| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria A. Poca | + 3683 word(s) | 3683 | 2021-01-20 03:30:30 | | | |
| 2 | Bruce Ren | -21 word(s) | 3662 | 2021-01-21 03:34:40 | | | | |
| 3 | Catherine Yang | Meta information modification | 3662 | 2021-09-28 11:19:07 | | |
Video Upload Options
In the past decade, there has been a clear trend towards better outcomes in patients with hydro-cephalus, especially those with normal pressure hydrocephalus (NPH). This is partly due to the availability of more sophisticated hardware and a better understanding of implants. However, there is little evidence to show the superiority of a specific type of valve over another. The most commonly reported consequence of hydrodynamic mismatch is shunt over-drainage. Simple dif-ferential pressure valves, with a fixed opening pressure or even adjustable valves, lead to non-physiologic intraventricular pressure (IVP) as soon as the patient moves into an upright pos-ture. These valves fail to maintain IVP within physiological limits due to the changes in hydro-static pressure in the drainage system. To solve this problem more complex third-generation hy-drostatic valves have been designed. These gravitational devices aim to reduce flow through a shunt system when the patient is upright but there are important technical differences between them.
1. Introduction
Hydrocephalus is a common disease treated in neurosurgical departments. Valve selection is an important factor for optimizing patient outcome. When differential-pressure valves (DPV) were introduced in the late 1950s, the neurosurgeon could select between high, medium, or low opening pressure valves. However, it soon became evident that over-drainage and its related complications (e.g., postural headaches, subdural bleeding, and subdural collections) were a direct consequence of the gravitationally-induced over-drainage when patients assume a standing or sitting position. The transition from the first generation of DPV to the more complex designs introduced later was a consequence of both neurosurgeons’ and engineers’ attempts to control the siphon effect that the DPV causes when the patient stands.
In the early 1970s, measurements of intracranial pressure (ICP) in patients revealed that pressure in the lateral ventricles was sub-atmospheric when the patient is erect. The first DPV generation systematically induced a significant increase in the magnitude of negative ICP when patients were sitting or standing [1][2]. The negative shunt-induced ICP was attributed to the gravity-induced ‘siphoning’ effects, an intrinsic flaw of DPVs that were designed to work on the differential pressure between the ICP and the pressure where the distal catheter was placed, usually in the right atrium or in the peritoneum. Siphoning induces shunt over-drainage and is the cause of many reported adverse effects after shunting [3].
In recent decades, a wide variety of valves, shunt designs, and devices for siphon-control or gravity-compensating devices have been introduced [4]. Today, we must choose among valves with different opening pressures, variable hydrodynamics, and adjustable (‘programmable’) valves with or without incorporated ‘antisiphon’ or gravitational devices. The use of gravitational devices incorporated into the valve or added in series into the distal catheter can produce a more physiological cerebrospinal fluid (CSF) drainage through the shunt, reducing the complications of over-drainage. A pragmatic randomized controlled study in patients with idiopathic NPH (iNPH) showed that the use of a gravitational valve prevented one over-drainage complication in every third patient undergoing shunting compared with a non-gravitational programmable valve [5].
One of the first gravitational valves designed was the Miethke® Dual-Switch valve (M-DSV; Aesculap, AG, Tuttlingen, Germany), which includes two valve chambers arranged in parallel: a low-opening pressure valve, designed for working in the supine position, and a second high-opening pressure valve, which starts working when the patient assumes the upright position. This device has been used for decades, especially in Europe and Japan [5]. The M-DSV has also been the main device used at our center since the second half of the 1990s. Subsequently, other smaller gravitational valves have displaced the use of the M-DSV in most centers.
The M-DSV is still available, is a good example of the gravitational device, and presents some differential characteristics that must be considered when selecting a valve for a specific patient. Here, we describe the characteristics of the M-DSV, its main advantages and drawbacks, and use our center learning curve with gravitational devices to make some recommendations for their use. The discussion of this gravitational valve allows us to emphasize the importance of using gravitational control in patients with hydrocephalus in which a shunt is implanted. We also want to discuss some of the issues that neurosurgeons should take into consideration when using gravitational devices in patients with hydrocephalus.
2. The Miethke® Dual-Switch Valve
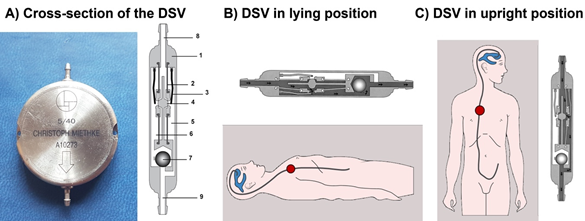
The M-DSV is a robust device with a titanium casing designed by biomedical engineers at the Technical University of Berlin in close cooperation with the neurosurgical department of the Virchow Klinikum, Free University Berlin (nowadays Charité, Humboldt University, Berlin) and the neurosurgical department of the University Hospital Essen. It is designed for subcutaneous implantation in the thoracic region of adult patients 6][7]. The device has a diameter of approximately 28 mm and is 6 mm thick, a thickness that is comparable to other valves and is distinctly thinner than a pacemaker, another device typically implanted subcutaneously in the thoracic region. Because of its design, subcutaneous pressure does not influence the M-DSV performance, as has been shown with other over-drainage-compensating devices, such as antisiphon valves [8]. M-DSV safety is also increased because it is better than other conventional valves for the drainage of viscous CSF, which might include proteins and cell debris [6][7]. This is because in the M-DSV, the area exposed to the fluid pressure is about 200-times larger than in other valves which systematically leads to higher acting (spring) forces which are consequently less affected by changed viscosity or debris [6]. The M-DSV includes two valve chambers arranged in parallel. The first, low-opening pressure valve is designed for working in the supine position, and the second, high-opening pressure valve starts working when the patient assumes the upright position. The pressure of both of these elements is controlled by ball-in-cone valves, and their main objective is to maintain ICP values within physiological ranges regardless of the patient’s posture (Figure 1) [7]. When patients are supine, the low-pressure chamber operates like a conventional DVP and is activated when the differential pressure is above the opening pressure (OP) of the valve. In the upright position, the low-pressure valve is closed by a tantalum ball. Afterward the control of the ventricular pressure in the upright position of the patient is shifted to the high-pressure-chamber of the M-DSV, and the postural changes move a tantalum ball [7] that opens the flow path for the high-pressure controller (Figure 1). The OP of this second valve is higher and takes into consideration the additional hydrostatic pressure of at least +35 cm H2O, which occurs in the upright position. The opening pressure for the upright position must be selected according to the height of the patient. At present, the M-DSV is available with an OP of 5–16 cm H2O for the supine position and 30–50 cmH2O for the upright position. For lumboperitoneal (LP) shunts, the available pressure settings for the lower-pressure valve is 10 cm H2O and for the upright position it is 30–50 cmH2O. Table 1 summarizes the pressures provided by the manufacturer.
Figure 1. (A) Cross-section of the Miethke® Dual-Switch valve (DSV). It has a solid titanium casing (1). Two titanium plates (3) are integrated into diaphragms made of silicone (2). Each plate, together with a ball (4), creates a valve seat, which is integrated into the casing as an opening and closing mechanism. Two different springs control the position of the plates. There is a stronger spring for the high-pressure chamber (5) and a weaker one for the low-pressure chamber (6). A heavy tantalum ball (7) is used to shift the flow path to either chamber. Titanium lips are used for the inlet (8) and outlet (9) tubing connection. When patients are supine (B), the low-pressure chamber operates like a conventional DPV and is activated when the differential pressure is above the opening pressure of the valve. In the upright position (C), the low-pressure valve is closed, and the postural changes move a tantalum ball that opens the flow path for the high-pressure controller [7].
Table 1. Closing pressures stated by the manufacturers (left) compared to the pressure values found in the tested shunts by Czosnyka at the UK Shunt Evaluation Laboratory (right, in parenthesis) for different models of Dual-Switch valves.
|
Pressure Settings for Ventriculoperitoneal Shunts |
|||
|
Low Pressure (Supine Position) mm H2O |
High Pressure (Upright Position) mm H2O |
Low Pressure (Supine Position) mm Hg |
High Pressure (Upright Position) mm Hg |
|
5* |
30 |
3.7 |
22.0 |
|
5* |
40 |
3.7 |
29.4 |
|
5* |
50 |
3.7 |
36.8 |
|
10 |
30 |
7.4/(7 ± 0.6) ** |
22.0/(22 ± 2.1) ** |
|
13 |
30 |
9.5/(9.5 ± 0.4) ** |
22.0 |
|
16 |
30 |
11.8/(12 ± 1.1) ** |
22.0 |
|
10 |
40 |
7.4 |
29.4/(28 ± 1.9) ** |
|
13 |
40 |
9.5 |
29.4 |
|
16 |
40 |
11.8 |
29.4 |
|
10 |
50 |
7.4 |
36.8/(38 ± 2.2) ** |
|
13 |
50 |
9.5 |
36.8 |
|
16 |
50 |
11.8 |
36.8 |
|
Pressure Settings for Lumboperitoneal Shunts |
|||
|
10 |
30 |
7.4 |
22.0 |
|
10 |
40 |
7.4 |
29.4 |
|
10 |
50 |
7.4 |
36.8 |
* Valves especially designed for patients with normal pressure hydrocephalus. Adapted from the UK Shunt Evaluation Laboratory Report [9]. ** Pressure values found in the tested shunts by Czosnyka et al. [10] Values here are given as mean ± standard deviation.
3. Dual-Switch Valve Performance Reported by the Manufacturer
In in vitro tests carried out by the manufacturers, the M-DSV has shown good and predictable performance [6][7]. In terms of sensitivity to changes in CSF viscosity, composition, and sticking, as well as the influence of the pressure of the subcutaneous tissues, the M-DSV has superior performance compared with other valves [6][7]. Stepwise changes conducted in the laboratory–simulating changes from the upright to the supine position–demonstrated that ICP remains consistently within physiological limits [6]. The authors emphasize that all in vitro testing carried out provided strong evidence for the capability of the M-DSV to maintain intraventricular pressure within physiological ranges, regardless of changes in CSF flow and posture. The investigations substantiate the theoretical concept that the M-DSV is less affected by atypical CSF compositions in contrast to all other clinically used valves for treatment of hydrocephalus.
4. Independent Evaluation of the Performance of the Dual-Switch Valve
The UK Shunt Evaluation Laboratory assess the hydrodynamic properties of all types of shunts available in the UK. This group has evaluated many valves available in the market, offering important and independent information that every neurosurgeon should consider when selecting a valve for a specific patient. The main aims of these authors have always been to: (1) assess hydrodynamic properties of a shunt regardless of the manufacturer; (2) check whether the shunt performs according to the manufacturer’s specifications and whether it complies with international standards (ISO/DIS7197); (3) characterize drainage capabilities with a more extensive range of tests than those listed in the ISO standard, and (4) summarize what is known from the literature about a shunt’s performance [9]. In these studies, the long-term stability of valve performance is tested in a laboratory environment that simulates the conditions within the human body, with the aim of demonstrating whether the shunt is susceptible to alterations in CSF drainage caused by postural changes, external pressure, change in ambient temperature, and/or the presence of a pulsating pattern in the inlet pressure, among other aspects [9].
In the report evaluation of the M-DSV, 11 valves were tested [9]. The authors demonstrated that M-DSV valves could limit drainage in a stepwise manner when the gravitational valve axis changed from horizontal to vertical. The performance was stable and durable, regardless of ambient pressure, temperature, or magnetic field. None of the parameters (OP, closing pressure (CP), and resistance of the shunt ()) were altered by changes in temperature (30–40 °C). Both OP and CP displayed minor variations (less than 1.5 mm Hg) in all tests. Good agreement of the operating pressures with the manufacturer’s data was recorded (differences of less than 1 mm Hg), and differences in measured parameters were minor among all tested valves. The valves did not show reflux when tested according to the ISO standard and did not exhibit a reversal of flow for an outlet-inlet differential pressure of up to 100 mm Hg. Table 2 summarizes the technical findings reported by this group.
Table 2. Evaluation of the performance and hydrodynamic properties of Miethke® Dual-Switch valves (M-DSV) by the U.K. Shunt Evaluation Laboratory.
|
Mechanical Durability |
All the shunts showed mechanical durability over the period of testing (>48 days) and good stability of hydrodynamic performance over a 28-day period. |
|
Opening and Closing Pressures |
Very well defined and stable in time, linearly changing with performance level. The distal catheter did not change the opening and closing pressure significantly. |
|
Pressure-Flow Performance Curves (PFPC) |
The PFPC, operating, opening, and closing pressures were stable, with minimal scatter, and were in accordance with data reported by the manufacturer for valves working in both horizontal and vertical positions. |
|
Hysteresis of the PFPC |
There was no hysteresis of PFPC. Slight hysteresis (but very narrow, 1 mm Hg of width) appeared after connection of the distal catheter. |
|
Pressure Changes Related to Body Posture |
The valves showed increased stepwise operational pressure in vertical position when compared to horizontal position, according to its fixed parameters. |
|
Hydrodynamic Resistance |
The valves have rather low and stable hydrodynamic resistance (2.2 ± 0.8 mm Hg/mL/min) and therefore are able to stabilize ICP according to the fixed settings. The addition of a distal catheter increases the shunt resistance to 2.9 ± 1.1 mm Hg/mL/min. |
|
Effect of Particles in Reagent |
Small particles (10 microns diameter) temporarily increased the valve’s hydrodynamic resistance to 7 mm Hg/ml/min. Large microspheres (with a diameter greater than erythrocytes—25 µm) were found to open the shunt temporarily (closing pressure decreased from around 9 to 3 mm Hg). In both cases, these effects were only temporary when the particles were washed out spontaneously and on the next day the valve returned to normal performance. |
|
ISO/DIS 7197 Compliances |
The M-DSV complied with this international standard for the testing of hydrocephalus valves. |
|
Resistance to Breakage and Leakage of Preassembled Junctions |
Preassembled junctions did not break when a test specimen was subjected to a load of 1 Kg of force for 1 minute. All junctions remained free from leakage when the water pressure was increased to 3 kPa (about 25 mm Hg). |
|
Reflux |
The valves did not show any reflux when tested according to the ISO standard. Valves did not exhibit flow reversal for an outlet-inlet differential pressure of up to 100 mm Hg. |
|
Magnetic Influence |
Magnetic field 3T did not have any influence on the shunt’s performance. |
|
External Pressure Influence |
No change in operating pressure occurred when an environmental pressure of 7 mm Hg was applied. |
|
Temperature Influence |
None of the parameters (opening, closing pressure, and resistance) were altered by a temperature change of 30 °C to 40 °C. |
Adapted from the UK Shunt Evaluation Laboratory Report [9,10].
5. The Effect of MRI on the Function of M-DSV
The MR-compatibility of CSF shunts has become increasingly important with the growing number of high-field MR scanners employed and the new, powerful magnetic fields that will be available soon. Lindner et al. [13] evaluated three M-DSV valves before and after exposure to 3T MRI. The M-DSV does not have ferromagnetic properties because it is made of titanium, tantalum, and silicone. In vivo, the valve is positioned in the thoracic region. All MRI experiments were performed on a 3T human-scale whole-body scanner (MAGNETOM Trio, Siemens, Erlangen, Germany) using a protocol analogous to clinical practice and with the valves mounted either on the side of a phantom aligned parallel to the axis of the main magnetic field or mounted perpendicularly [13]. Due to the chemical constituents of the valves, displacement or extensive heating of the devices was not expected, and the authors centered their evaluations on the functioning of the valves as the crucial parameter. The authors found that 3T magnetic fields did not influence the stability and safety of the M-DSV, with clear evidence that the valves continued to function correctly after MR scanning [13].
6. Pressure Selection of the Two Valves in the M-DSV
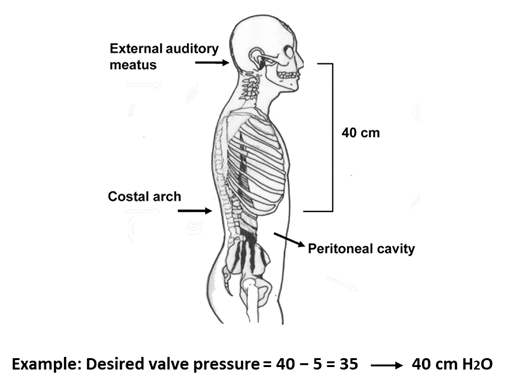
The M-DSV is available with several OPs for the supine position and for the upright position as listed in Table 1. The most reliable procedure for selecting a valve OP is based on the results of continuous ICP monitoring before surgery. In practice, however, when ICP recording is unavailable, most clinicians rely on the manufacturer’s recommendations [14]. According to the manufacturer, the recommended standard pressure setting for the lower-pressure valve (DualSwitch valve®, Instructions for use. C. Miethke GmbH & Co. KG) is 10 cm H2O (5 cm H2O for patients with NPH). The high-pressure side of the valve is calculated as a function of the patient’s height and is chosen so that with the patient upright, a ventricular pressure of at least −5 cm H2O is maintained (DualSwitch valve®, Instructions for use). Adequate pressure is calculated as follows: (1) measurement of the distance between the third ventricle (at the level of the foramen of Monro, roughly measured from the external auditory meatus) and the diaphragm (roughly measured at the level of the costal arch); (2) subtract 5 cm from the measured distance; (3) choose a valve with a high-pressure setting that exceeds the final measured value by the smallest amount (Figure 2). When these criteria are met, patient ICP would be expected to be maintained between −5 and +5 cmH2O even after the placement of the shunt [14].
Figure 2. Method of selecting a suitable pressure for the high-pressure valve in the Miethke® Dual-Switch valve. (1) Measure the distance between the third ventricle (external auditory meatus) and the patient’s diaphragm (costal arch); (2) subtract 5 cm from the measured distance; (3) choose a valve whose high-pressure setting exceeds the final measured value by the smallest amount.
Hertel et al. used a different strategy in which the opening pressure for the upright position (UPP) was dependent upon the patient’s height (180 cm or less, UPP = 30 cm or 40 cm H2O; height over 180 cm, UPP = 50 cm H2O) [15]. However, these authors did not consider the weight of the patient, which also determines the expected intra-abdominal pressure [16] and may explain the functional underdrainage found by some authors when using hydrostatic valves [17][18].
7. Intracranial Pressure Changes after M-DSV Implantation
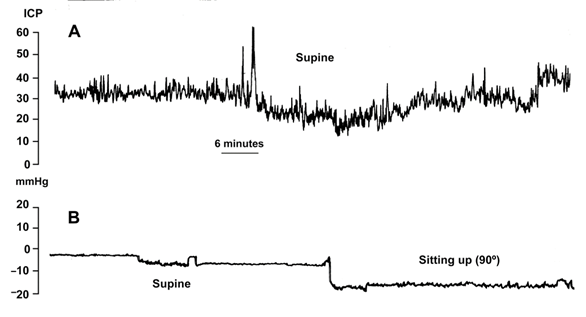
The M-DSV was designed to adjust ICP according to the supine or upright position of the patient, avoiding over-drainage. However, switching between pressure settings is not gradual but instead occurs at an angle of approximately 60–70°. Consequently, when changing patient position from horizontal to vertical, there is a short period in which ICP reaches a non-physiological negative value due to a delayed switching of the tantalum ball. Thus, if the patient changes slowly from the supine to the upright position, a situation of moderate over-drainage can occur. In this period the tantalum ball only diminishes the flow throughout the valve as the ball starts to close the valve-seat. Although this phenomenon of temporary over-drainage has no serious clinical consequences, it explains the headaches that some patients might present during the first days after shunt implantation when they assume the sitting or standing position. To avoid headaches, during the first two days after valve implantation the patient’s bed should preferably be kept flat and not partly raised. Figure 3 illustrates the posture-induced ICP changes in a patient with idiopathic intracranial hypertension before and after the implantation of a 10/40 cm H2O M-DSV.
Figure 3. Intracranial pressure (ICP) readings from a patient with idiopathic intracranial hypertension before (A) and after the implantation of a 10/40-cm H2O Miethke® Dual-Switch valve (B). In the posture-induced ICP changes after shunting (B), the patient remained supine for 1 h and then sat up and remained sitting for 3 h.
8. Surgical aspects in the implantation of the M-DSV
Antisiphon devices (ASDs) control over-drainage by increasing the resistance of the shunt (Rshunt). Gravitational devices (GDs) control over-drainage by increasing the OP of the valve through the movement of inbuilt metallic balls when the patient moves from the recumbent position. Consequently, the correct function of any GD depends on an adequate device’s vertical implantation. Any degree of deviation from the vertical axis equal to or more than 45° might eliminate the beneficial effect of the device. A retro-auricular implantation of other gravitational valves, such as GAV and PaediGAV (Aesculap, AG, Tuttlingen, Germany), is common in both adults and pediatric patients. However, a correct vertical alignment should always be ensured because the movement of the head with relation to the body axis might compromise the performance of the device. In adults, we prefer to implant GDs based on ball technology in the chest to avoid or minimize this problem (Figure 4A). Additionally, the M-DSV has been specifically designed to be implanted in the thorax. When this valve is implanted, the angle between the vertical axis of the valve should not deviate more than 15° from the gravitational axis to avoid interference with the mechanical design of the valve.
Another recommended precaution when implanting an M-DSV in the thorax is that it should be placed on a flat surface. In male patients, the valve can be placed at the infraclavicular or sub-mammary level. However, because of females’ variable breast size, it is better to place the valve at the sub-mammary level, on the costal grid, and at enough distance so that the bra does not coincide with the valve. Finally, given the dimensions and weight of the valve (28×6 mm, 11.6 g), it must be sutured in the muscular plane with a non-absorbable suture to ensure that the device does not move (Figure 4A).
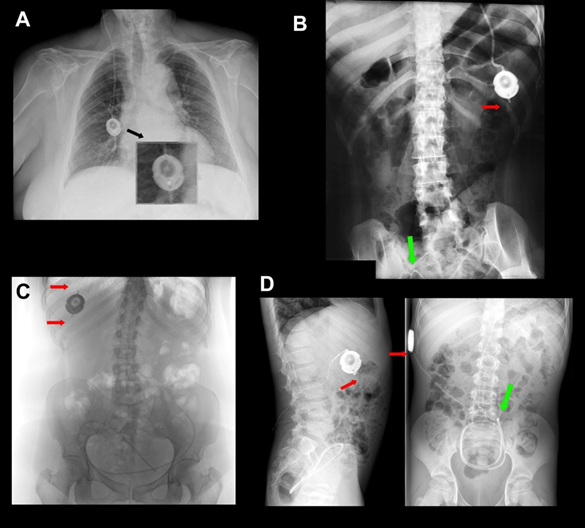
Figure 4. (A) A 10/50-cm H2O Miethke® Dual-Switch valve (M-DSV) implanted in a 75-year-old woman affected by normal pressure hydrocephalus (NPH). Note how the vertical axis of the valve is perfectly aligned with the vertical axis of the patient’s spine. This is the recommended position when this type of valve is inserted. The device is anchored to the muscular plane by a 0-silk suture. (B-C) Chest X-ray of the thoracic implantation site of the M-DSV in several patients showing fractures of the catheters (red arrows). (B) Fracture of the distal catheter of the valve in a patient aged 23 years with hydrocephalus associated with spina bifida with abdominal migration of the catheter (green arrow). A fracture of the distal catheter was diagnosed six years after implantation of the valve. (C) Fracture of the proximal and distal catheters of the valve in a patient with hydrocephalus associated with a Chiari type 1 malformation (red arrows). (D) Early distal catheter fracture (red arrow) when using a lumbo-peritoneal M-DSV. Although it would be expected that this problem would happen a few years after the implantation of the valve, in our center, we observed this problem only ten months after valve implantation in a 12-year-old child. The valve was replaced, and the child presented this problem for the second time one year after this second implantation. Greater mobility in children, when compared to adults, reinforces the fact that this type of valve has been designed for adults and should not be implanted in children.
References
- Fox, J.L.; McCullough, D.C.; Green, R.C. Effect of cerebrospinal fluid shunts on intracranial pressure and on cerebrospinal fluid dynamics: 2. A new technique of pressure measurements: results and concepts 3.A concept of hydrocephalus. J. Neurol. Neurosurg. Psychiatry 1973, 36, 302–312, doi:10.1136/jnnp.36.2.302.
- Aschoff, A.; Kremer, P.; Hashemi, B.; Kunze, S. The scientific history of hydrocephalus and its treatment. Neurosurg. Rev. 1999, 22, 67–93, doi:10.1007/s101430050035.
- Portnoy, H.D.; Schulte, R.R.; Fox, J.L.; Croissant, P.D.; Tripp, L. Anti-siphon and reversible occlusion valves for shunting in hydrocephalus and preventing post-shunt subdural hematomas. J Neurosurg 1973, 38, 729–738.
- Miyake, H. Shunt Devices for the Treatment of Adult Hydrocephalus: Recent Progress and Characteristics. Neurol. medi-co-chirurgica 2016, 56, 274–283, doi:10.2176/nmc.ra.2015–0282.
- Lemcke, J.; Meier, U.; Müller, C.; Fritsch, M.J.; Kehler, U.; Langer, N.; Kiefer, M.; Eymann, R.; Schuhmann, M.U.; Speil, A.; et al. Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: a pragmatic, randomised, open label, multicentre trial (SVASONA). J. Neurol. Neurosurg. Psychiatry 2013, 84, 850–857, doi:10.1136/jnnp-2012-303936.
- Miethke, C.; Affeld, K. A. New Valve for the Treatment of Hydrocephalus — Ein neues Ventil zur Behandlung des Hydro-cephalus. Biomed. Tech. Eng. 1994, 39, 181–187, doi:10.1515/bmte.1994.39.7-8.181.
- Sprung, C.; Miethke, C.; A Trost, H.; Lanksch, W.R.; Stolke, D. The dual-switch valve. A new hydrostatic valve for the treatment of hydrocephalus. Child’s Nerv. Syst. 1996, 12, 573–581.
- Drake, J.M.; Da Silva, M.C.; Rutka, J.T. Functional Obstruction of an Antisiphon Device by Raised Tissue Capsule Pressure. Neurosurg. 1993, 32, 137–139, doi:10.1227/00006123-199301000-00023.
- Czosnyka M, Czosnyka, Z.H., Pickard, J.D. Evaluation report of Aesculap-Miethke Dual-Switch and Paedi-GAV valves. Cambridge, UK: Academic Neurosurgical Unit 2003; 144: 525–538.
- Czosnyka, Z.; Czosnyka, M.; Richards, H.K.; Pickard, J.D. Evaluation of three new models of hydrocephalus shunts. Opera-tive Neuromodulation 2005, 95, 223–227, doi:10.1007/3-211-32318-x_46.
- Chari, A.; Czosnyka, M.; Richards, H.K.; Pickard, J.D.; Czosnyka, Z.H. Hydrocephalus shunt technology: 20 years of expe-rience from the Cambridge Shunt Evaluation Laboratory. J. Neurosurg. 2014, 120, 697–707, doi:10.3171/2013.11.jns121895.
- Czosnyka, Z.; Czosnyka, M.; Pickard, J.D.; Chari, A. Who Needs a Revision? 20 Years of Cambridge Shunt Lab. Operative Neuromodulation 2016, 122, 347–351, doi:10.1007/978-3-319-22533-3_68.
- Lindner, D.; Preul, C.; Trantakis, C.; Moeller, H.; Meixensberger, J. Effect of 3T MRI on the function of shunt valves—Evaluation of Paedi GAV, Dual Switch and proGAV. Eur. J. Radiol. 2005, 56, 56–59, doi:10.1016/j.ejrad.2005.03.029.
- Udayakumaran, S.; Roth, J.; Kesler, A.; Constantini, S. Miethke DualSwitch Valve in lumboperitoneal shunts. Acta Neurochir. 2010, 152, 1793–1800, doi:10.1007/s00701-010-0724-4.
- Hertel, F.; Züchner, M.; Decker, C.; Schill, S.; Bosniak, I.; Bettag, M. The Miethke Dual Switch Valve: Experience in 169 Adult Patients with Different Kinds of Hydrocephalus: An Open Field Study. Minim. Invasive Neurosurg. 2008, 51, 147–153, doi:10.1055/s-2008-1065337.
- Sahuquillo, J.; Arikan, F.; Poca, M.A.; Noguer, M.; Martínez-Ricarte, F. INTRA-ABDOMINAL PRESSURE: THE NEGLECT-ED VARIABLE IN SELECTING THE VENTRICULOPERITONEAL SHUNT FOR TREATING HYDROCEPHALUS. Neuro-surg. 2008, 62, 143–150, doi:10.1227/01.neu.0000311071.33615.e1.
- Sprung, C.; Miethke, C.; Schlosser, H.-G.; Brock, M. The enigma of underdrainage in shunting with hydrostatic valves and possible solutions. Operative Neuromodulation 2005, 95, 229–235, doi:10.1007/3-211-32318-x_47.
- Meier, U.; Kiefer, M.; Sprung, C. Evaluation of the Miethke dual- switch valve in patients with normal pressure hydrocepha-lus. Surg. Neurol. 2004, 61, 119–127, doi:10.1016/j.surneu.2003.05.003.