| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kentaro Matsuzaki | + 1082 word(s) | 1082 | 2021-01-13 06:46:20 | | | |
| 2 | Vicky Zhou | Meta information modification | 1082 | 2021-01-22 06:17:27 | | |
Video Upload Options
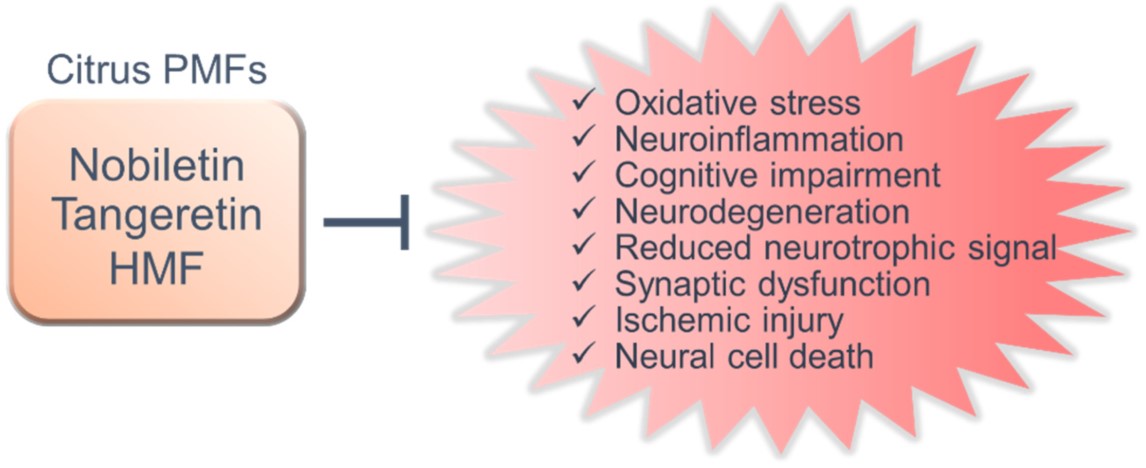
Several reports have shown that polymethoxylated flavones (PMFs) derived from citrus fruit, such as nobiletin, tangeretin, and 3,3′,4′,5,6,7,8-heptamethoxyflavone, are promising molecules for the prevention of neurodegenerative and neurological disorders. In various animal models, PMFs have been shown to have a neuroprotective effect and improve cognitive dysfunction with regard to neurological disorders by exerting favorable effects against their pathological features, including oxidative stress, neuroinflammation, neurodegeneration, and synaptic dysfunction as well as its related mechanisms.
1. Introduction
The number of patients with neurodegenerative diseases and neurological disorders associated with dementia, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and cerebrovascular dementia, is increasing [1][2]. Currently, approximately 50 million people worldwide have dementia, and that number is expected to triple by 2050 [1][3]. Because AD is the most common form of neurodegenerative disease [1][4][5], the development of new treatments for AD is anticipated. The earliest pathological symptom of AD involves the accumulation of β-amyloid (Aβ) plaques in the brain [5][6]. Briefly, Aβ plaques reportedly start to form more than 20 years before the onset of AD symptoms [6]. About 10 years after the start of Aβ accumulation, tau hyperphosphorylation and aggregation lead to the formation of neurofibrillary tangles in the brain [6]. A few years later, mild cognitive impairment develops, and the onset of AD occurs several years after that [6]. Thus, the mechanism of AD onset is supported by the amyloid cascade hypothesis, which states that abnormal accumulation of Aβ is responsible for cognitive decline [6]. However, abnormal accumulation of Aβ/tau in the brain occurs long before AD onset, and thus far, no clinical trials have successfully treated patients after AD onset. Researchers have recently shown that neuroinflammation and oxidative stress in the brain play major roles in the development of AD [7][8]. Interestingly, Venegas et al. (2017) reported that the overactivation of microglia in the brain elicits an inflammatory response that triggers Aβ accumulation [9]. It has also been reported that age-induced oxidative stress promotes Aβ accumulation in the brain, which suggests that AD onset might be caused by neuroinflammation or oxidative stress that precedes plaque formation in the brain [10]. Furthermore, studies have reported that lifestyle-related diseases, such as diabetes, dyslipidemia, and obesity, increase the risk of developing AD [11][12][13]. Therefore, preventing neuroinflammation, oxidative stress, lifestyle-related diseases, and the accumulation of Aβ/tau in the brain is a promising strategy for the prevention and treatment of AD. Neurodegenerative diseases and neurologic disorders have some pathological similarities at the intracellular and molecular levels, including oxidative stress, inflammation, and cognitive decline [7][8][14]. It is widely accepted that oxidative stress and neuroinflammation contribute to the progression of not only AD but also other neurodegenerative and/or neurological disorders, such as PD, cerebrovascular dementia, epilepsy, and depression [2][14]. However, therapeutic agents for these diseases have not yet been elucidated. Consequently, the development of functional foods to prevent neurodegenerative diseases and neurological disorders is highly expected.
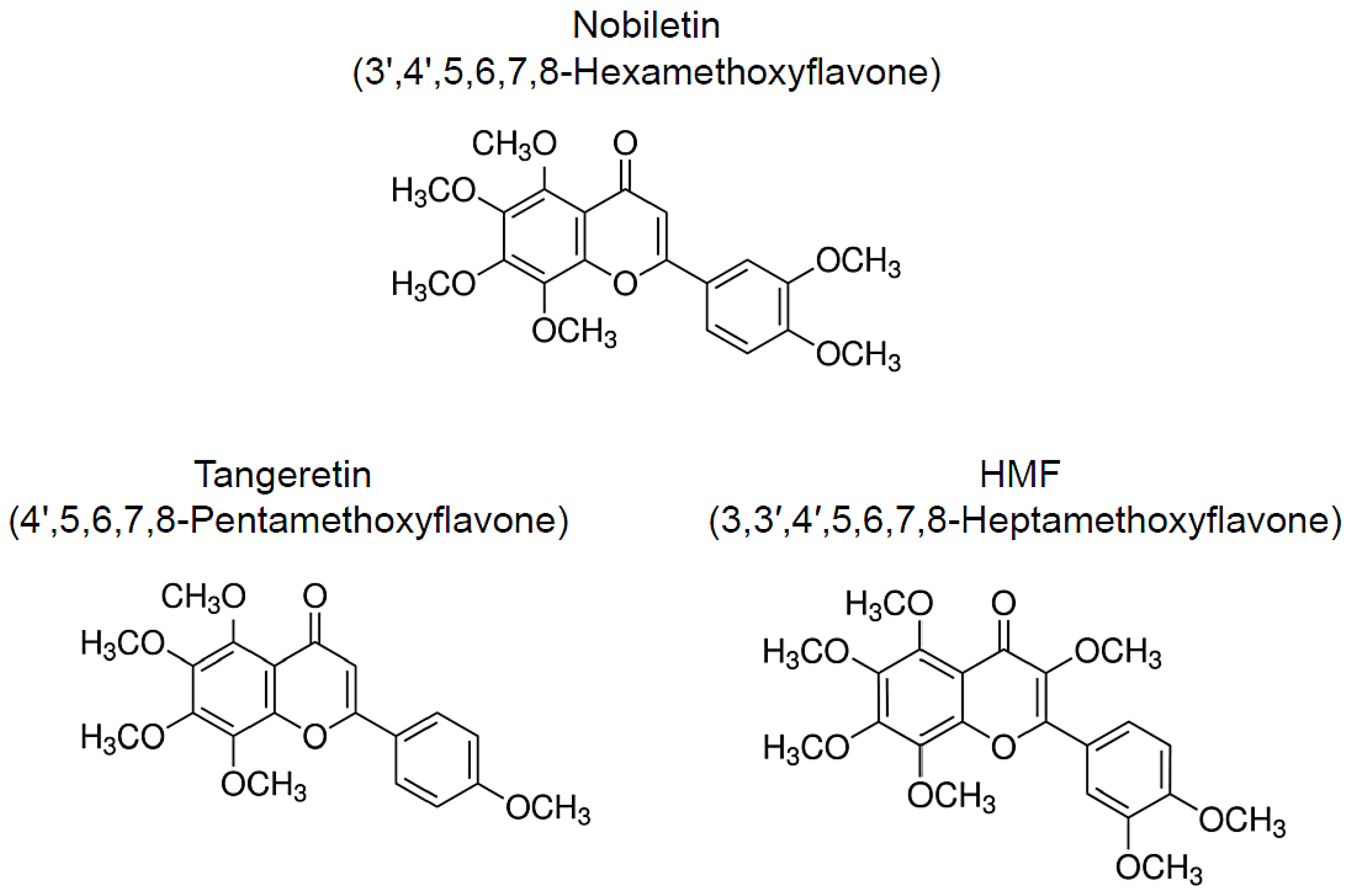
Numerous natural resources contain bioactive substances, which allows them to function as treatments and means of preventing geriatric and neurodegenerative diseases [14][15][16][17][18][19]. Citrus peels are a rich source of polymethoxylated flavones (PMFs) and have been widely used as a crude drug in traditional herbal medicines. The following compounds are major PMFs in citrus fruits (Figure 1): nobiletin (3′,4′,5,6,7,8-hexamethoxyflavone, C21H22O8), tangeretin (4′,5,6,7,8-pentamethoxyflavone, C20H20O7), and 3,3′,4′,5,6,7,8-heptamethoxyflavone (C22H24O9, HMF) [20]. Because of their anti-inflammatory and antioxidant effects, these citrus-derived PMFs have the potential to prevent neurodegenerative and neurological disorders [21][22][23][24][25][26]. In addition, these PMFs have shown beneficial effects against hyperlipidemia [27][28], obesity [29][30], diabetes [31][32], cardiovascular dysfunction [33][34], and cancer [35][36][37]. In this study, we focus on the neuroprotective and ameliorative effects of citrus-derived PMFs, nobiletin, tangeretin, and HMF, against central nervous system dysfunction in several rat and murine models of central nervous system disorders and related diseases as well as their mechanisms of action.
Figure 1. Chemical structure of citrus-derived polymethoxylated flavones, nobiletin, tangeretin, and 3,3′,4′,5,6,7,8-heptamethoxyflavone (HMF).
2. Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders
PMFs significantly prevent and/or improve cognitive dysfunction and motor dysfunction in animal models. These action mechanisms involve diverse functions, such as antioxidant effects, anti-inflammatory effects, inhibition of Aβ pathology, suppression of neurodegeneration and neuronal cell death, regulation of neurotrophic signals and synaptic plasticity (Figure 2). Furthermore, citrus PMFs exert antidementia effects after oral, subcutaneous and intraperitoneal administration in animal models of neurodegenerative diseases and neuronal disorders. Nobiletin, tangeretin, HMF, and its bioactive metabolites can also cross the blood–brain barrier [150][38][39]. PMFs are generally quite safe, which is a major advantage. It has been shown that chronic administration of the extract of Citrus reticulata Blanco, Citrus reticulata or Citrus sinensis, which contain high concentrations of PMFs, have no harmful effects on animals [40][41], and humans [42][43].
Citrus peels and/or extract have been reported to have various beneficial effects on humans, e.g., body weight control [43], promote cardiovascular health [44], improve hepatic steatosis [45] and cancer prevention [46][47]. Although consisting of only a few cases, one clinical study has demonstrated that PMF-rich citrus peel extract prevents the progression of cognitive dysfunction in AD patients on donepezil therapy [48]. Based on this evidence, it is important to develop functional foods or drugs that could prevent or ameliorate central nervous system disorders.
The three types of PMFs exerted neuroprotective and neurotrophic effects in various experimental models. These natural compounds share common mechanisms such as antioxidant and anti-inflammatory effects. The antioxidant and anti-inflammatory effects of PMFs may be an important mechanism of neuroprotective effect in various neurological models. On the other hand, PMFs seem to activate neural function through activation of several intracellular signal cascade and gene expression. Therefore, structure-activity relationship studies may be needed in order to understand the precise mechanism of action of the neuroprotective effects of PMFs.
We further mention herein the facts that there is one exciting discovery of PMFs’ function which deserve additional discussion. Circadian rhythms are biological activity rhythm driven by internal circadian clocks and are a fundamental mechanism to regulate various pathways and pathophysiology [49]. Circadian disruption induces the development of numerous diseases, including obesity, metabolic syndrome, neuroinflammation and cognitive impairment [49][50][51]. In addition, disruption of circadian rhythms is a common occurrence in elderly individuals, and is more severe in patients with neurodegenerative diseases, such as AD and PD [50][51]. Interestingly, nobiletin, and to a certain degree also tangeretin, has been reported to activate circadian rhythms, and confer protection against metabolic disease, aging and delirium [52][53][54][55][56][57]. The regulation of circadian rhythms by PMFs may partly be involved in the improvement of neuronal function in several neurological disease model animals.
References
- Australia, D.; Baker, S.; Banerjee, S. Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to dementia. In Alzheimer’s Disease International; Alzheimer’s Disease International: London, UK, 2019.
- Robinson, L.; Tang, E.; Taylor, J.-P. Dementia: Timely diagnosis and early intervention. BMJ 2015, 350, h3029.
- World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Switzerland, Geneva, 2019.
- Selkoe, D.J.; Lansbury, P.J. Alzheimer’s disease is the most common neurodegenerative disorder. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Siegel, J.G., Agranoff, B.W., Albers., R.W., Fisher, S.K., Uhler, M.D., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999.
- Rabbito, A.; Dulewicz, M.; Kulczyńska-Przybik, A.; Mroczko, B. Biochemical Markers in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1989.
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216.
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405.
- Mhatre, M.; Floyd, R.A.; Hensley, K. Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: Common links and potential therapeutic targets. J. Alzheimers Dis. 2004, 6, 147–157.
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 2017, 552, 355–361.
- Ohyagi, Y.; Asahara, H.; Chui, D.H.; Tsuruta, Y.; Sakae, N.; Miyoshi, K.; Yamada, T.; Kikuchi, H.; Taniwaki, T.; Murai, H.; et al. Intracellular Abeta42 activates p53 promoter: A pathway to neurodegeneration in Alzheimer’s disease. FASEB J. 2005, 19, 255–257.
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74.
- Picone, P.; Di Carlo, M.; Nuzzo, D. Obesity and Alzheimer’s disease: Molecular bases. Eur. J. Neurosci. 2020, 52, 3944–3950.
- Vinciguerra, F.; Graziano, M.; Hagnäs, M.; Frittita, L.; Tumminia, A. Influence of the Mediterranean and Ketogenic Diets on Cognitive Status and Decline: A Narrative Review. Nutrients 2020, 12, 1019.
- Ohizumi, Y. A New Strategy for Preventive and Functional Therapeutic Methods for Dementia—Approach Using Natural Products. Yakugaku Zasshi 2015, 135, 449–464.
- Matsuzaki, K.; Yano, S.; Sumiyoshi, E.; Shido, O.; Katsube, T.; Tabata, M.; Okuda, M.; Sugimoto, H.; Yoshino, K.; Hashimoto, M. Long-Term Ultra-High Hydrostatic Pressurized Brown Rice Intake Prevents Bone Mineral Density Decline in Elderly Japanese Individuals. J. Nutr. Sci. Vitaminol. 2019, 65, S88–S92.
- Islam, R.; Matsuzaki, K.; Sumiyoshi, E.; Hossain, M.E.; Hashimoto, M.; Katakura, M.; Sugimoto, N.; Shido, O. Theobromine Improves Working Memory by Activating the CaMKII/CREB/BDNF Pathway in Rats. Nutrients 2019, 11, 888.
- Sumiyoshi, E.; Matsuzaki, K.; Sugimoto, N.; Tanabe, Y.; Hara, T.; Katakura, M.; Miyamoto, M.; Mishima, S.; Shido, O. Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial. Nutrients 2019, 11, 2800.
- Hashimoto, M.; Hossain, S.; Matsuzaki, K.; Shido, O.; Yoshino, K. The journey from white rice to ultra-high hydrostatic pressurized brown rice: An excellent endeavor for ideal nutrition from staple food. Crit. Rev. Food Sci. Nutr. 2020, 16, 1–19.
- Hashimoto, M.; Tanabe, Y.; Hossain, S.; Matsuzaki, K.; Ohno, M.; Kato, S.; Katakura, M.; Shido, O. Intake of Alpha-Linolenic Acid-Rich Perilla frutescens Leaf Powder Decreases Home Blood Pressure and Serum Oxidized Low-Density Lipoprotein in Japanese Adults. Molecules 2020, 25, 2099.
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192.
- Choi, S.Y.; Hwang, J.H.; Ko, H.C.; Park, J.G.; Kim, S.J. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-kappaB and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155.
- Hirata, Y.; Masuda, Y.; Kakutani, H.; Higuchi, T.; Takada, K.; Ito, A.; Nakagawa, Y.; Ishii, H. Sp1 is an essential transcription factor for LPS-induced tissue factor expression in THP-1 monocytic cells, and nobiletin represses the expression through inhibition of NF-κB, AP-1, and Sp1 activation. Biochem. Pharmacol. 2008, 75, 1504–1514.
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.-T.; Li, H.-B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.-Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907.
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M.; et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066.
- Wang, Z.; Yang, B.; Chen, X.; Zhou, Q.; Li, H.; Chen, S.; Yin, D.; He, H.; He, M. Nobiletin Regulates ROS/ADMA/DDAHII/eNOS/NO Pathway and Alleviates Vascular Endothelium Injury by Iron Overload. Biol. Trace Elem. Res. 2020, 198, 87–97.
- He, W.; Li, Y.; Liu, M.; Yu, H.; Chen, Q.; Chen, Y.; Ruan, J.; Ding, Z.; Zhang, Y.; Wang, T. Citrus aurantium L. and Its Flavonoids Regulate TNBS-Induced Inflammatory Bowel Disease through Anti-Inflammation and Suppressing Isolated Jejunum Contraction. Int. J. Mol. Sci. 2018, 19, 3057.
- Lee, A.Y.; Park, W.; Kang, T.W.; Cha, M.H.; Chun, J.M. Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. J. Ethnopharmacol. 2018, 221, 151–159.
- Nichols, L.A.; Jackson, D.E.; Manthey, J.A.; Shukla, S.D.; Holland, L.J. Citrus flavonoids repress the mRNA for stearoyl-CoA desaturase, a key enzyme in lipid synthesis and obesity control, in rat primary hepatocytes. Lipids Health Dis. 2011, 10, 36.
- Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Morrow, M.R.; Sawyez, C.G.; Edwards, J.Y.; Drangova, M.; Huff, M.W. Intervention with citrus flavonoids reverses obesity and improves metabolic syndrome and atherosclerosis in obese Ldlr-/- mice. J. Lipid Res. 2018, 59, 1714–1728.
- Chou, Y.C.; Ho, C.T.; Pan, M.H. Immature Citrus reticulata Extract Promotes Browning of Beige Adipocytes in High-Fat Diet-Induced C57BL/6 Mice. J. Agric. Food Chem. 2018, 66, 9697–9703.
- Nguyen-Ngo, C.; Salomon, C.; Quak, S.; Lai, A.; Willcox, J.C.; Lappas, M. Nobiletin exerts anti-diabetic and anti-inflammatory effects in an in vitro human model and in vivo murine model of gestational diabetes. Clin. Sci. 2020, 134, 571–592.
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708.
- Parkar, N.A.; Bhattm, L.K.; Addepalli, V. Efficacy of nobiletin, a citrus flavonoid, in the treatment of the cardiovascular dysfunction of diabetes in rats. Food Funct. 2016, 7, 3121–3129.
- Sunagawa, Y.; Funamoto, M.; Suzuki, A.; Shimizu, K.; Sakurai, R.; Katanasaka, Y.; Miyazaki, Y.; Asakawa, T.; Kan, T.; Inagaki, J.; et al. A Novel Target Molecule of Nobiletin Derived from Citrus Peels has a Therapeutic Potency Against the Development of Heart Failure. Eur. Cardiol. 2017, 12, 105.
- Zhang, M.; Zhang, R.; Liu, J.; Wang, H.; Wang, Z.; Liu, J.; Shan, Y.; Yu, H. The Effects of 5,6,7,8,3′,4′-Hexamethoxyflavone on Apoptosis of Cultured Human Choriocarcinoma Trophoblast Cells. Molecules 2020, 25, 946.
- Goan, Y.G.; Wu, W.T.; Liu, C.I.; Neoh, C.A.; Wu, Y.J. Involvement of Mitochondrial Dysfunction, Endoplasmic Reticulum Stress, and the PI3K/AKT/mTOR Pathway in Nobiletin-Induced Apoptosis of Human Bladder Cancer Cells. Molecules 2019, 24, 2881.
- Sp, N.; Kang, D.Y.; Joung, Y.H.; Park, J.H.; Kim, W.S.; Lee, H.K.; Song, K.D.; Park, Y.M.; Yang, Y.M. Nobiletin Inhibits Angiogenesis by Regulating Src/FAK/STAT3-Mediated Signaling through PXN in ER+; Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 935.
- Saigusa, D.; Shibuya, M.; Jinno, D.; Yamakoshi, H.; Iwabuchi, Y.; Yokosuka, A.; Mimaki, Y.; Naganuma, A.; Ohizumi, Y.; Tomioka, Y.; et al. High-performance liquid chromatography with photodiode array detection for determination of nobiletin content in the brain and serum of mice administrated the natural compound. Anal. Bioanal. Chem. 2011, 400, 3635–3641.
- Takiyama, M.; Matsumoto, T.; Watanabe, J. LC-MS/MS detection of citrus unshiu peel-derived flavonoids in the plasma and brain after oral administration of yokukansankachimpihange in rats. Xenobiotica 2019, 49, 1494–1503.
- Nakajima, A.; Nemoto, K.; Ohizumi, Y. An evaluation of the genotoxicity and subchronic toxicity of the peel extract of Ponkan cultivar ‘Ohta ponkan’ (Citrus reticulata Blanco) that is rich in nobiletin and tangeretin with anti-dementia activity. Regul. Toxicol. Pharmacol. 2020, 114, 104670.
- Vanhoecke, B.W.; Delporte, F.; Van Braeckel, E.; Heyerick, A.; Depypere, H.T.; Nuytinck, M.; De Keukeleire, D.; Bracke, M.E. A safety study of oral tangeretin and xanthohumol administration to laboratory mice. In Vivo 2005, 19, 103–107.
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98.
- Wang, X.; Li, D.; Liu, F.; Cui, Y.; Li, X. Dietary citrus and/or its extracts intake contributed to weight control: Evidence from a systematic review and meta-analysis of 13 randomized clinical trials. Phytother. Res. 2020, 34, 2006–2022.
- Oben, J.; Enonchong, E.; Kothari, S.; Chambliss, W.; Garrison, R.; Dolnick, D. Phellodendron and Citrus extracts benefit cardiovascular health in osteoarthritis patients: A double-blind, placebo-controlled pilot study. Nutr. J. 2008, 7, 16.
- Ferro, Y.; Montalcini, T.; Mazza, E.; Foti, D.; Angotti, E.; Gliozzi, M.; Nucera, S.; Paone, S.; Bombardelli, E.; Aversa, I.; et al. Randomized Clinical Trial: Bergamot Citrus and Wild Cardoon Reduce Liver Steatosis and Body Weight in Non-diabetic Individuals Aged Over 50 Years. Front. Endocrinol. 2020, 11, 494.
- Zhang, L.; Xu, X.; Jiang, T.; Wu, K.; Ding, C.; Liu, Z.; Zhang, X.; Yu, T.; Song, C. Citrus aurantium Naringenin Prevents Osteosarcoma Progression and Recurrence in the Patients Who Underwent Osteosarcoma Surgery by Improving Antioxidant Capability. Oxidative Med. Cell. Longev. 2018, 2018, 1–16.
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025.
- Seki, T.; Kamiya, T.; Furukawa, K.; Azumi, M.; Ishizuka, S.; Takayama, S.; Nagase, S.; Arai, H.; Yamakuni, T.; Yaegashi, N. Nobiletin-rich Citrus reticulata peels, a kampo medicine for Alzheimer’s disease: A case series. Geriatr. Gerontol. Int. 2013, 13, 236–238.
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84.
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318.
- Kress, G.J.; Liao, F.; Dimitry, J.M.; Cedeno, M.R.; Fitzgerald, G.A.; Holtzman, D.M.; Musiek, E.S. Regulation of amyloid-β dynamics and pathology by the circadian clock. J. Exp. Med. 2018, 215, 1059–1068.
- Nohara, K.; Nemkov, T.; D’Alessandro, A.; Yoo, S.-H.; Chen, Z. Coordinate Regulation of Cholesterol and Bile Acid Metabolism by the Clock Modifier Nobiletin in Metabolically Challenged Old Mice. Int. J. Mol. Sci. 2019, 20, 4281.
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923.
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621.
- Petrenko, V.; Gandasi, N.R.; Sage, D.; Tengholm, A.; Barg, S.; Dibner, C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc. Natl. Acad. Sci. USA 2020, 117, 2484–2495.
- Shinozaki, A.; Misawa, K.; Ikeda, Y.; Haraguchi, A.; Kamagata, M.; Tahara, Y.; Shibata, S. Potent Effects of Flavonoid Nobiletin on Amplitude, Period, and Phase of the Circadian Clock Rhythm in PER2::LUCIFERASE Mouse Embryonic Fibroblasts. PLoS ONE 2017, 12, e0170904.
- Gile, J.; Scott, B.; Eckle, T. The Period 2 Enhancer Nobiletin as Novel Therapy in Murine Models of Circadian Disruption Resembling Delirium. Crit. Care Med. 2018, 46, e600–e608.