
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wided Najahi Missaoui | + 1864 word(s) | 1864 | 2021-01-12 05:21:49 | | | |
| 2 | Vicky Zhou | Meta information modification | 1864 | 2021-01-21 07:35:26 | | |
Video Upload Options
The field of nanotechnology has grown over the last two decades and made the transition from the benchtop to applied technologies. Nanoscale-sized particles, or nanoparticles, have emerged as promising tools with broad applications in drug delivery, diagnostics, cosmetics and several other biological and non-biological areas. These advances lead to questions about nanoparticle safety. Despite considerable efforts to understand the toxicity and safety of these nanoparticles, many of these questions are not yet fully answered. Nevertheless, these efforts have identified several approaches to minimize and prevent nanoparticle toxicity to promote safer nanotechnology.
1. Introduction: Nanoparticles and Nanotechnology
The field of nanotechnology has advanced exponentially in the last decade and many products containing nanoparticles are now used in various applications such as in food science, cosmetics and pharmaceuticals [1]. Nanoparticles (NPs) are defined as particles with one dimension ranging between 1 and 100 nm. NPs exhibit different properties depending on their size and surface functionalities [2]. The small size and large surface area account for the extensive use of NPs in various areas such as cosmetics, electronics and both diagnostic and therapeutic medical applications [3]. The exponential growth and increasing interest in nanotechnology have been enhanced by the ability to image nanomaterials using techniques with atomic resolution capabilities such as scanning tunneling microscopy, scanning transmission electron microscopy and tandem electron microscopy [4][5][6]. Along with the application of NPs, there has been a growth in scientific publications, as shown in Figure 1.
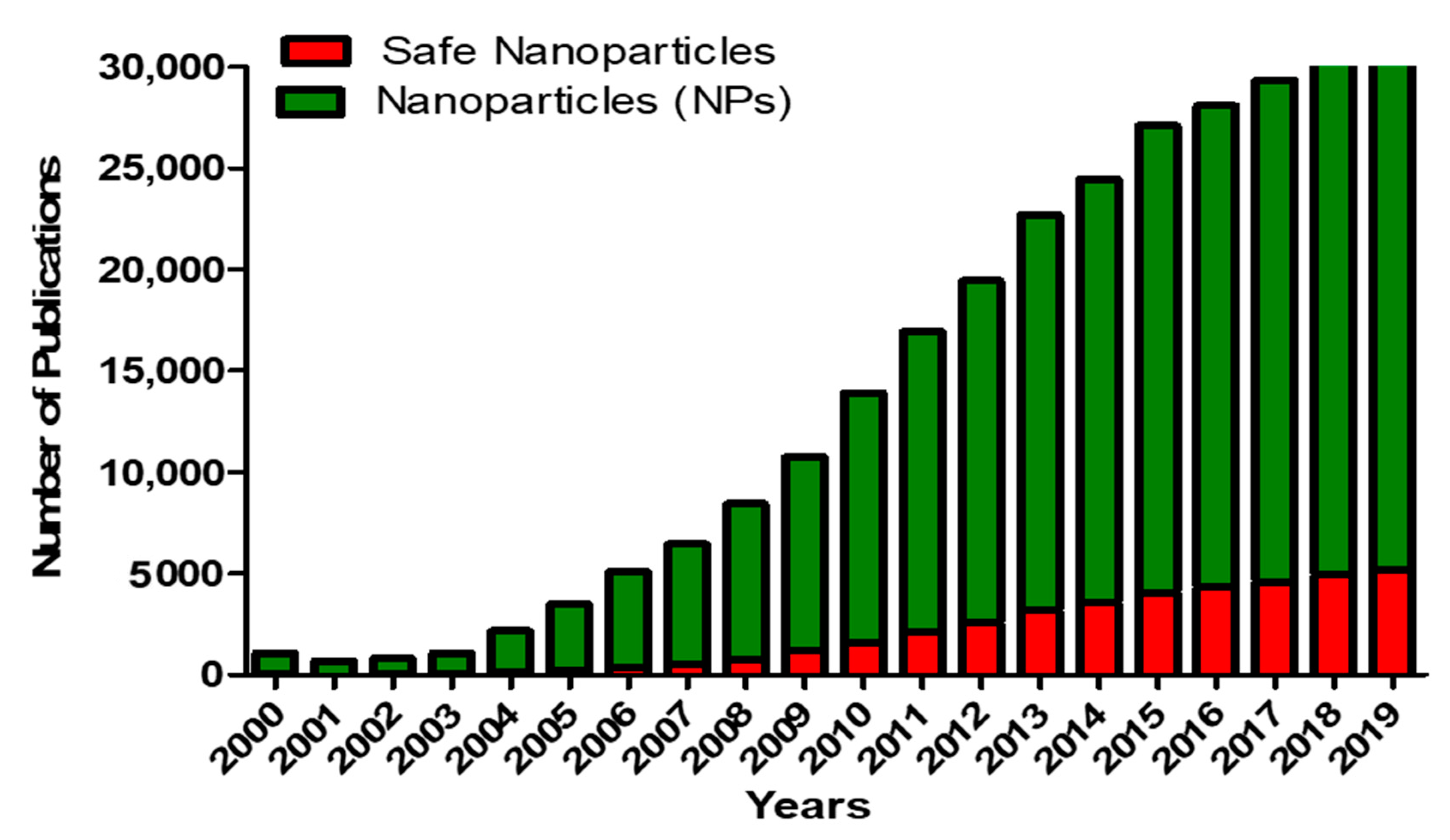
The exponential growth of nanotechnology has led to studies focusing on the associated risks of NPs and nanotechnology in general. However, and despite our increased exposure to NPs, information regarding NP safety is lagging behind as compared to the research on the application of NPs (Figure 1) [3].
NPs are used as pharmaceutical drug carriers with applications in both diagnostics and therapy. These NPs, including polymeric NPs, nanoemulsions, liposomes and solid NPs, are suggested to have potential clinical applications. Their clinical applicability depends on different parameters such as their physical and chemical properties, drug loading efficiency, drug release and most importantly low or no toxicity of the carrier itself [7].
Despite the potential for clinical application, some studies have suggested that NPs can be toxic. These studies have demonstrated the ability of NPs to accumulate in cells and induce organ-specific toxicity. These studies, combined with the ever-increasing human exposure, demonstrate an urgent need for the design of safe NPs and the development of strict guidelines for their development with regards to toxicity testing.
2. Approaches to Produce Non-Cytotoxic Nanoparticles
One promising approach toward decreasing the risk of some NPs, especially lipid-based NPs, is the use of “next-generation lipids” that combine high potency and biodegradable properties. Martin et al. have developed biodegradable lipids by incorporating biocleavable ester functions within the hydrophobic alkyl chains. This class of biodegradable lipids showed rapid elimination from plasma and improved tolerability in preclinical studies with high in vivo potency [8].
Surface coating strategies are also being suggested as one of the major surface modification strategies to decrease the risk of NPs and design safer nanotechnology. Surface coating refers to any modification, functionalization or stabilization applied to NPs in order to selectively alter their properties. The surface of NPs can be covered with various substances such as polymers in single- or multi-layers that can be either complete or incomplete [9]. This is because the coating material, if chosen correctly, provides biocompatibility and affects the behavior (e.g., colloidal stability) and the fate (e.g., degradation, excretion, accumulation) of NPs following their administration in the complex environment of biological fluids, cells and organisms.
There are numerous coating materials and coating techniques that are available for NPs. However, the most important criteria for these coating materials is to maintain high colloidal stability from the production steps of NPs, including stability in salt- and protein-containing media, such as buffer solutions or cell culture media, to their in vitro testing in biological cells and in vivo testing in animal models [9]. Another advantage of this strategy is that surface coating is reversible by non-covalent modification. Since bioavailability and potential toxicological effects of NPs are dependent on their dispersion state, various noncovalent coatings can be used to alter the dispersion state of NPs to alter their toxicity [10]. Coating strategies can be used for various types of NPs including polymeric, lipid-based and inorganic NPs. Examples of coating materials include polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), polyvinyl alcohol (PVA), poly(N–isopropylacrylamide) (PNIPAM), zwitterionic polymers such as poly(carboxybetaine) (PCB), poly(sulfo-betaine) (PSB) and phosphorylcholine-based copolymers and polysaccharides such as dextran and chitosan [9][11][12][13]. As an example, single-walled and multi-walled carbon nanotubes (CNTs) have been used in drug delivery as drug nanocarriers, in tissue engineering, water purification and in sensors [14][15]. CNTs have been also shown to induce inflammation, fibrosis and promote cancer progression as a result of their surface chemistry, length and aggregation state [15][16]. Wang et al. have shown that surface coating of CNTs using a nonionic triblock copolymer, PF108, improved the dispersion state of CNTs and reduced their agglomeration, cellular uptake and pro-fibrogenic effects. The authors assessed the protective effects of PF108 coating against the toxicity of CNTs in vitro using bronchial epithelial BEAS-2B cells and phagocytic THP-1 cells and in vivo using mice lungs [17]. The decrease in toxicity correlated to a decrease in pro-inflammatory cytokine (IL-1β) production by THP-1 cells and pro-fibrogenic TGF-β1 production by BEAS-2B cells, as compared to non-coated CNTs. In vivo studies demonstrated that PF108-coated CNTs reduced their deposition in the lung and protected against pulmonary fibrosis compared to uncoated CNTs. The stability of the PF108 coating on CNTs was maintained even under acidic lysosomal conditions [17]. Another study conducted by Mutlu et al. demonstrated that CNTs coated with PF108 protected against lung toxicity and were cleared from the lungs after 90 days compared to non-coated CNTs, which aggregated and induced granulomatous lung inflammation and fibrosis [18]. These studies suggest that surface coating with pluronic F108 (PF108) can provide protection against particle-induced toxicity and may be an effective strategy for the design of safer NPs.
Doping is a widely used and effective strategy for inorganic NPs. This technique alters the crystal structure of materials through the addition of impurities to improve chemical and physical properties [19][20][21]. Examples of dopants include aluminum, titanium and iron. These dopants, when incorporated evenly into the nanoparticles, have shown the ability to alter the density of reactive chemical entities on the surface of NPs and therefore reduce the binding energy of metal ions to oxygen [22][23]. Doping of NPs can decrease NP dissolution and cause a reduction in toxic ions released, and therefore alter the reactive surfaces, resulting in a decrease in ROS generation [23][24][25].
Doping has increased the potential use of inorganic NPs in nanomedicine. Inorganic NPs have been studied extensively; however, they also display significant toxicity to healthy cells and organs, which limits their clinical applications [3][26]. Doping has been shown to improve the antimicrobial potential of silver-based NPs, which, when doped with titanium oxide (TiO2), enhanced their antibacterial activities against Escherichia coli and Bacillus subtilis [27].
Flame spray pyrolysis (FSP) is a well-established technique used in NP doping. FSP uses a rapid combustion method, liquid precursor, self-sustaining flame with a high local temperature and large temperature gradient that allow for the synthesis of homogenous crystalline nanoscale materials [28]. Even though ZnO NPs have wide industrial applications, such as in cosmetics (e.g., sunscreens) and electronics [29], ZnO-induced pulmonary inflammations have been reported in humans. This phenomenon is known as “metal fume fever” that takes place when welders are exposed to metal fumes containing high concentrations of ZnO [30]. This suggests that reduction of dissolution of ZnO could possibly decrease these toxic effects [31]. George et al. (2010) synthesized Fe-doped ZnO NPs by FSP and assessed their cytotoxicity in vitro using RAW 264.7 and BEAS-2B mammalian cells and found that ZnO dissolution was decreased, which correlated to reduced cytotoxicity [22]. In vivo studies showed the reduced toxicity of Fe-doped ZnO nanoparticles in zebrafish embryos and rodent lungs [24]. While doping seems to be promising in reducing toxic effects of NPs, further studies are needed to determine if doping has any interference with the efficacy of encapsulated drugs and therefore their clinical applications.
Other modifications of surface properties of NPs that have been suggested to reduce their risk include alteration of charge density and hydrophobicity, which is reported to improve the efficacy of some NPs in biomedical applications including targeted drug delivery [32][33][34][35]. Adjustment of surface chemistry properties of NPs can be achieved by covalent binding of functional groups such as anionic, nonionic and cationic groups onto their surface [35][36][37][38][39][40]. Li et al. (2013) synthesized and assessed the toxicity of CNTs functionalized with anionic, nonionic and cationic surface groups in vitro and in vivo. CNTs with the anionic groups (carboxylate and polyethylene glycol), displayed the lowest pro-fibrogenic effects and uptake in THP-1 and BEAS-2B cells [37]. Cationic CNTs interact with anionic groups on cell membranes, which appears to enhance their cellular uptake [41][42][43]. When Goodman et al. (2004) compared the toxicity of gold NPs with cationic functional groups (ammonium groups) in comparison to NPs with anionic groups (carboxylate group), their data showed reduced cytotoxicity and cellular uptake with anionic NPs [44]. These studies suggest that changing the surface properties of CNTs and gold nanoparticles with anionic groups could potentially decrease their toxicity.
The hypothesis that altering the surface properties of NPs can reduce their toxicity is further supported by studies with iron oxide NPs, whose toxicity is attributed to the release of hydroxyl radicals resulting from reactions at their surface [45]. Surface functionalization of iron oxide NPs with organic compounds such as aldehyde, carboxyl and amino groups, stabilized the high chemical activity of these NPs, which resulted in a decrease in toxicity while increasing their biological compatibility [46][47][48][49]
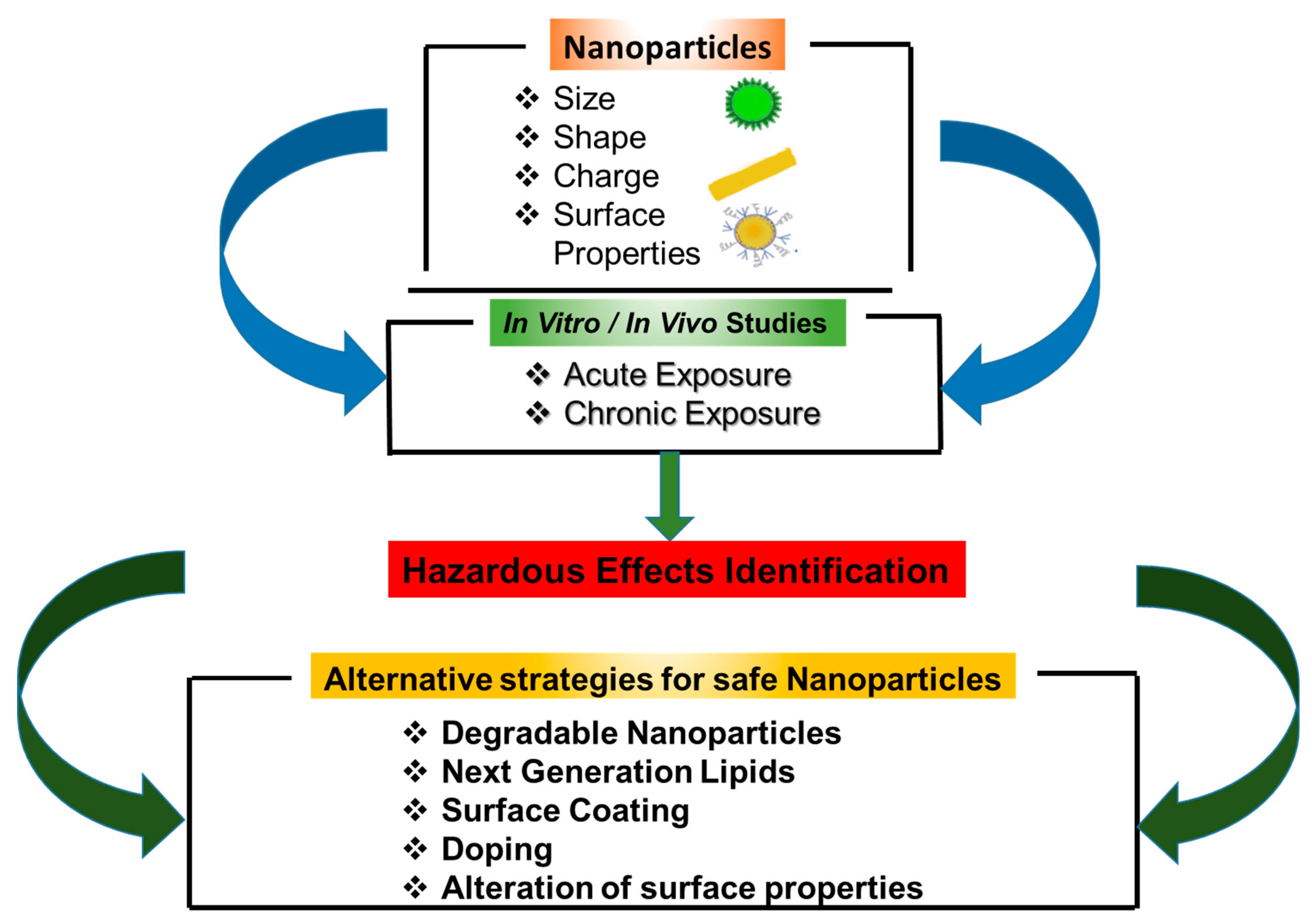
One common theme amongst techniques to reduce or prevent the toxicity of NPs is the modification of physicochemical properties of NPs, such as dissolution and release of toxic metal ions and agglomeration (Figure 2). However, further studies are needed to assess the effectiveness of these strategies under different exposure conditions and environments. This is especially needed due to the increasing number of new NPs being developed and their expanded use in the fields of biomedical application, drug delivery, diagnosis and imaging. There is also a need to develop well-thought-out and standardized procedures for synthesizing NPs that are suited for their different applications. One caveat to this is that many techniques for NP manufacturing and synthesis are based on studies conducted under non-GLP (Good Laboratory Practices)/non-GMP (Good Manufacturing Practices) environments, using small scale batches. As such, large scale-up production of NPs can create unforeseen impurities, necessitating a re-evaluation of safety protocols as well as assessment of finalized products. A further issue complicating the assessment of the toxicity of NPs is their potential toxicity in combination with drugs and other materials, which has received minimal attention.
References
- Kumar, V.; Kumari, A.; Guleria, P.; Yadav, S.K. Evaluating the toxicity of selected types of nanochemicals. Rev. Environ. Contam. Toxicol. 2012, 215, 39–121.
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health effects--pros and cons. Environ. Health Perspect. 2006, 114, 1818–1825.
- Missaoui, W.N.; Arnold, R.D.; Cummings, B.S. Toxicological status of nanoparticles: What we know and what we don’t know. Chem. Biol. Interact. 2018, 295, 1–12.
- Sharma, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Nanostructured Materials for Food Applications: Spectroscopy, Microscopy and Physical Properties. Bioengineering (Basel) 2019, 6.
- Jin, S.E.; Bae, J.W.; Hong, S. Multiscale observation of biological interactions of nanocarriers: From nano to macro. Microsc. Res. Tech. 2010, 73, 813–823.
- Banerjee, R.; Katsenovich, Y.; Lagos, L.; McIintosh, M.; Zhang, X.; Li, C.Z. Nanomedicine: Magnetic nanoparticles and their biomedical applications. Curr. Med. Chem. 2010, 17, 3120–3141.
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580.
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013, 21, 1570–1578.
- Schubert, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586.
- Wang, X.; Xia, T.; Ntim, S.A.; Ji, Z.; Lin, S.; Meng, H.; Chung, C.H.; George, S.; Zhang, H.; Wang, M.; et al. Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung. ACS Nano 2011, 5, 9772–9787.
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636.
- Pombo García, K.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: Recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small 2014, 10, 2516–2529.
- Shen, C.R.; Wu, S.T.; Tsai, Z.T.; Wang, J.J.; Yen, T.C.; Tsai, J.S.; Shih, M.F.; Liu, C.L. Characterization of quaternized chitosantem. ionic-coated “stealth” nanoparticles forel potential magnetic resonance imaging contrast agent for cell tracking. Polym. Int. 2011, 60, 945–950.
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539.
- Tang, S.; Tang, Y.; Zhong, L.; Murat, K.; Asan, G.; Yu, J.; Jian, R.; Wang, C.; Zhou, P. Short- and long-term toxicities of multi-walled carbon nanotubes in vivo and in vitro. J. Appl. Toxicol. 2012, 32, 900–912.
- Boyles, M.S.; Young, L.; Brown, D.M.; MacCalman, L.; Cowie, H.; Moisala, A.; Smail, F.; Smith, P.J.; Proudfoot, L.; Windle, A.H.; et al. Multi-walled carbon nanotube induced frustrated phagocytosis, cytotoxicity and pro-inflammatory conditions in macrophages are length dependent and greater than that of asbestos. Toxicol. Vitro 2015, 29, 1513–1528.
- Wang, X.; Xia, T.; Duch, M.C.; Ji, Z.; Zhang, H.; Li, R.; Sun, B.; Lin, S.; Meng, H.; Liao, Y.P.; et al. Pluronic F108 coating decreases the lung fibrosis potential of multiwall carbon nanotubes by reducing lysosomal injury. Nano Lett. 2012, 12, 3050–3061.
- Mutlu, G.M.; Budinger, G.R.; Green, A.A.; Urich, D.; Soberanes, S.; Chiarella, S.E.; Alheid, G.F.; McCrimmon, D.R.; Szleifer, I.; Hersam, M.C. Biocompatible nanoscale dispersion of single-walled carbon nanotubes minimizes in vivo pulmonary toxicity. Nano Lett. 2010, 10, 1664–1670.
- Rao, G.T.; Babu, B.; Stella, R.J.; Manjari, V.P.; Ravikumar, R.V. Spectral investigations on undoped and Cu(2)(+) doped ZnO-CdS composite nanopowders. Spectrochim. Acta A Mol. Biomol. Spectrosc 2015, 139, 86–93.
- Adeleye, A.S.; Pokhrel, S.; Madler, L.; Keller, A.A. Influence of nanoparticle doping on the colloidal stability and toxicity of copper oxide nanoparticles in synthetic and natural waters. Water Res. 2018, 132, 12–22.
- Ahmad, J.; Siddiqui, M.A.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A.; Khan, S.T.; Wahab, R.; Al-Khedhairy, A.A.; Al-Salim, A.; Musarrat, J.; et al. Copper doping enhanced the oxidative stress-mediated cytotoxicity of TiO2 nanoparticles in A549 cells. Hum. Exp. Toxicol. 2018, 37, 496–507.
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Madler, L.; et al. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano 2010, 4, 15–29.
- Sun, B.; Pokhrel, S.; Dunphy, D.R.; Zhang, H.; Ji, Z.; Wang, X.; Wang, M.; Liao, Y.P.; Chang, C.H.; Dong, J.; et al. Reduction of Acute Inflammatory Effects of Fumed Silica Nanoparticles in the Lung by Adjusting Silanol Display through Calcination and Metal Doping. ACS Nano 2015, 9, 9357–9372.
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased dissolution of ZnO by iron doping yields nanoparticles with reduced toxicity in the rodent lung and zebrafish embryos. ACS Nano 2011, 5, 1223–1235.
- Naatz, H.; Lin, S.; Li, R.; Jiang, W.; Ji, Z.; Chang, C.H.; Koser, J.; Thoming, J.; Xia, T.; Nel, A.E.; et al. Safe-by-Design CuO Nanoparticles via Fe-Doping, Cu-O Bond Length Variation, and Biological Assessment in Cells and Zebrafish Embryos. ACS Nano 2017, 11, 501–515.
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater 2019, 31, e1805730.
- Yuan, Y.; Ding, J.; Xu, J.; Deng, J.; Guo, J. TiO2 nanoparticles co-doped with silver and nitrogen for antibacterial application. J. Nanosci. Nanotechnol. 2010, 10, 4868–4874.
- Teoh, W.Y.; Amal, R.; Madler, L. Flame spray pyrolysis: An enabling technology for nanoparticles design and fabrication. Nanoscale 2010, 2, 1324–1347.
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780.
- Liu, J.; Feng, X.; Wei, L.; Chen, L.; Song, B.; Shao, L. The toxicology of ion-shedding zinc oxide nanoparticles. Crit. Rev. Toxicol. 2016, 46, 348–384.
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater 2016, 308, 328–334.
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176.
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544.
- Paramasivam, G.; Kayambu, N.; Rabel, A.M.; Sundramoorthy, A.K.; Sundaramurthy, A. Anisotropic noble metal nanoparticles: Synthesis, surface functionalization and applications in biosensing, bioimaging, drug delivery and theranostics. Acta Biomater. 2017, 49, 45–65.
- Nicol, J.R.; Dixon, D.; Coulter, J.A. Gold nanoparticle surface functionalization: A necessary requirement in the development of novel nanotherapeutics. Nanomedicine (Lond) 2015, 10, 1315–1326.
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951.
- Li, R.; Wang, X.; Ji, Z.; Sun, B.; Zhang, H.; Chang, C.H.; Lin, S.; Meng, H.; Liao, Y.P.; Wang, M.; et al. Surface charge and cellular processing of covalently functionalized multiwall carbon nanotubes determine pulmonary toxicity. ACS Nano 2013, 7, 2352–2368.
- Nel, A.E.; Madler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557.
- Atale, S.S.; Dyawanapelly, S.; Jagtap, D.D.; Jain, R.; Dandekar, P. Understanding the nano-bio interactions using real-time surface plasmon resonance tool. Int. J. Biol. Macromol. 2019, 123, 97–107.
- Cai, P.; Zhang, X.; Wang, M.; Wu, Y.L.; Chen, X. Combinatorial Nano-Bio Interfaces. ACS Nano 2018, 12, 5078–5084.
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2013, 46, 622–631.
- Kettiger, H.; Schipanski, A.; Wick, P.; Huwyler, J. Engineered nanomaterial uptake and tissue distribution: From cell to organism. Int. J. Nanomed. 2013, 8, 3255–3269.
- Zarska, M.; Novotny, F.; Havel, F.; Sramek, M.; Babelova, A.; Benada, O.; Novotny, M.; Saran, H.; Kuca, K.; Musilek, K.; et al. Two-Step Mechanism of Cellular Uptake of Cationic Gold Nanoparticles Modified by (16-Mercaptohexadecyl)trimethylammonium Bromide. Bioconjug. Chem. 2016, 27, 2558–2574.
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900.
- Voinov, M.A.; Sosa Pagan, J.O.; Morrison, E.; Smirnova, T.I.; Smirnov, A.I. Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J. Am. Chem. Soc. 2011, 133, 35–41.
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater 2015, 16, 023501.
- Thomas, R.; Park, I.K.; Jeong, Y.Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930.
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415.
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Banobre-Lopez, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Health Mater 2018, 7.




