
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ki Choon Choi | + 1378 word(s) | 1378 | 2021-01-14 09:03:33 | | | |
| 2 | Catherine Yang | + 1 word(s) | 1379 | 2021-01-21 03:27:12 | | |
Video Upload Options
Co-culture system provides a novel platform to study interaction between different cell types in an in-vitro method. The co-cultures techniques have played key role in the understanding of cell–cell communication and relevant for drug response analysis.
1. Introduction
In vitro co-culture techniques can be used to mimic in vivo environments and to observe interactions among cells (autocrine) and between cells (paracrine) [1]. Co-culture systems may be used to explore the mechanisms of action of drugs and their potential targets; they help to bridge the gap between mono-culture methods and animal models. Co-culture systems can be divided into two main categories, indirect methods and direct methods [2]. In the indirect methods, cells are physically separated into two different populations using trans-well inserts and/or overflow culture chambers that allow communication only via secretory factors. On the other hand, direct co-culture methods allow cell–cell interactions between different type of cells, which is typically achieved by spatially controlling the positions of adherent cells within a culture dish [3]. Generally, co-culture systems are used to study the secretory and transcription factors which are partially/fully involved in induction of cell differentiation, regulation of cellular proliferation, and production of metabolites for signaling cascades. A co-culture system can be used to reduce the amount of drug needed for a study, identify the target organs of a drug, and predict the adverse effects of drug metabolites [4]. Thus, this technique shows great potential for cell toxicological studies in the future and is now used chiefly for pharmacodynamic research.
Co-culture systems are widely used to study cross-talk between different cell lines, including adipocytes, endothelial cells, fibroblasts, macrophages, and muscle cells. Also, it is a crucial tool for understanding the various metabolic connections between adipose and other tissues [5]. The co-culture studies can provide realistic insights on cell–cell interactions via secretory factors that are effect in various metabolic functions such as energy homeostasis, muscle atrophy, and obesity and related co-morbidities. Previously, Ruiz-Ojeda et al. (2016) [6] investigated the co-culture relationship between adipocytes and macrophages and how they communicate under conditions that mimic obesity, insulin resistance, or inflammation. Similarly, an indirect co-culture system used to study intercellular communication between muscle and adipocyte cells was developed by Choi et al. (2013) [7]. Pre-adipocyte differentiation is regulated by differentiation myoblasts in the co-culture system, while pre-adipocytes promote adipogenic gene expression in muscle satellite cells co-cultured with pre-adipocytes. Muscle and fat tissue are major paracrine and endocrine organs that communicate with each other regarding muscle development, regulation of energy homeostasis, and insulin sensitivity [8]. For example, exercise-induced improvements in muscle function influence carbohydrate and fatty acid metabolism in the whole body as well as peripheral insulin sensitivity. Skeletal muscle communicates with other tissue types (i.e., adipose) to regulate, either directly or indirectly, whole-body energy homeostasis through myokine release [9] (Figure 1). Muscle-derived secretory proteins—including IL-6, irisin, myostatin—and some peptides, known as myokines, regulate adipogenesis via paracrine and endocrine effects [10]. Recently, Chu et al. (2016) [11] reported that porcine pre-adipocyte differentiation was inhibited in a C2C12 co-culture cells and that the expression levels of early differentiation marker genes in adipocytes were lower than those in mono-cultured adipocyte cells. Recently, Shahin-Shamsabadi [12] developed a 3D bio-fabrication method using adipocytes and myoblasts, that analyzed specifically either in direct physical contact or in close proximity such that the paracrine interaction between the cells. The physical contact between cells have been flouted in co-culture systems using transwell inserts and can be used in studies for the development of anti-obesity drugs. Anan et al. [13] studied the method for analyzing the direct interaction between adipose tissue and cardiomyocytes. The HL-1 cells suppressed the development of CD44+/CD105+ mesenchymal stem cell-like cells and lipid-laden preadipocytes from ATFs. In addition, the HL-1 cells stimulated the secretion of adiponectin in adipose tissue fragments (ATF), whereas they decreased production of leptin in a co-culture experiment.
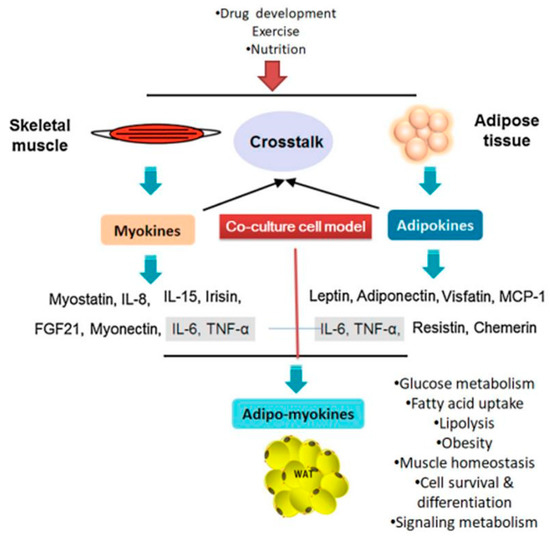
Figure 1. Cross-talk between muscle and adipose tissues is regulated by various secretory factors (adapted and modified from Li et al. (2017) [14]). WAT: white adipose tissue; TNF-α- tumor necrosis growth factor- alpha; FGF21: fibroblast growth factor 21; IL: interleukin.
2. Adipocytes/Muscle Cells Co-Culture Models
Co-culture models have been used to examine diverse cellular functions, such as interactions between muscle and nerve cells, angiogenesis, adipocyte/muscle cell cross-talk, immune cell functions, etc. [15]. Interactions between co-cultured myoblasts and adipocytes have been implicated in facilitation of muscle growth, tissue repair and muscle regeneration. These findings have led to the discovery of adipo-myokine secretory factors (AMSFs), which are produced by adipocytes and myocytes to induce differentiation and proliferation. Table 1 summarizes the findings of recent studies in co-culture systems using adipose and muscle cells.
At present, our knowledge of the interactions between adipocytes and myocytes largely stems from studies of the effects of individual adipokine factors on cultured muscle cells or adipocytes and vice versa. A wide variety of adipocyte mediated secretory molecules regulate muscle metabolism without affecting other tissues. Furthermore, in an in vitro co-culture system, myocytes were exposed to a group of free fatty acids (FFAs) and adipo-myokines [16][17] that communicate signals to other organs. These studies have fueled attention towards analyses of metabolic communication between fat and muscle cells. Adipose tissue is known to protect other cell types from lipotoxicity by providing a safe place to store surplus energy. However, obesity-related dysregulation of adipose tissue promotes lipid oversupply to several non-adipocyte tissue types. This can contribute to the development of metabolic diseases, such as cardiovascular disease and liver and bone disorders. Adipose tissue is an important endocrine organ that communicates with the brain and peripheral tissues to bring about changes in whole-body energy homeostasis through a network of circulating adipokines [18]. These signaling factors include peptide hormones; chemokines such as leptin, adiponectin, resistin, visfatin, and apelin; and pro-inflammatory cytokines including interleukins (IL-1β, IL-6 and IL-15) and tumor necrosis factor-α (TNF-α). Obesity may lower the levels of circulating insulin-sensitizing adipokines such as adiponectin while increasing the levels of pro-inflammatory response molecules such as IL-6 and TNF-α in adipose tissues [19].
Table 1. Examples of co-culture model systems consisted of adipose and muscle cells.
| Co-Culture Model | Compounds Used | Study Findings | Ref. |
|---|---|---|---|
| Pre-adipocytes-myoblasts | Arginine and/or trans 10, cis-12 CLA | Increased adipogenic gene expression in myoblasts | [7] |
| Bovine adipocytes and pre-adipocytes | Adipogenic induction medium | Increase lipolytic response and glycerol release | [20] |
| 3T3-L1 adipocyte–C2C12 cells | Ferulic acid | Increase lipolytic profile and glycerol release | [21] |
| C2C12 myoblasts-3T3-L1 adipocytes | Adipocytes medium induced IL-6 | Suppress the differentiation of C2C12 cells | [22] |
| Differentiated C2C12 with 3T3-L1 cells | Calcitriol | Decreased anti-inflammatory cytokines production | [23] |
| 3T3-L1 (adipocyte)-L6 muscle cell line | Differentiation media without additives | Co-culture adipocyte cells increased GPDH activity | [24] |
| Human fat and skeletal muscle cells | Differentiation medium with 1 pmol/L insulin | adipocyte induce a paracrine perturbation in muscle cells | [25] |
| 3T3-L1 preadipocytes-differentiated C2C12 | DMEM differentiation medium | C2C12 suppressed the mRNA, protein expression of glucocorticoids receptor | [11] |
| C2C12 myocytes and 3T3-L1 adipocytes | Adipocyte conditioned medium with Leucine | Modulation of muscle and adipocyte energy metabolism | [26] |
| C2C12 myocytes and 3T3-L1 pre-adipocytes | Zinc oxide nanoparticles | Increased expression of antioxidant enzymes and mRNA expression | [27] |
| 3T3-L1 adipocytes with RAW 264 macrophage | Dietary calcium | Reduce the inflammatory cytokine and oxidative stress in adipocytes | [28] |
| 3T3-L1 adipocytes with splenocytes cells | Lipopolysaccharides (LPS) | Elevated cytokine secretion (TNF-a, IL-6, MCP-1) | [29] |
| Murine adipocytes-C2C12 cells | Leucine and calcitriol | Decrease energy storage in adipocytes and increasing fatty acid utilization in C2C12 | [30] |
| 3T3-L1 pre-adipocytes and C2C12 muscle cells | DMEM/FBS growth medium | Promote the mitochondrial biogenesis bydirect activation of SIRT1 in both cells | [31] |
| 3T3-L1 pre-adipocytes and L6 muscle cells | DMEM/F12 supplemented with BSA | Oxygen species production and level of Glut1 mRNA and protein increased in L6 cells | [32] |
| Primary human adipocyte and skeletal myotubes | Low serum differentiation medium | Understating the metabolic function of intra muscular adipogenesis (lipolytic activity) | [17] |
| 3T3-L1 cells with J-6 cells | Defined Medium for co-cultured cells | Low level of IGF-1 IGF-II are not likely to play a role in intercellular communication between these cells | [1] |
| Porcine pre-adipocytes and muscle satellite cells | DMEM/F12 medium | Induce cell growth and proliferation meanwhile, inhibited the cell differentiation | [33] |
| Skeletal muscle (L6)-adipocyte (3T3-L1) | Specific differentiation medium for both cells | IL-6 cytokine plays main role in cross-talk between these cells | [34] |
| 3T3-L1 and L6 cell line | Differentiation medium containing 5% HS | Adipocyte differentiation inhibited and suppress the lipogenic gene expression | [35] |
References
- Dodson, M.V.; Vierck, J.L.; Hossner, K.L.; Byrne, K.; McNamara, J.P. The development and utility of a defined muscle and fat co-culture system. Tissue Cell 1997, 29, 517–524.
- Levorson, E.J.; Santoro, M.; Kasper, F.K.; Mikos, A.G. Direct and indirect co-culture of chondrocytes and mesenchymal stem cells for the generation of polymer/extracellular matrix hybrid constructs. Acta Biomater. 2014, 10, 1824–1835.
- Venter, C.; Niesler, C. A triple co-culture method to investigate the effect of macrophages and fibroblasts on myoblast proliferation and migration. Biotechniques 2018, 64, 52–58.
- Stanford, K.I.; Goodyear, L.J. Muscle-Adipose Tissue Cross-Talk. Cold Spring Harb. Perspect. Med. 2018, 8.
- Gupta, R.K. Adipocytes. Curr. Biol. 2014, 24, R988–R993.
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040.
- Choi, S.H.; Chung, K.Y.; Johnson, B.J.; Go, G.W.; Kim, K.H.; Choi, C.W.; Smith, S.B. Co-culture of bovine muscle satellite cells with preadipocytes increases PPARγ and C/EBPβ gene expression in differentiated myoblasts and increases GPR43 gene expression in adipocytes. J. Nutr. Biochem. 2013, 24, 539–543.
- Chen, W.; Wang, L.; You, W.; Shan, T. Myokines mediate the cross-talk between skeletal muscle and other organs. J. Cell. Physiol. 2020.
- Oh, K.-J.; Lee, D.S.; Kim, W.K.; Han, B.S.; Lee, S.C.; Bae, K.-H. Metabolic Adaptation in Obesity and Type II Diabetes: Myokines, Adipokines and Hepatokines. Int. J. Mol. Sci. 2016, 18, 8.
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharm. 2017, 34, 49–55.
- Chu, W.; Wei, W.; Yu, S.; Han, H.; Shi, X.; Sun, W.; Gao, Y.; Zhang, L.; Chen, J. C2C12 myotubes inhibit the proliferation and differentiation of 3T3-L1 preadipocytes by reducing the expression of glucocorticoid receptor gene. Biochem. Biophys. Res. Commun. 2016, 472, 68–74.
- Shahin-Shamsabadi, A.; Selvaganapathy, P.R. A 3D self-assembled in vitro model to simulate direct and indirect interactions between adipocytes and skeletal muscle cells. Adv. Biosyst. 2020, 4, 2000034.
- Anan, M.; Uchihashi, K.; Aoki, S.; Matsunobu, A.; Ootani, A.; Node, K.; Toda, S. A promising culture model for analyzing the interaction between adipose tissue and cardiomyocytes. Endocrinology 2011, 152, 1599–1605.
- Li, F.; Li, Y.; Duan, Y.; Hu, C.-A.A.; Tang, Y.; Yin, Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. 2017, 33, 73–82.
- Fernandez, L.A.; MacSween, J.M.; Robson, D.A. Growth of B cell colonies independent of T cell contact. Immunol. Lett. 1989, 22, 167–171.
- Dyck, D.J.; Heigenhauser, G.J.; Bruce, C.R. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol. 2006, 186, 5–16.
- Kovalik, J.-P.; Slentz, D.; Stevens, R.D.; Kraus, W.E.; Houmard, J.A.; Nicoll, J.B.; Lea-Currie, Y.R.; Everingham, K.; Kien, C.L.; Buehrer, B.M.; et al. Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes 2011, 60, 1882–1893.
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. AMS 2013, 9, 191–200.
- Trayhurn, P.; Wood, I.S. Signalling role of adipose tissue: Adipokines and inflammation in obesity. Biochem. Soc. Trans. 2005, 33, 1078–1081.
- Strieder-Barboza, C.; Thompson, E.; Thelen, K.; Contreras, G.A. Technical note: Bovine adipocyte and preadipocyte co-culture as an efficient adipogenic model. J. Dairy Sci. 2019, 102, 3622–3629.
- Kuppusamy, P.; Soundharrajan, I.; Hwang, I.; Kim, D.; Choi, K.C.; National Institute of Animal Science, Rural Development Ad-ministration, Cheonan, Korea. Unpublished work. 2020.
- Seo, K.; Suzuki, T.; Kobayashi, K.; Nishimura, T. Adipocytes suppress differentiation of muscle cells in a co-culture system. Anim. Sci. J. 2019, 90, 423–434.
- Choi, H.; Myung, K. Calcitriol enhances fat synthesis factors and calpain activity in co-cultured cells. Cell Biol. Int. 2014, 38, 910–917.
- Choi, C.-W.; Cho, W.-M.; Yeon, S.-H.; HwangBo, S.; Song, M.-K.; Park, S.; Baek, K.-H. Comparison between Single and Co-culture of Adipocyte and Muscle Cell Lines in Cell Morphology and Cytosolic Substances. J. Anim. Sci. Technol. 2012, 54, 103–109.
- Dietze, D.; Koenen, M.; Röhrig, K.; Horikoshi, H.; Hauner, H.; Eckel, J. Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes 2002, 51, 2369–2376.
- Sun, X.; Zemel, M.B. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr. Metab. 2009, 6, 26.
- Pandurangan, M.; Veerappan, M.; Kim, D.H. Cytotoxicity of zinc oxide nanoparticles on antioxidant enzyme activities and mRNA expression in the cocultured C2C12 and 3T3-L1 cells. Appl. Biochem. Biotechnol. 2015, 175, 1270–1280.
- Sun, X.; Zemel, M.B. Calcitriol and calcium regulate cytokine production and adipocyte-macrophage cross-talk. J. Nutr. Biochem. 2008, 19, 392–399.
- Nitta, C.F.; Orlando, R.A. Crosstalk between immune cells and adipocytes requires both paracrine factors and cell contact to modify cytokine secretion. PLoS ONE 2013, 8, e77306.
- Sun, X.; Zemel, M.B. Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells. Lipids 2007, 42, 297–305.
- Bruckbauer, A.; Zemel, M.B. Effects of dairy consumption on SIRT1 and mitochondrial biogenesis in adipocytes and muscle cells. Nutr. Metab. 2011, 8, 91.
- Kudoh, A.; Satoh, H.; Hirai, H.; Watanabe, T.; Shimabukuro, M. Preliminary Evidence for Adipocytokine Signals in Skeletal Muscle Glucose Uptake. Front Endocrinol. 2018, 9, 295.
- Yan, J.; Gan, L.; Yang, H.; Sun, C. The proliferation and differentiation characteristics of co-cultured porcine preadipocytes and muscle satellite cells in vitro. Mol. Biol. Rep. 2013, 40, 3197–3202.
- Seyoum, B.; Fite, A.; Abou-Samra, A.B. Effects of 3T3 adipocytes on interleukin-6 expression and insulin signaling in L6 skeletal muscle cells. Biochem. Biophys. Res. Commun. 2011, 410, 13–18.
- Park, S.; Baek, K.; Choi, C. Suppression of adipogenic differentiation by muscle cell-induced decrease in genes related to lipogenesis in muscle and fat co-culture system. Cell Biol. Int. 2013, 37, 1003–1009.





