their processed products, e.g., wines and beverages[8][9]. Moreover, as potential dietary antioxidants, PAs are considered beneficial to human health. It has been widely accepted that the PAs from food plants and medicinal plants are associated with various bioactivities, displaying immunomodulatory, anti-inflammatory, anti-cancer, anti-microbial, and hypolipidemic properties while reducing the risk of cardiovascular disease and ameliorating obesity [10][11][12][13][14][15][16][17][18][19][20][21][22].
Developmental regulation is considered the driving factor for PAs biosynthesis, which can also be influenced by environmental fluctuations. In grape berry, the PAs accumulate in the skin prior to véraison, after which the total PAs content decreases due to intermediate metabolites deviation[4]. Many crops have shown a similar decreasing trend in PAs levels during the fruit ripening process, including bilberries, persimmons, and blueberries[23][24][25]. PAs contribute to the astringency and bitterness of the young fruits, preventing them from being eaten before they are ripe[26]. The accumulation of PAs is associated with the resistance of plants against various biotic and abiotic stimuli, such as low temperature[27][28], drought[29], wounding[30][31], UV radiation[30], and fungal pathogens[32][33][34]. Therefore, increasing attention has been focused on the metabolic engineering and regulatory mechanism of PAs in horticultural plants. The structural genes and key factors that regulate PAs biosynthesis were identified and characterized in many plants, particularly in horticultural crops rich in PAs, such as grapevines, poplars, and apples. Studies involving PAs biosynthesis regulation in response to environmental stresses revealed that the regulatory network was based on the regulators belonging to the MYB, MYC-like basic helix-loop-helix (bHLH), WD40-repeat proteins, and the MYB-bHLH-WD40 (MBW) complexes. However, research regarding more specific regulators in this complicated network is required to understand the interaction between these vital regulators during PAs biosynthesis to ultimately improve the quality of crops.
2. Structure and Biosynthesis of Proanthocyanidins
PAs consist of a mixture of flavan-3-ol units and flavan-3,4-diols (leucoanthocyanidins) in complicated ways[35]. Flavan-3-ol monomeric units include (+)-catechins, (−)-epicatechins, and their derivatives: (+)-gallocatechin, (+)-catechin gallate, (+)-gallocatechin gallate (−)-epicatechin gallate, (−)-epigallocatechin, and (−)-epigallocatechin gallate (Figure 1). Both (+)-catechins and (−)-epicatechins are believed to act as the starter units for PAs polymerization, and flavan-3-ols and flavan-3,4-diols serve as extension units for further PAs extension[1][36][37]. Different numbers of subunits polymerized to form oligomeric or polymeric PAs[1][38]. The degree of polymerization of PAs varies among species and tissues. Based on the interflavanic linkages, the subunits linked by C4-C8 and/or C4-C6 bonds are known as B-type PAs, and those with additional C2-O-C7 or C2-O-C5 bond in the structure are classified into A-type PAs (Figure 1)[1]. In addition, esterification, glycosylation, and other modifications have also been found in PAs with more complex structures. B-type PAs are widely distributed in plants and foods, such as senna alata leaves, pine trees, black wattle, beans, barley, sorghum, seeds, fruits, berries, nuts, cinnamon, chocolate, and wine[39][40][41][42][43][44][45]. A-type PAs are mainly found in bilberry, peanuts, plums, cranberries, curry, and cinnamon[46][47][48][49].
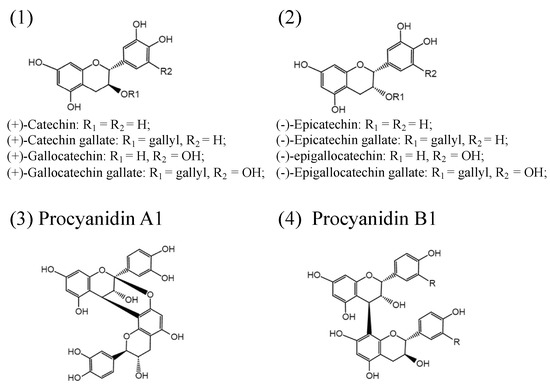
Figure 1. Structures of the flavan-3-ols (1,2); simple A1-type proanthocyanidins (3); simple B1-type proanthocyanidins (4).
The pathway of flavonoid biosynthesis has been well studied in various plants (Figure 2). Flavonols, anthocyanins, and PAs are the main products of the flavonoid biosynthetic pathway, which belongs to the plant secondary metabolism[50][51][52]. They share the common upstream steps in the biosynthetic pathway of flavonoids, and their precursor is dihydroflavonols which can be converted to leucoanthocyanidins by dihydroflavonol-4-reductase (DFR) and to flavonols by flavonol synthase (FLS). Leucoanthocyanidins can be further catalyzed to produce anthocyanidins by the oxygenation reaction of anthocyanidin synthase (ANS) or leucoanthocyanidin dioxygenase (LDOX). Leucoanthocyanidins and anthocyanidins can be catalyzed by leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) to produce 2,3-trans-flavan-3-ols (such as (+)-catechin) and 2,3-cis-flavan-3-ols (such as (−)-epicatechin) which are the key subunits involved in the last steps of PAs biosynthesis.
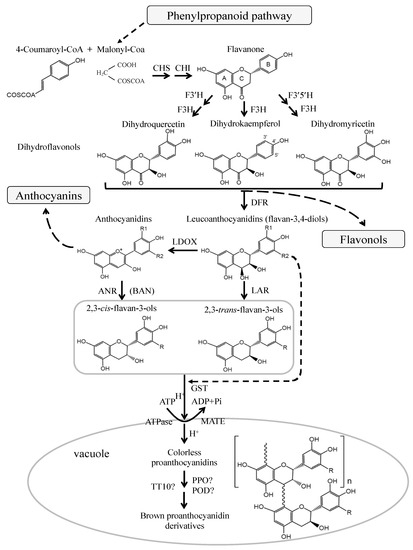
Figure 2. Biosynthetic pathway of flavonoid (adapt from[1][38]). The enzymes are: CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′,5′-hydroxylase; DFR, dihydroflavonol-4-reductase; LAR, leucoanthocyanidin reductase; ANS, anthocyanidin synthase; LDOX, leucoanthocyanidin dioxygenase; GST, glutathione S-transferase; MATE, multidrug and toxic compound extrusion; PPO, polyphenol oxidase; POD, peroxidase; TT10, transparent testa 10.
To date, LAR and ANR genes have been cloned from several plant species, and their functions have been demonstrated both genetically and biochemically[3][36][53][54][55][56][57][58][59][60]. As is well known, leucoanthocyanidins are converted to (+)-catechins through the catalysis of LAR. Interestingly, ectopic expression of tea CsLAR, and cacao TcLAR in tobacco showed that the production of (−)-epicatechin was higher than that of catechins[57][61]. More recent studies also found that LAR from Medicago truncatula and grapevine can convert 4β-(S-cysteinyl)-epicatechin into (−)-epicatechins, thereby regulating the degree of PAs polymerization[59][62]. In addition to catalyzing the synthesis of (−)-epicatechins from anthocyanidins, ANR is proven to convert anthocyanidins to a mixture of (−)-epicatechins and (+)-catechins[61][63]. This suggests that LAR and ANR may possess more features to be explored [61][62][63].
Most of the enzymes for flavonoid biosynthesis are located on the endoplasmic reticulum and tonoplast of cells. PAs are synthesized in the cytoplasm, and then transferred and accumulated into the vacuole and apoplast [64][65][66]. In seed, PAs are mostly accumulated in the inner endothelial layer of seed testa [35][67][36], and the brown color of the seed coat may result from the oxidation of PAs[68]. The seeds without PAs content are called transparent testa (tt) phenotype and tannin deficient seeds, which exhibit yellow or pale brown in color[54][69][70]. The PA-deficient mutants have become an ideal material for investigating the biosynthesis and transportation of PAs. MATE (multidrug and toxic compound extrusion) and GST (glutathione S-transferase) are proteins responsible for flavonoids transportation. AtTT12, a member of the MATE family, localizes the PAs in the vacuoles of the testa of Arabidopsis seeds[71]. AtTT13/AtAHA10 encodes a protein belonging to the ATPase family, which is required for the deposition of PA precursors in vacuole and functions as a proton pump, providing the driving force for TT12-mediated transportation of PA precursors[72]. VvMATE1 and VvMATE2 are transport proteins located in the tonoplast and Golgi complex, respectively, and involved in the transport and accumulation of PAs through different ways in grapevine [66]. GST proteins were thought to deliver anthocyanins into vacuoles, e.g., ZmBz2 in maize, PhAN9 in petunia, AtTT19 in Arabidopsis, and VviGSTs in grape[37][73][74]. After heterologous overexpression of PhAN9 in Arabidopsis tt19 mutant, the anthocyanin pigment in seedlings was complemented, but no complementation was observed in the pale brown seeds of the tt19 mutant[37]. Moreover, PAs precursors were dispersed in the cytoplasm in tt12 mutant[71], but accumulated as membrane-like structures in endothelium cells of tt19, indicating that TT19 may function as a subsequent transporter to deliver the vesicles containing PAs precursors to the central vacuole[37][75]. VviGST3, a homolog to AtTT19, was highly expressed in the seed during grape berry development, and the complementation in seed color was observed in VviGST3 overexpressed tt19 mutant, suggesting that VviGST3 may be associated with the transport of PAs[76]. In addition, heterologous expression of pear PcGSTF12 in the Arabidopsis tt19 mutant showed that PcGSTF12 did not complement the mutant seed color to the normal brown, but it promoted the procyanidin A3 accumulation in the mutant and affected the transcription of PAs- and anthocyanin-related genes. The results of this study indicated that PcGSTF12 may be involved in the transport and accumulation of PAs and anthocyanins[77].
The vacuole is the final destination for the transportation of PAs, and the polymerization of PAs probably takes place here. Although little is known about the polymerization mechanisms of PAs, the enzymatic and nonenzymatic polymerization reactions are widely discussed[1][78][79]. Plant polyphenol oxidases, TT10 and plant peroxidases may take part in PAs polymerization and oxidation[1][64][80][81]. For instance, AtTT10 encodes a putative laccase that oxidizes epicatechin into yellow or brown oligomers, which may involve in PAs oxidative browning in the seed testa [64].
3. Conclusions and Further Prospects
PAs have attracted attention not only because they possess beneficial pharmacological properties but also because of their special role in the regulation of fruit quality and plant defense. The principal focus of research in the biosynthetic pathway and regulatory mechanism of PAs has been explored for years, and some encouraging progress has been made. However, there are still many issues that need to be further explored. The external environment in which plants live is constantly changing, there is a diversity of species, and the complex genetic background of woody plants, results in different regulatory mechanisms of PAs in various species in response to developmental cues and various stresses (Figure 3). In all species analyzed to date, a large number of MYBs regulate the synthesis of PAs that are involved in plant response to stress and signals. Nevertheless, the bHLH and WD40 cofactors of the MBW complex are also involved in the regulation of other pathways, so their specificity for PAs-regulation is less than that of MYB TFs, and few studies have focused on bHLH TFs, WD40 protein, and the proteins from other families that also participate in the biosynthesis of PAs. The highly induced transcription and accumulation of PAs are considered to be a systemic response of plant to adverse conditions[82]. Further work needs to reveal how the biotic and abiotic stresses trigger the transcription of these TFs, how the transcriptional and post-transcriptional regulation modifies these TFs, and how the crosstalk between these TFs and various signals and hormones is conducted.
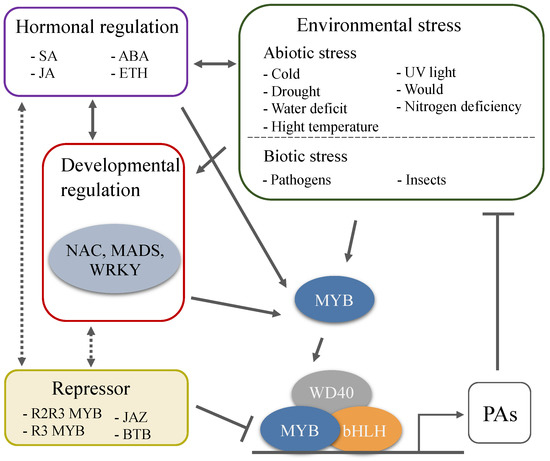
Figure 3. Environmental and developmental regulation of PAs biosynthesis through the MBW complexes. A large number of MYBs regulate the synthesis of PAs that are involved in plant response to stress and signals. Plant hormones are response to the environmental and developmental cues, and are the key factors involved in PAs biosynthesis. Environmental stresses affect the accumulation of PAs, and the high levels of PAs further enhance plant tolerance to extreme environmental challenges.
Metabolic engineering and molecular biology methods can be used to produce PA-rich crops that can enhance the tolerance of crops to various environmental stress, and improve the quality and nutritional value of crops. PAs have a great impact on the sensory properties of fruits by contributing to the astringency and bitterness, and may also influence the digestive system in humans and animals[1][83]. For example, the accumulation of PAs in persimmon fruit leads to astringency[84], which brings unpleasant tactile sensation. Understanding the regulation of PAs biosynthesis network can provide further insights into more targeted gene modifications, and are of great importance for breeding. However, it should be noted that one factor might affect the biosynthesis of many other metabolites. For example, the overexpression of VvMYBPA1 in tobacco upregulates PAs metabolism while downregulating anthocyanin biosynthesis[85]. Therefore, the comprehensive and in-depth analysis of the molecular regulation mechanism of plant resistance mediated by PAs has a positive significance for improving our knowledge of regulatory network controlling PAs synthesis and provides a theoretical foundation for genetic improvement and breeding of the plants with moderate levels of PAs.
References
- Smulikowska, S.; Pastuszewska, B.; Świȩch, E.; Ochtabińska, A.; Mieczkowska, A.; Nguyen, V.C.; Buraczewska, L. Tannin content affects negatively nutritive value of pea for monogastrics. J. Anim. Feed Sci. 2001, 10, 511–523.
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A final frontier in flavonoid research? New Phytol. 2005, 165, 9–28.
- Kennedy, J.A.; Hayasaka, Y.; Vidal, S.; Waters, E.J.; Jones, G.P. Composition of grape skin proanthocyanidins at different stages of berry development. J. Agric. Food Chem. 2001, 49, 5348–5355.
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin Synthesis and Expression of Genes Encoding Leucoanthocyanidin Reductase and Anthocyanidin Reductase in Developing Grape Berries and Grapevine Leaves. Plant Physiol. 2005, 139, 652–663.
- Debeaujon, I.; Léon-Kloosterziel, K.M.; Koornneef, M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–413.
- Bogs, J.; Jaffé, F.W.; Takos, A.M.; Walker, A.R.; Robinson, S.P. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007, 143, 1347–1361.
- Jia, L.G.; Sheng, Z.W.; Xu, W.F.; Li, Y.X.; Liu, Y.G.; Xia, Y.J.; Zhang, J.H. Modulation of anti-oxidation ability by proanthocyanidins during germination of arabidopsis thaliana seeds. Mol. Plant. 2012, 5, 472–481.
- Zhao, P.; Li, X.; Jia, J.; Yuan, G.; Chen, S.; Qi, D.; Cheng, L.; Liu, G. bHLH92 from sheepgrass acts as a negative regulator of anthocyanin/proanthocyandin accumulation and influences seed dormancy. J. Exp. Bot. 2019, 70, 269–284.
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330–335.
- Gonzalo-Diago, A.; Dizy, M.; Fernández-Zurbano, P. Taste and mouthfeel properties of red wines proanthocyanidins and their relation to the chemical composition. J. Agric. Food Chem. 2013, 61, 8861–8870.
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144.
- Serafini, M.; Bugianesi, R.; Maiani, G.; Valtuena, S.; De Santis, S.; Crozier, A. Plasma antioxidants from chocolate. Nature 2003, 424, 1013.
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61, 1601082.
- Erasto, P.; Majinda, R. Bioactive proanthocyanidins from the root bark of Cassia abbreviata. Int. J. Biol. Chem. Sci. 2012, 5, 2170–2179.
- Sobeh, M.; Mahmoud, M.F.; Abdelfattah, M.A.O.; Cheng, H.; El-Shazly, A.M.; Wink, M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018, 213, 38–47.
- Natella, F.; Belelli, F.; Gentili, V.; Ursini, F.; Scaccini, C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J. Agric. Food Chem. 2002, 50, 7720–7725.
- Cos, P.; Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.; Vlietinck, A. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2012, 11, 1345–1359.
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104.
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52.
- Toden, S.; Ravindranathan, P.; Gu, J.; Cardenas, J.; Yuchang, M.; Goel, A. Oligomeric proanthocyanidins (OPCs) target cancer stem-like cells and suppress tumor organoid formation in colorectal cancer. Sci. Rep. 2018, 8, 1–13.
- Sieniawska, E. Activities of tannins-From in vitro studies to clinical trials. Nat. Prod. Commun. 2015, 10, 1877–1884.
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262.
- Bladé, C.; Arola, L.; Salvadó, M.J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59.
- Jaakola, L.; Määttä, K.; Pirttilä, A.M.; Törrönen, R.; Kärenlampi, S.; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002, 130, 729–739.
- Akagi, T.; Ikegami, A.; Tsujimoto, T.; Kobayashi, S.; Sato, A.; Kono, A.; Yonemori, K. DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol. 2009, 151, 2028–2045.
- Zifkin, M.; Jin, A.; Ozga, J.A.; Irina Zaharia, L.; Schernthaner, J.P.; Gesell, A.; Abrams, S.R.; Kennedy, J.A.; Peter Constabel, C. Gene expression and metabolite profiling of developing highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol. 2012, 158, 200–224.
- Sanoner, P.; Guyot, S.; Marnet, N.; Molle, D.; Drilleau, J.F. Polyphenol profiles of french cider apple varieties (Malus domestica sp.). J. Agric. Food Chem. 1999, 47, 4847–4853.
- Wang, N.; Qu, C.; Jiang, S.; Chen, Z.; Xu, H.; Fang, H.; Su, M.; Zhang, J.; Wang, Y.; Liu, W.; et al. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. 2018, 96, 39–55.
- An, J.P.; Li, R.; Qu, F.J.; You, C.X.; Wang, X.F.; Hao, Y.J. R2R3-MYB transcription factor MdMYB23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. Plant J. 2018, 96, 562–577.
- Malisch, C.S.; Salminen, J.P.; Kölliker, R.; Engström, M.T.; Suter, D.; Studer, B.; Lüscher, A. Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant’s ontogenetic stage. J. Agric. Food Chem. 2016, 64, 9307–9316.
- Mellway, R.D.; Tran, L.T.; Prouse, M.B.; Campbell, M.M.; Peter Constabel, C. The wound-, pathogen-, and ultraviolet B-Responsive MYB134 gene encodes an R2R3 MYB transcription factor that regulates proanthocyanidin synthesis in poplar. Plant Physiol. 2009, 150, 924–941.
- Shen, Y.; Sun, T.; Pan, Q.; Anupol, N.; Chen, H.; Shi, J.; Liu, F.; Deqiang, D.; Wang, C.; Zhao, J.; et al. RrMYB5- and RrMYB10-regulated flavonoid biosynthesis plays a pivotal role in feedback loop responding to wounding and oxidation in Rosa rugosa. Plant Biotechnol. J. 2019, 17, 2078–2095.
- Yuan, L.; Wang, L.; Han, Z.; Jiang, Y.; Zhao, L.; Liu, H.; Yang, L.; Luo, K. Molecular cloning and characterization of PtrLAR3, a gene encoding leucoanthocyanidin reductase from Populus trichocarpa, and its constitutive expression enhances fungal resistance in transgenic plants. J. Exp. Bot. 2012, 63, 2513–2524.
- Ullah, C.; Unsicker, S.B.; Fellenberg, C.; Constabel, C.P.; Schmidt, A.; Gershenzon, J.; Hammerbacher, A. Flavan-3-ols are an effective chemical defense against rust infection. Plant Physiol. 2017, 175, 1560–1578.
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The transcription factor MYB115 contributes to the regulation of proanthocyanidin biosynthesis and enhances fungal resistance in poplar. New Phytol. 2017, 215, 351–367.
- Abrahams, S.; Tanner, G.J.; Larkin, P.J.; Ashton, A.R. Identification and biochemical characterization of mutants in the proanthocyanidin pathway in Arabidopsis. Plant Physiol. 2002, 130, 561–576.
- Xie, D.Y.; Sharma, S.B.; Paiva, N.L.; Ferreira, D.; Dixon, R.A. Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 2003, 299, 396–399.
- Kitamura, S.; Shikazono, N.; Tanaka, A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004, 37, 104–114.
- Constabel, C.P. Molecular controls of proanthocyanidin synthesis and structure: Prospects for genetic engineering in crop plants. J. Agric. Food Chem. 2018, 66, 9882–9888.
- Ramsay, A.; Mueller-Harvey, I. Senna alata leaves are a good source of propelargonidins. Nat. Prod. Res. 2016, 30, 1548–1551.
- Gu, L.; Kelm, M.; Hammerstone, J.F.; Beecher, G.; Cunningham, D.; Vannozzi, S.; Prior, R.L. Fractionation of polymeric procyanidins from lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC-MS fluorescent detection method. J. Agric. Food Chem. 2002, 50, 4852–4860.
- Gabetta, B.; Fuzzati, N.; Griffini, A.; Lolla, E.; Pace, R.; Ruffilli, T.; Peterlongo, F. Characterization of proanthocyanidins from grape seeds. Fitoterapia 2000, 71, 162–175.
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521.
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617.
- Karonen, M.; Loponen, J.; Ossipov, V.; Pihlaja, K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 522, 105–112.
- Chen, X.; Xiong, J.; Huang, S.; Li, X.; Zhang, Y.; Zhang, L.; Wang, F. Analytical profiling of proanthocyanidins from Acacia mearnsii bark and in vitro assessment of antioxidant and antidiabetic potential. Molecules 2018, 23, 2891.
- Suvanto, J.; Karppinen, K.; Riihinen, K.; Jaakola, L.; Salminen, J.P. Changes in the proanthocyanidin composition and related gene expression in bilberry (Vaccinium myrtillus L.) Tissues. J. Agric. Food Chem. 2020, 68, 7378–7386.
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 2000, 54, 173–181.
- Yu, J.; Ahmedna, M.; Goktepe, I.; Dai, J. Peanut skin procyanidins: Composition and antioxidant activities as affected by processing. J. Food Compos. Anal. 2006, 19, 364–371.
- Appeldoorn, M.M.; Sanders, M.; Vincken, J.P.; Cheynier, V.; Le Guernevé, C.; Hollman, P.C.H.; Gruppen, H. Efficient isolation of major procyanidin A-type dimers from peanut skins and B-type dimers from grape seeds. Food Chem. 2009, 117, 713–720.
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.-M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430.
- Routaboul, J.M.; Dubos, C.; Beck, G.; Marquis, C.; Bidzinski, P.; Loudet, O.; Lepiniec, L. Metabolite profiling and quantitative genetics of natural variation for flavonoids in Arabidopsis. J. Exp. Bot. 2012, 63, 3749–3764.
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34.
- Tanner, G.J.; Francki, K.T.; Abrahams, S.; Watson, J.M.; Larkin, P.J.; Ashton, A.R. Proanthocyanidin biosynthesis in plants. Purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J. Biol. Chem. 2003, 278, 31647–31656.
- Abrahams, S.; Lee, E.; Walker, A.R.; Tanner, G.J.; Larkin, P.J.; Ashton, A.R. The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J. 2003, 35, 624–636.
- Paolocci, F.; Robbins, M.P.; Madeo, L.; Arcioni, S.; Martens, S.; Damiani, F. Ectopic expression of a basic helix-loop-helix gene transactivates parallel pathways of proanthocyanidin biosynthesis. Structure, expression analysis, and genetic control of leucoanthocyanidin 4-reductase and anthocyanidin reductase genes in Lotus cornicu. Plant Physiol. 2007, 143, 504–516.
- Pang, Y.; Peel, G.J.; Wright, E.; Wang, Z.; Dixon, R.A. Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol. 2007, 145, 601–615.
- Liu, Y.; Shi, Z.; Maximova, S.; Payne, M.J.; Guiltinan, M.J. Proanthocyanidin synthesis in Theobroma cacao: Genes encoding anthocyanidin synthase, anthocyanidin reductase, and leucoanthocyanidin reductase. BMC Plant Biol. 2013, 13, 202.
- Matsui, K.; Hisano, T.; Yasui, Y.; Mori, M.; Walker, A.R.; Morishita, T.; Katsu, K. Isolation and characterization of genes encoding leucoanthocyanidin reductase (FeLAR) and anthocyanidin reductase (FeANR) in buckwheat (Fagopyrum esculentum). J. Plant Physiol. 2016, 205, 41–47.
- Liu, C.; Wang, X.; Shulaev, V.; Dixon, R.A. A role for leucoanthocyanidin reductase in the extension of proanthocyanidins. Nat. Plants 2016, 2, 16182.
- Li, H.; Tian, J.; Yao, Y.Y.; Zhang, J.; Song, T.T.; Li, K.T.; Yao, Y.C. Identification of leucoanthocyanidin reductase and anthocyanidin reductase genes involved in proanthocyanidin biosynthesis in Malus crabapple plants. Plant Physiol. Biochem. 2019, 139, 141–151.
- Pang, Y.; Abeysinghe, I.S.B.; He, J.; He, X.; Huhman, D.; Mudith Mewan, K.; Sumner, L.W.; Yun, J.; Dixon, R.A. Functional characterization of proanthocyanidin pathway enzymes from tea and their application for metabolic engineering. Plant Physiol. 2013, 161, 1103–1116.
- Yu, K.; Jun, J.H.; Duan, C.; Dixon, R.A. VvLAR1 and VvLAR2 are bifunctional enzymes for proanthocyanidin biosynthesis in grapevine. Plant Physiol. 2019, 180, 1362–1374.
- Pourcel, L.; Routaboul, J.M.; Kerhoas, L.; Caboche, M.; Lepiniec, L.; Debeaujon, I. TRANSPARENT TESTA10 encodes a laccase-like enzyme involved in oxidative polymerization of flavonoids in Arabidopsis seed coat. Plant Cell 2005, 17, 2966–2980.
- Kitamura, S.; Matsuda, F.; Tohge, T.; Yonekura-Sakakibara, K.; Yamazaki, M.; Saito, K.; Narumi, I. Metabolic profiling and cytological analysis of proanthocyanidins in immature seeds of Arabidopsis thaliana flavonoid accumulation mutants. Plant J. 2010, 62, 549–559.
- Pérez-Díaz, R.; Ryngajllo, M.; Pérez-Díaz, J.; Peña-Cortés, H.; Casaretto, J.A.; González-Villanueva, E.; Ruiz-Lara, S. VvMATE1 and VvMATE2 encode putative proanthocyanidin transporters expressed during berry development in Vitis vinifera L. Plant Cell Rep. 2014, 33, 1147–1159.
- Devic, M.; Guilleminot, J.; Debeaujon, I.; Bechtold, N.; Bensaude, E.; Koornneef, M.; Pelletier, G.; Delseny, M. The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development. Plant J. 1999, 19, 387–398.
- Koornneef, M. Mutations affecting the testa colour in Arabidopsis. Arab. Inf. Serv. 1990, 27, 1–4.
- Shirley, B.W.; Kubasek, W.L.; Storz, G.; Bruggemann, E.; Koornneef, M.; Ausubel, F.M.; Goodman, H.M. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995, 8, 659–671.
- Debeaujon, I.; Peeters, A.J.M.; Léon-Kloosterziel, K.M.; Koornneef, M. The transparent testa 12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 2001, 13, 853–871.
- Appelhagen, I.; Nordholt, N.; Seidel, T.; Spelt, K.; Koes, R.; Quattrochio, F.; Sagasser, M.; Weisshaar, B. TRANSPARENT TESTA 13 is a tonoplast P3A-ATPase required for vacuolar deposition of proanthocyanidins in Arabidopsis thaliana seeds. Plant J. 2015, 82, 840–849.
- Marrs, K.A.; Alfenito, M.R.; Lloyd, A.M.; Walbot, V. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene bronze-2. Nature 1995, 375, 397–400.
- Alfenito, M.R.; Souer, E.; Goodman, C.D.; Buell, R.; Mol, J.; Koes, R.; Walbot, V. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 1998, 10, 1135–1149.
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242.
- Pérez-Díaz, R.; Madrid-Espinoza, J.; Salinas-Cornejo, J.; González-Villanueva, E.; Ruiz-Lara, S. Differential roles for VviGST1, VviGST3, and VviGST4 in proanthocyanidin and anthocyanin transport in vitis vinífera. Front. Plant Sci. 2016, 7, 1166.
- Zhang, Z.; Tian, C.; Zhang, Y.; Li, C.; Li, X.; Yu, Q.; Wang, S.; Wang, X.; Chen, X.; Feng, S. Transcriptomic and metabolomic analysis provides insights into anthocyanin and procyanidin accumulation in pear. BMC Plant Biol. 2020, 20, 1–14.
- Stafford, H.A. The Enzymology of proanthocyanidin biosynthesis. In Chemistry and Significance of Condensed Tannins; Springer: Berlin/Heidelberg, Germany, 1989; pp. 47–70.
- Xie, D.Y.; Dixon, R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry 2005, 66, 2127–2144.
- Hosny, M.; Rosazza, J.P.N. Novel oxidations of (+)-catechin by horseradish peroxidase and laccase. J. Agric. Food Chem. 2002, 50, 5539–5545.
- López-Serrano, M.; Ros Barceló, A. Comparative study of the products of the peroxidase-catalyzed and the polyphenoloxidase-catalyzed (+)-catechin oxidation. Their possible implications in strawberry (Fragaria × ananassa) browning reactions. J. Agric. Food Chem. 2002, 50, 1218–1224.
- Peters, D.J.; Constabel, C.P. Molecular analysis of herbivore-induced condensed tannin synthesis: Cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J. 2002, 32, 701–712.
- Peters, D.J.; Constabel, C.P. Molecular analysis of herbivore-induced condensed tannin synthesis: Cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J. 2002, 32, 701–712.
- Smulikowska, S.; Pastuszewska, B.; Świȩch, E.; Ochtabińska, A.; Mieczkowska, A.; Nguyen, V.C.; Buraczewska, L. Tannin content affects negatively nutritive value of pea for monogastrics. J. Anim. Feed Sci. 2001, 10, 511–523.
- Ikegami, A.; Kitajima, A.; Yonemori, K. Inhibition of flavonoid biosynthetic gene expression coincides with loss of astringency in pollination-constant, non-astringent (PCNA)-type persimmon fruit. J. Hortic. Sci. Biotechnol. 2005, 80, 225–228.
- Passeri, V.; Martens, S.; Carvalho, E.; Bianchet, C.; Damiani, F.; Paolocci, F. The R2R3MYB VvMYBPA1 from grape reprograms the phenylpropanoid pathway in tobacco flowers. Planta 2017, 246, 185–199.
- Ikegami, A.; Kitajima, A.; Yonemori, K. Inhibition of flavonoid biosynthetic gene expression coincides with loss of astringency in pollination-constant, non-astringent (PCNA)-type persimmon fruit. J. Hortic. Sci. Biotechnol. 2005, 80, 225–228.
- Passeri, V.; Martens, S.; Carvalho, E.; Bianchet, C.; Damiani, F.; Paolocci, F. The R2R3MYB VvMYBPA1 from grape reprograms the phenylpropanoid pathway in tobacco flowers. Planta 2017, 246, 185–199.




