| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cosmin Iulian Codrea | + 2628 word(s) | 2628 | 2021-01-22 09:47:05 | | | |
| 2 | Karina Chen | Meta information modification | 2628 | 2021-01-26 06:39:03 | | |
Video Upload Options
Bone tissue engineering aims at delivering novel methods for treating bone tissue deficiencies of-ten resulting from polytrauma, pathological fractures, and osteonecrosis as there is an increasing need to provide functional replacement grafts for the patients.
1. Bone Tissue
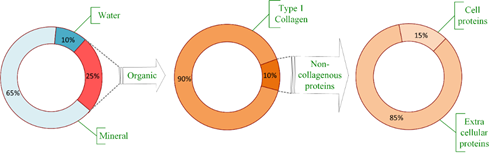
Bones are living organs constantly modeling and remodeling throughout life and serve as reservoir for calcium, phosphate and many other bodily elements, thereby, assuring their homeostasis [9]. Bone consists of an organic matrix, mineral components, and water, as presented in Figure 1. The bulk part of the organic matrix consists of type I collagen, but other non-collagenous proteins are also present, mainly extracellular and a small part within the cells [1]. Bone composition, by weight, is approximately 65% mineral (mostly as hydroxyapatite (85%), but also calcium carbonate and calcium fluoride [9]), organic part is approximately 20% to 25%, mostly type I collagen (about 90%) [1], while the water content varies between 10% and 20% depending on several factors including age, species, bone health, etc. [2].
Figure 1. Bone composition. Realized based on [3].
Bone tissue can assume a compact (cortical bone) or trabecular (cancellous bone) structure [4], but indifferently, all experience remodeling throughout life associated with bone resorption, a process performed by osteoclasts subsequent bone formation through the action of osteoblasts [5].
Cortical bone consists of a dense matrix constructed by recurrent osteon units with collagen fibers assembled in a concentric manner around a central canal containing blood vessels [6]. This structure formed by collagen fibers is oriented along with the directions of the loading lines, greatly confirmed with the piezoelectric assistance involved in bone formation [7]. It forms a dense outer shell [8] consisting of well-organized lamellae of hierarchical structures [5] and provides torsion, bending resistance, and compressive strength [8]. They possess remarkable strength but lower capacity to bear loads beyond the elastic deformation range in contrast to trabecular (cancellous) bone, consisting in unparallel fibrillar units of variable porosity (ranging between 50–90%) [5].
Cancellous (trabecular) bone is found in the porous interior [9], possesses a network of interlinked trabeculae containing marrow, organized in a hierarchical manner extended between solid material, trabeculae, lamellae, and collagen-hydroxyapatite composite and having disorganized collagen network [6]. The trabeculae exhibit considerable surface area in which nutrients diffusion and growth factors circulation is easily done, allowing, thus, cancellous bone to play a metabolically active role and permit a more persistent remodeling compared to cortical bone [9]. The mechanical capacity of cancellous bone derives to a great extent from its bone mineral density (BMD), while the stiffness of cortical bone derives to a great extent from its porosity [9].
Bone tissue is distinct due to its capacity to heal free of scar tissue, which determines an unscarred full restoration of bone tissue integrity [10]. Fractured bone repair itself by repeating various steps of endochondral and intramembranous bone formation and healing is devoid of scar tissue. Development of hematoma implies inflammation at the injured site and the action of signaling molecules involved in regulating new bone development (interleukins, tumor necrosis factor-a, fibroblast growth factors, platelet-derived growth factor, vascular endothelial growth factor (VEGF), bone morphogenetic proteins (BMPs), etc.) [8]. Inflammatory cells (macrophages, monocytes, lymphocytes, and polymorphonuclear cells) and fibroblasts infiltrate bone tissue in a process mediated by prostaglandins [2]. Throughout fracture repair, mesenchymal stem cells (MSCs) from the bone marrow are engage at the fracture site in interaction with the local cells influencing the healing efficacy [11]. The emergence of intramembranous bone starts right away at the cortical tissue and periosteum. Later, the fracture stabilization occurs through the action of the outer soft tissues through callus formation. Afterwards, chondrocyte proliferation happens. Ingrowing of blood vessels starts to bring chondroclasts to the site, which reabsorb calcified cartilage, and osteoblastic progenitors, which launch new bone tissue formation [9]. The recruitment of osteoblast on the surface and the emergence of new bone tissue are moreover promoted by the modifications in the ionic dynamics of the microenvironment [12]. Mechanical continuity of the cortical bone tissue is reached through later remodeling of recently formed bone. If the needed regeneration of bone starts due to damage or disease, hematoma building and a quick inflammatory response occurs as a way to facilitate host cell attraction and the discharge of decisive signaling molecules [8]. Hematoma building and inflammation process starts bone healing, which further elapses via the major stages of anti-inflammatory signaling, revascularization, soft callus development, its mineralization and remodeling in the end, macrophages playing an essential role in the process [10], influencing, thus, the success or failure of a bone implant [13]. Macrophages control cell migration to the injured site through the cytokines and growth factors they produce (tumor necrosis factor-a, IL-1, IL-6, IL-8, IL-12, TGF-b, platelet-derived growth factor, and insulin-like growth factor-1), and have effect on cell proliferation, collagen synthesis and angiogenesis [14]. Monocytes, neutrophils, and natural killer cells are also present in the affected site [15]. Inflammatory markers are the following cytokines: TNF-α, IL-1β and iNOS [16]. Osteogenesis, osteoclastogenesis, and angiogenesis are greatly regulated by immune cells and their signaling molecules [17].
Initial inflammatory action is necessary to start the bone healing process but persistent inflammatory action against the implant usually leads to granulation (formation of connective tissue) and formation of a fibrotic capsule around the implant with undesirable results [18] or increased healing time [19].
Bone tissue extracellular matrix is formed out of a non-mineralized organic constituent, largely type-1 collagen with numerous post-translational modifications, but also non-collagenous proteins particularly involved in improved fusion between the mineral crystals and the organic matter [20], and a mineralized inorganic constituent (made up of carbonated apatite mineral particles in the form of 4-nm-thick plates) [21]. The nano-composite structure (collagen fibers reinforced by hydroxyapatite) is necessary for compressive strength and toughness towards fracture [21], thus, bearing an impact without cracking [20]. The toughness and flexibility of the collagen fibers adjust bone ductility.
The metaphyseal component of bone is largely made up of cancellous tissue, which is metabolically more active compared to cortical tissue. As a consequence of this attribute, osteoporosis can impact its mass and structural integrity at a higher rate [22], mainly because diffusion is much higher as surface to volume ratio is superior in contrast to the cortical one.
Healing rates are age dependent, as in young people fractures usually heal about the weight-bearing level in approximately six weeks, and about complete mechanical integrity level after approximately one year, and in the elderly individuals the healing rates can be much lower [23]. In pathological or large fractures and defects, the processes of healing and repair can be unsuccessful, due to deficient blood delivery, bone cells or bone forming minerals, but also due to infection in the bone or its adjacent tissues, and systemic affections causing delay or even lack of union [24]. Important factors that affect the process of bone tissue healing are also mechanical stability, proportions of the affected site, severity and incidence of adjacent tissue injuries [25].
After implantation of bone scaffolds in the body, injured blood vessels quickly lead to protein adsorption on scaffold surface and formation of fibrin-rich clot with the role of a transitory surface matrix at the tissue-scaffold interface occurs. It emits various pro-inflammatory cytokines and chemokines, triggering the mobilization of inflammatory and osteoprogenitor cells towards the infixed scaffold. The scaffold is recognized as a foreign body by the immune system, inducing specific immune reactions. The scaffold active regulates the kind and extent of immune system reactions during bone regeneration [13]. To counteract chronic inflammatory action against implants, anti-inflammatory factors such as corticosteroids and prostaglandins were investigated but adverse effects such as hepatotoxicity, cardiotoxicity, and immunological impairment in long-term use were reported. Extended inflammation may cause fibrosis, granuloma formation and further encapsulation and failure of the implant [18].
2. Bone Healing and Types of Bone Grafts
Bone grafts are made of biomaterial implanted in order to assist healing alone or with the aid of other materials, because of the expected properties of osteogenesis, osteoinduction, and osteoconduction either alone or combined [23]. Bone grafts are especially needed if a larger bone lost is recorded. It is worth to mention that in fact, only the need of blood is higher than the need of bone grafts. It is also important to mention that synthetic bone grafts have some advantages against autografts, and this is why many synthetic grafts are studied worldwide [26].
Autografts are obtained by taking bone from another part of the patient’s own body [2] and still serves as a point of reference to which other grafts may be compared [27] because of their histocompatibility, absence of immunogenic reactions and all other properties demanded of a bone graft material, respectively osteoinduction (growth factors to drive the regeneration process, i.e., bone morphogenetic proteins), osteogenesis (i.e., osteoprogenitor cells, functionally active osteoblastic cells to produce new bone matrix) and osteoconduction (i.e., three-dimensional, porous matrix) [21]. Autografts integrate into the host bone faster [28] and are the most effective method for bone regeneration [2], unfortunately donor site morbidity is a problematic consideration [29]. Important health risks such as major vessel or visceral injuries during harvesting are also among the limitations in using autografts [24].
Allografts consist of taking bone from the donor and has a lower rate of incorporation within the bone [2]. They are linked to risks of immune reactions and infection carrying, have low osteoinductive capacity and have no bone cells, since donor grafts are devitalized and processed through irradiation or freeze-drying [30]. The handicap of restricted donor supply and the need for immunosuppressive therapy [31] and batch to batch variation [32] add to the ones previously mentioned.
Xenografts are derived from species other than the recipient, are osteoconductive, relatively inexpensive, do not lengthen the healing time, and the need for a second surgical site for bone harvesting is eliminated [33]. They carry a rare risk of transmission of zoonotic diseases [34], causing an immune reaction, and are unable to gain adequate height and width for large defects [33]. Remains of bovine bone from xenografts can still be unresorbed even after three years as some histological analyses have proved. They are osteoconductive rather than osteoinductive [35] and are considered to be more liable to rejection, even in an aggressive manner [34].
Biomaterial scaffolds (engineered scaffolds) address the limits of autografts, allografts and xenografts. Novel approaches offered by tissue engineering have been conducted with the aim to create grafts for repairing and regenerating damaged tissues [36] through the use of a provisional biomaterial scaffold at the injured site in order to stimulate healing and ensure certain recovery of functionality [37] and harness bone innate regenerative capacity, as bone has a natural potential to repair, remodel, and regenerate itself [23].
Scaffolds need to be engineered with features that provide cells with signals for regeneration in order to cancel the need for extended in vitro culture anterior to implantation. Hence, considerable efforts are committed to advance biomimetic biomaterials to a more complex stage, making them able to incorporate multi-functionality and possess bioinstructive and stimuli-responsive properties [23].
Scaffolds should possess cytocompatibility and imitate biochemical and mechanical properties of the veritable bone, furthering similar biological functions in order to prevail limitations such as donor deficit, immunogenic reaction, and infection carrying, with the main strategies considering the admixture of cells, biologically active molecules, and impermanent 3D fine-tuned porous design. Degradation capability is necessary to integrate the scaffold and should progress at a rate capable of maintaining mechanical support in implantation sites. Apart of that, it plays a critical role in not only in ensuring the means for metabolite diffusion and the development of new blood vessels, but also in releasing the agents loaded into the biomaterial . Scaffolds enable osteogenic cells adhesion of and provide a suitable microenvironment for them in order to secrete osteoid, the matrix of recently formed tissue and deliver signaling molecules to the bone regeneration space (osteoconduction).
3. Biomaterial Scaffolds
Scaffold development usually starts by choosing the scaffold material [37] as chemical composition of the biomaterial plays an essential part in the favorable outcome of the process [8]. We distinguish three classes of scaffolds in line with their material composition: metals, polymers, and bio-ceramics, but they may consist also of a combination of these three material types as composites [8][27]. Common materials used and approved, such as stainless steel, poly(lactic-co-glycolic acid), and hydroxyapatite can be appealing as they are already safely used as components in existing products [37]. However, they still have their disadvantages such as the necessity for several surgical intervention, stress shielding, wear particle osteolysis [32] and no potential for drug delivery in the case of biocompatible metals and their alloys [12] and inferior mechanical properties that limit its use in load bearing bone parts in the case of bioactive glasses [32]. A further classification is made by considering the origin of the material (natural or synthetic) and its degradation potential under physiologic conditions (resorbable versus non-resorbable) [38].
Other characteristics to take into account is the heterogeneousness of the scaffold, that bone is biologically and biomechanically variable, the ease to handle it as a one-step procedure, its ability to reach suitable mechanical properties in order to bear the load of normal movements, and its ability to allow vascularization in order to obtain a normal bone structure [25], which can thus prevent the scaffold to undergo ischemia and cell death [34].
Scaffold material aims to support the viability of the adequate cell category and simultaneously operate as a nonpermanent replacement of the bone extracellular matrix, as a substitute matrix that will preferably be displaced by newly functional bone tissue [39], while reproducing the properties of normal bone tissue formation [40]. Scaffolds designed to aid regeneration need to encourage migration, cell adhesion, proliferation, and differentiation [29] and ideally some essential features and functions should be certainly considered: architecture, cytocompatibility, bioactivity, biodegradability, and mechanical characteristics [8].
Material characteristics, such as biomaterial porosity and its surface properties, for instance nano/micro topography, play a decisive role in osteoinduction [8] and the expected histogenesis [39]. The architectural features consider a controlled porous microstructure and high porous interconnectivity, a controlled degradation rate, mechanical stability, and osteoconductive properties [29]. In addition, for an effective interaction between scaffolds and the cellular component, other design parameters are necessarily acknowledged, such as surface properties, permeability, geometric properties, and mechanical strength, all of which influence nutrients and oxygen transport throughout the scaffolds [41] and influence cell-material interactions [42] controlling, thus, bone regeneration.
Host reactions to the implanted scaffold are greatly influenced by the physical (Figure 2) and chemical properties of the scaffold and design strategies should consider and evaluate this impact [13].
The ability of cells to penetrate, proliferate and differentiate are greatly influenced by pore size, distribution and scaffold geometry, and also the rate of scaffold degradation [43].
Porosity: interconnection between pores promotes the loading of cells into biomaterials due to large internal surface area, which ensures attachment and spreading sites. Because the biomaterial is designed as an open network structure, diffusion of oxygen, metabolites, and growth factors is possible, thus, enabling cells viability and proliferation, and the space needed for the deposition of proteins secreted by the cells. Porosity of the bone tissue facilitate the penetration of host cells and the growth of blood vessels that provide the means to feed the emerging tissue [39]. This microstructure permits the ingrowth of cells, which lead to tissue regeneration. Uniform cell seeding and nutrient exchange are dependent on the interconnectivity of pores [29]. By raising the available surface inside a pore to which cells can adhere on the implant, known as the specific surface area (SSA), a quicker growth of hydroxyapatite appears and thus bone bonding will be more quickly as well [44].
Scaffold architecture needs to consider, in order to assure well-adjusted biological and physical properties of scaffolds, the following key parameters: total porosity, pore morphology, distribution, and size [45]. Given the size of the bone cells of about 20 to 35 µm, materials with a porosity of about 50 to 150 µm seem to be the optimal for bone grafting because, in this case, the bone cells can easily penetrate inside, and can assure an in depth osteointegration of these synthetic grafts. If the pore size increases, it means that the mechanical properties decrease and also the time requested for the feeling of these pores with new bone increase, which overall means a slower healing and a higher risk of secondary failure [46][47][48].
References
- Burr, D.B.; Akkus, O. Chapter 1—Bone morphology and organization. In Basic and Applied Bone Biology; Burr, D.B., Allen, M.R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 3–25.
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 27.
- Burr, D.B.; Akkus, O. Chapter 1—Bone morphology and organization. In Basic and Applied Bone Biology; Burr, D.B., Allen, M.R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 3–25.
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408.
- Xie, Y.; Zhang, L.; Xiong, Q.; Gao, Y.; Ge, W.; Tang, P. Bench-to-bedside strategies for osteoporotic fracture: From osteoimmunology to mechanosensation. Bone Res. 2019, 7, 25.
- Gonzalez-Fernandez, T.; Sikorski, P.; Leach, J.K. Bio-instructive materials for musculoskeletal regeneration. Acta Biomater. 2019, 96, 20–34.
- Silva, C.C.; Thomazini, D.; Pinheiro, A.G.; Aranha, N.; Figueiró, S.D.; Góes, J.C.; Sombra, A.S.B. Collagen–hydroxyapatite films: Piezoelectric properties. Mater. Sci. Eng. B 2001, 86, 210–218.
- Tripathy, N.; Perumal, E.; Ahmad, R.; Song, J.E.; Khang, G. Chapter 40—Hybrid composite biomaterials. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 695–714.
- Xie, Y.; Zhang, L.; Xiong, Q.; Gao, Y.; Ge, W.; Tang, P. Bench-to-bedside strategies for osteoporotic fracture: From osteoimmunology to mechanosensation. Bone Res. 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]Adler, R.A. Osteoporosis in men: A review. Bone Res. 2014, 2, 14001.
- Schlundt, C.; El Khassawna, T.; Serra, A.; Dienelt, A.; Wendler, S.; Schell, H.; van Rooijen, N.; Radbruch, A.; Lucius, R.; Hartmann, S.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89.
- Chandran, S.; John, A. Osseointegration of osteoporotic bone implants: Role of stem cells, Silica and Strontium—A concise review. J. Clin. Orthop. Trauma 2019, 10, S32–S36.
- Gil Mur, F.J. 8—Accelerating mineralization of biomimetic surfaces. In Biomineralization and Biomaterials; Aparicio, C., Ginebra, M.-P., Eds.; Woodhead Publishing: Boston, MA, USA, 2016; pp. 267–289.
- He, J.; Chen, G.; Liu, M.; Xu, Z.; Chen, H.; Yang, L.; Lv, Y. Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater. Sci. Eng. C 2020, 108, 110411.
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The Early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part B Rev. 2010, 16, 427–434.
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275.
- Li, L.; Yu, M.; Li, Y.; Li, Q.; Yang, H.; Zheng, M.; Han, Y.; Lu, D.; Lu, S.; Gui, L. Synergistic anti-inflammatory and osteogenic n-HA/resveratrol/chitosan composite microspheres for osteoporotic bone regeneration. Bioact. Mater. 2021, 6, 1255–1266.
- Chang, J.; Zhang, X.; Dai, K. Chapter 2—Biomaterial-induced microenvironment and host reaction in bone regeneration. In Bioactive Materials for Bone Regeneration; Chang, J., Zhang, X., Dai, K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 105–181.
- Li, L.; Yu, M.; Li, Y.; Li, Q.; Yang, H.; Zheng, M.; Han, Y.; Lu, D.; Lu, S.; Gui, L. Synergistic anti-inflammatory and osteogenic n-HA/resveratrol/chitosan composite microspheres for osteoporotic bone regeneration. Bioact. Mater. 2021, 6, 1255–1266.
- Kyllönen, L.; D′Este, M.; Alini, M.; Eglin, D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater. 2015, 11, 412–434.
- Xie, Y.; Zhang, L.; Xiong, Q.; Gao, Y.; Ge, W.; Tang, P. Bench-to-bedside strategies for osteoporotic fracture: From osteoimmunology to mechanosensation. Bone Res. 2019, 7, 25.
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408.
- Komrakova, M.; Weidemann, A.; Dullin, C.; Ebert, J.; Tezval, M.; Stuermer, K.M.; Sehmisch, S. The impact of strontium ranelate on metaphyseal bone healing in ovariectomized rats. Calcif. Tissue Int. 2015, 97, 391–401.
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95.
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18.
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 27.
- de Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9.
- Shuai, C.; Yu, L.; Feng, P.; Gao, C.; Peng, S. Interfacial reinforcement in bioceramic/biopolymer composite bone scaffold: The role of coupling agent. Colloids Surf. B Biointerfaces 2020, 193, 111083.
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247.
- Rambhia, K.J.; Ma, P.X. Chapter 48—Biomineralization and bone regeneration. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 853–866.
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408
- Zhu, Y.; Wagner, W.R. Chapter 30—Design principles in biomaterials and scaffolds. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 505–522.
- Prabha, R.D.; Ding, M.; Bollen, P.; Ditzel, N.; Varma, H.K.; Nair, P.D.; Kassem, M. Strontium ion reinforced bioceramic scaffold for load bearing bone regeneration. Mater. Sci. Eng. C 2020, 109, 110427.
- Herford, A.S.; Stoffella, E.; Stanford, C.M. Chapter 5—Bone grafts and bone substitute materials. In Principles and Practice of Single Implant and Restorations; Torabinejad, M., Sabeti, M.A., Goodacre, C.J., Eds.; W.B. Saunders: Saint Louis, MI, USA, 2014; pp. 75–86.
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18.
- Arpağ, O.F.; Damlar, I.; Altan, A.; Tatli, U.; Günay, A. To what extent does hyaluronic acid affect healing of xenografts? A histomorphometric study in a rabbit model. J. Appl. Oral Sci. 2018, 26, e20170004.
- Maia, F.R.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L. Chapter 32—Natural origin materials for bone tissue engineering: Properties, processing, and performance. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 535–558.
- Zhu, Y.; Wagner, W.R. Chapter 30—Design principles in biomaterials and scaffolds. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 505–522.
- Francois, E.L.; Yaszemski, M.J. Chapter 43—Preclinical bone repair models in regenerative medicine. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 761–767
- McHale, M.K.; Bergmann, N.M.; West, J.L. Chapter 38—Histogenesis in three-dimensional scaffolds. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 661–674.
- Tripathy, N.; Perumal, E.; Ahmad, R.; Song, J.E.; Khang, G. Chapter 40—Hybrid composite biomaterials. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 695–714.
- Bambole, V.; Yakhmi, J.V. Chapter 14—Tissue engineering: Use of electrospinning technique for recreating physiological functions. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 387–455.
- Liu, W.; Cheng, M.; Wahafu, T.; Zhao, Y.; Qin, H.; Wang, J.; Zhang, X.; Wang, L. The in vitro and in vivo performance of a strontium-containing coating on the low-modulus Ti35Nb2Ta3Zr alloy formed by micro-arc oxidation. J. Mater. Sci. Mater. Med. 2015, 26, 203.
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9.
- Kaur, P.; Singh, K.J.; Kaur, S.; Kaur, S.; Singh, A.P. Sol-gel derived strontium-doped SiO2–CaO–MgO–P2O5 bioceramics for faster growth of bone like hydroxyapatite and their in vitro study for orthopedic applications. Mater. Chem. Phys. 2020, 245, 122763.
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9.
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818.
- Develioğlu, H.; Koptagel, E.; Gedik, R.; Dupoirieux, L. The effect of a biphasic ceramic on calvarial bone regeneration in rats. J. Oral Implantol. 2005, 31, 309–312.
- Ficai, A.; Andronescu, E.; Voicu, G.; Ficai, D. Advances in Collagen/Hydroxyapatite Composite Materials; InTech: Rijeka, Croatia, 2011.