| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Johnson Rajasingh | + 1070 word(s) | 1070 | 2021-01-13 07:12:24 | | | |
| 2 | Nicole Yin | Meta information modification | 1070 | 2021-01-20 09:38:14 | | |
Video Upload Options
Mesenchymal stem cells (MSCs) are multipotent cells which can proliferate and replace dead cells in the body. MSCs also secrete immunomodulatory molecules, creating a regenerative microenvironment that has an excellent potential for tissue regeneration.
1. Introduction
MSCs have the potential for self-renewal and limited differentiation. They exist in many different tissues and organs such as adipose tissue, bone marrow, skin, fallopian tube, cord blood, liver and lungs[1]. MSCs were defined as stromal cells of the bone marrow and showed the properties of hematopoietic stem-like cells but were unable to differentiate into hematopoietic cells[2].
2. Mechanism of Actions of Adult MSCs
2.1. Trans-Differentiation
Trans-differentiation is defined as a process by which a cell is made to differentiate from one cell type to another distinct lineage through genetic reprogramming[3]. MSCs have the ability to trans-differentiate into various germ layers such as ectoderm, mesoderm and endoderm. Bone marrow derived MSCs trans-differentiate under specific conditions into ectodermal derivatives such as neurons. These MSC-derived neurons were used to treat diseases associated with neurodegeneration by implanting them into a precise location[4][5]. Furthermore, MSCs were able to trans-differentiate into spindle-shaped Schwann cells, which were then used to treat neurodegenerative demyelination disorders and also to treat skin injuries[6][7]. These studies have proven that MSCs are able to trans-differentiate into cells belonging to the ectodermal germ layer. Moreover, MSCs have been shown to trans-differentiate under specific conditions into mesoderm germ layer cells such as adipocytes, osteocytes and chondrocytes. MSCs were able to trans-differentiate into osteoblasts and osteocytes that can be used for bone repair and regeneration[8]. Several studies have shown that MSCs can be trans-differentiated into adipocytes and chondrocytes[3][9][10]. MSCs also trans-differentiated into hepatocytes, which represents the endoderm lineage, and these cell types were used to treat liver diseases[11].
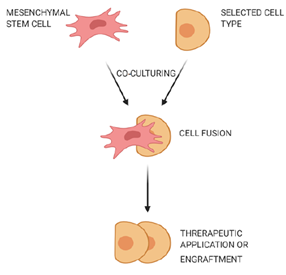
2.2. Cell Fusion
Cell fusion is defined as the process of one cell interacting with neighboring cells to form a multicellular aggregate with a common function (Figure 1). In this process, the genetic information is fused and expressed as molecular markers that show characteristic features of the fused cells[12]. Fusion of human MSCs with rodent cerebellar Purkinje cells was observed, and the fused cells were used to improve the therapy of neurodegenerative disorders or cerebellar related issues[13]. MSCs could also fuse with human gastrointestinal epithelial cells to form aggregates while expressing characteristic features of both cell types[14].
Figure 1. Fusion of mesenchymal stem cells with non-stem cells. Mesenchymal stem cells interact with neighboring cells to form a multicellular aggregate with improved characteristics, and these fused cells can be used for treatment of neurodegenerative or gastrointestinal disorders.
2.3. Mitochondrial Transfer
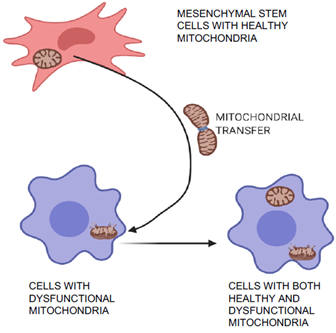
Mitochondrial transfer is one of the unique phenomena of MSCs in which the cells can transfer mitochondria to neighboring injured cells, which are then restored (Figure 2) and can aid in tissue repair and regeneration[15][16]. Initially, transfer was proved by co-culture experiments when mitochondria from human MSCs were found in recipient cells that had been devoid of mitochondria[15]. Mitochondrial transfer was observed to occur through formation of intracellular nanotubes, gap junctions, cell fusion, microvesicles, and direct uptake of isolated mitochondria[17][18][19][20]. Mitochondrial transfer from MSCs plays a crucial role in regeneration of several tissues including lung, heart, kidney, and brain[21]. Many stress signals, such as the release of damaged mitochondria, mitochondrial DNA, and an increased level of reactive oxygen species induced the transfer of mitochondria from MSCs to recipient cells[20][22]. The expression of Miro1 protein in MSCs was shown to play an important role during tunnel tube formation for mitochondrial transfer to the injured cell under stress[23][24]. This feature of MSCs was found to repair cardiomyocytes during myocardial infarction[25][26], damaged corneal epithelium in the eye[27] [48], renal tubular cells[28], brain-cortical cells[29], and lung cells[30].
Figure 2. Mitochondrial transfer and repair of damaged cells. MSCs can transfer healthy mitochondria to the injured neighboring cells with dysfunctional mitochondria. This characteristic feature of MSCs help to regenerate several tissues including lung, heart, kidney, and brain.
2.4. Extracellular Vesicles or Microvesicles
Extracellular vesicles are released by many types of cells, including MSCs. The size of these vesicles ranges from 30–120 nm and they are called exosomes. The most important capabilities of these vesicles are to transport essential macromolecules like protein and genetic material to neighboring cells by endocytosis[31]. However, currently, these vesicles pose difficulties with extraction, quality, and reproducibility from differing cell types. MSC-derived extracellular vesicles or exosomes were utilized for cell-free therapy[32][33][34]. MSC-derived exosomes have been employed to overcome the transplant-associated problem of Graft vs. Host Disease (GvHD)[35]. Studies have shown that MSC-derived paracrine factors induce signaling, promote improved skin wound healing[36], and also mediate essential physiological functions[37]. Although some therapeutic benefits of MSCs are obtained through trans-differentiation and most of the benefits are mediated through paracrine mechanisms[38], it is worth noting that therapeutic effects of microvesicles and exosomes derived from MSCs differ depending on the tissue or organ in which they resided[39] . Thus, further studies are needed to compare exosomes derived from MSCs of differing origins.
3. Induced Pluripotent Stem Cell-Derived MSCs (iMSCs)
Organ or tissue repair with cell therapy has emerged as a promising treatment alternative in patients with degenerative diseases. Researchers are currently developing a variety of therapies with stem cells obtained from many different sources and which can provide trophic and paracrine support or even replace dying cells[40]. A fundamental goal of regenerative medicine is to repair the failing organ by replenishing functional cells. A variety of autologous and allogeneic cell types have been tested for repair of diseases in humans, including cardiac diseases, and have shown a wide range of results, from significant improvement to no improvement[41][42][43]. MSCs isolated from adult organs hold promise, but scalability and senescence are major issues. During insult to any organ, cells are lost or become dysfunctional, and the post-insult milieu can have a negative impact on the health of autologous MSCs and their therapeutic capabilities. Thus, manipulation of autologous cells into primitive iPSCs and then further differentiation into specific required cell types is an attractive approach in stem cell transplantation therapy (Figure 3). Studies have shown that iMSCs promote healing in inflammatory bowel disease[44] and limb ischemia[45]. Moreover, iMSCs promote angiogenesis in ischemia models[46] and prevent allergic airway inflammation[47].
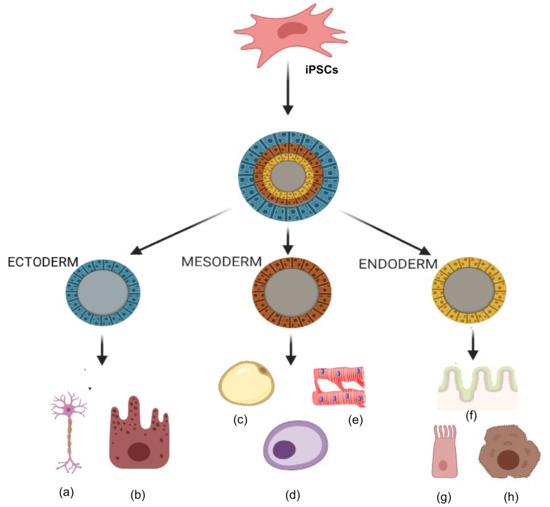
Figure 3. Tri-lineage differentiation capabilities of induced pluripotent stem cells (iPSCs). Ability of iPSCs to differentiate into three germ layers (ectoderm, mesoderm and endoderm) in types of cells such as (a) neurons, (b) epithelial cells, (c) adipocytes, (d) osteocytes, (e) cardiomyocytes, (f) gut epithelium, (g) lung cells, and (h) hepatocytes.
References
- Mozhdeh Mohammadian; Karim Shamsasenjan; Parisa Lotfi Nezhad; Mehdi Talebi; Mehdi Jahedi; Hossein Nickkhah; Neda Minayi; Ali Akbar Movassaghpour; Mesenchymal Stem Cells: New Aspect in Cell-Based Regenerative Therapy. Advanced Pharmaceutical Bulletin 2013, 3, 433-437, 10.5681/apb.2013.070.
- Maureen Owen; A. J. Friedenstein; Stromal Stem Cells: Marrow-Derived Osteogenic Precursors. Novartis Foundation Symposia 2007, 136, 42-60, 10.1002/9780470513637.ch4.
- Song, L.; Tuan, R.S. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 980–982.
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370.
- Ribeiro, J.; Pereira, T.; Caseiro, A.R.; Armada-da-Silva, P.; Pires, I.; Prada, J.; Amorim, I.; Amado, S.; França, M.; Gonçalves, C.; et al. Evaluation of biodegradable electric conductive tube-guides and mesenchymal stem cells. World J. Stem Cells 2015, 7, 956–975.
- Keilhoff, G.; Goihl, A.; Langnäse, K.; Fansa, H.; Wolf, G. Transdifferentiation of mesenchymal stem cells into Schwann cell-like myelinating cells. Eur. J. Cell Biol. 2006, 85, 11–24.
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008, 180, 2581–2587.
- Terhi J. Heino; Terhi J. Heino And Teuvo A. Hentunen; Differentiation of Osteoblasts and Osteocytes from Mesenchymal Stem Cells. Current Stem Cell Research & Therapy 2008, 3, 131-145, 10.2174/157488808784223032.
- Fink, T.; Zachar, V. Adipogenic differentiation of human mesenchymal stem cells. Methods Mol. Biol. 2011, 698, 243–251.
- Song, L.; Baksh, D.; Tuan, R.S. Mesenchymal stem cell-based cartilage tissue engineering: Cells, scaffold and biology. Cytotherapy 2004, 6, 596–601.
- Shin-Yeu Ong; Hui Dai; Kam W. Leong; Inducing hepatic differentiation of human mesenchymal stem cells in pellet culture. Biomaterials 2006, 27, 4087-4097, 10.1016/j.biomaterials.2006.03.022.
- Leonard M. Eisenberg; Carol A. Eisenberg; Stem cell plasticity, cell fusion, and transdifferentiation. Birth Defects Research Part C: Embryo Today: Reviews 2003, 69, 209-218, 10.1002/bdrc.10017.
- Kevin Kemp; D. Gordon; D. C. Wraith; E. Mallam; E. Hartfield; J. Uney; A. Wilkins; N. Scolding; Fusion between human mesenchymal stem cells and rodent cerebellar Purkinje cells. Neuropathology and Applied Neurobiology 2011, 37, 166-178, 10.1111/j.1365-2990.2010.01122.x.
- Jonathan Ferrand; Danièle Noël; Philippe Lehours; Martina Prochazkova-Carlotti; Lucie Chambonnier; Armelle Ménard; Francis Megraud; Christine Varon; Human Bone Marrow-Derived Stem Cells Acquire Epithelial Characteristics through Fusion with Gastrointestinal Epithelial Cells. PLoS ONE 2011, 6, e19569, 10.1371/journal.pone.0019569.
- Spees, J.L.; Olson, S.D.; Whitney, M.J.; Prockop, D.J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. USA 2006, 103, 1283–1288.
- Hsu, Y.C.; Wu, Y.T.; Yu, T.H.; Wei, Y.H. Mitochondria in mesenchymal stem cell biology and cell therapy: From cellular differentiation to mitochondrial transfer. Semin. Cell Dev. Biol. 2016, 52, 119–131.
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010.
- Gerdes, H.H.; Bukoreshtliev, N.V.; Barroso, J.F. Tunneling nanotubes: A new route for the exchange of components between animal cells. FEBS Lett. 2007, 581, 2194–2201.
- Sinha, P.; Islam, M.N.; Bhattacharya, S.; Bhattacharya, J. Intercellular mitochondrial transfer: Bioenergetic crosstalk between cells. Curr. Opin. Genet. Dev. 2016, 38, 97–101.
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 107.
- Paliwal, S.; Chaudhuri, R.; Agrawal, A.; Mohanty, S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018, 25, 31.
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350.
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Kumar, M.; Rehman, R.; Tiwari, B.K.; Jha, K.A.; Barhanpurkar, A.P.; et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014, 33, 994–1010.
- Zhang, Y.; Yu, Z.; Jiang, D.; Liang, X.; Liao, S.; Zhang, Z.; Yue, W.; Li, X.; Chiu, S.M.; Chai, Y.H.; et al. iPSC-MSCs with High Intrinsic MIRO1 and Sensitivity to TNF-α Yield Efficacious Mitochondrial Transfer to Rescue Anthracycline-Induced Cardiomyopathy. Stem Cell Rep. 2016, 7, 749–763.
- Masuzawa, A.; Black, K.M.; Pacak, C.A.; Ericsson, M.; Barnett, R.J.; Drumm, C.; Seth, P.; Bloch, D.B.; Levitsky, S.; Cowan, D.B.; et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H966–H982.
- McCully, J.D.; Cowan, D.B.; Pacak, C.A.; Toumpoulis, I.K.; Dayalan, H.; Levitsky, S. Injection of isolated mitochondria during early reperfusion for cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H94–H105.
- Dan Jiang; Fei Gao; Yuelin Zhang; David Sai Hung Wong; Qing Li; Hung-Fat Tse; Goufeng Xu; Zhendong Yu; Qizhou Lian; Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death & Disease 2016, 7, e2467-e2467, 10.1038/cddis.2016.358.
- Egor Y. Plotnikov; Tatyana G. Khryapenkova; Svetlana I. Galkina; Gennady T. Sukhikh; Dmitry B. Zorov; Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Experimental Cell Research 2010, 316, 2447-2455, 10.1016/j.yexcr.2010.06.009.
- Valentina A. Babenko; Denis N. Silachev; Ljubava D. Zorova; Irina B. Pevzner; Anastasia A. Khutornenko; E. Y. Plotnikov; Gennady T. Sukhikh; Dmitry B. Zorov; Improving the Post-Stroke Therapeutic Potency of Mesenchymal Multipotent Stromal Cells by Cocultivation With Cortical Neurons: The Role of Crosstalk Between Cells. STEM CELLS Translational Medicine 2015, 4, 1011-1020, 10.5966/sctm.2015-0010.
- Xiang Li; Yuelin Zhang; Sze C. Yeung; Yingmin Liang; Xiaoting Liang; Yue Ding; Mary S. M. Ip; Hung-Fat Tse; Judith C. W. Mak; Qizhou Lian; et al. Mitochondrial Transfer of Induced Pluripotent Stem Cell–Derived Mesenchymal Stem Cells to Airway Epithelial Cells Attenuates Cigarette Smoke–Induced Damage. American Journal of Respiratory Cell and Molecular Biology 2014, 51, 455-465, 10.1165/rcmb.2013-0529oc.
- Xia Li; Alexander L. Corbett; Erfan Taatizadeh; Nishat Tasnim; Jonathan P. Little; Cathie Garnis; Mads Daugaard; Emma Guns; Mina Hoorfar; Isaac T.S. Li; et al. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioengineering 2019, 3, 011503, 10.1063/1.5087122.
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858.
- Barani, B.; Rajasingh, S.; Rajasingh, J. Exosomes: Outlook for Future Cell-Free Cardiovascular Disease Therapy. Adv. Exp. Med. Biol. 2017, 998, 285–307.
- Bei, Y.; Das, S.; Rodosthenous, R.S.; Holvoet, P.; Vanhaverbeke, M.; Monteiro, M.C.; Monteiro, V.V.S.; Radosinska, J.; Bartekova, M.; Jansen, F.; et al. Extracellular Vesicles in Cardiovascular Theranostics. Theranostics 2017, 7, 4168–4182.
- Lambros Kordelas; Vera Rebmann; A-K Ludwig; Stefan Radtke; Johannes Ruesing; Thorsten R Doeppner; Matthias Epple; Peter A Horn; Dietrich W. Beelen; Bernd Giebel; et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014, 28, 970-973, 10.1038/leu.2014.41.
- Arno, A.I.; Amini-Nik, S.; Blit, P.H.; Al-Shehab, M.; Belo, C.; Herer, E.; Tien, C.H.; Jeschke, M.G. Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Res. Ther. 2014, 5, 28.
- Ferraris, V.A. How do cells talk to each other?: Paracrine factors secreted by mesenchymal stromal cells. J. Thorac. Cardiovasc. Surg 2016, 151, 849–851.
- Hocking, A.M.; Gibran, N.S. Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp. Cell Res. 2010, 316, 2213–2219.
- Zhao, T.; Sun, F.; Liu, J.; Ding, T.; She, J.; Mao, F.; Xu, W.; Qian, H.; Yan, Y. Emerging Role of Mesenchymal Stem Cell-derived Exosomes in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2019, 14, 482–494.
- Dai, W.; Hale, S.L.; Martin, B.J.; Kuang, J.Q.; Dow, J.S.; Wold, L.E.; Kloner, R.A. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short- and long-term effects. Circulation 2005, 112, 214–223.
- Schachinger, V.; Erbs, S.; Elsasser, A.; Haberbosch, W.; Hambrecht, R.; Holschermann, H.; Yu, J.; Corti, R.; Mathey, D.G.; Hamm, C.W.; et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 2006, 355, 1210–1221.
- Losordo, D.W.; Kibbe, M.R.; Mendelsohn, F.; Marston, W.; Driver, V.R.; Sharafuddin, M.; Teodorescu, V.; Wiechmann, B.N.; Thompson, C.; Kraiss, L.; et al. A Randomized, Controlled Pilot Study of Autologous CD34+ Cell Therapy for Critical Limb Ischemia. Circ. Cardiovasc. Interv. 2012.
- Jeevanantham, V.; Butler, M.; Saad, A.; Abdel-Latif, A.; Zuba-Surma, E.K.; Dawn, B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: A systematic review and meta-analysis. Circulation 2012, 126, 551–568.
- Yang, H.; Feng, R.; Fu, Q.; Xu, S.; Hao, X.; Qiu, Y.; Feng, T.; Zeng, Z.; Chen, M.; Zhang, S. Human induced pluripotent stem cell-derived mesenchymal stem cells promote healing via TNF-α-stimulated gene-6 in inflammatory bowel disease models. Cell Death Dis. 2019, 10, 718.
- Lian, Q.; Zhang, Y.; Zhang, J.; Zhang, H.K.; Wu, X.; Zhang, Y.; Lam, F.F.; Kang, S.; Xia, J.C.; Lai, W.H.; et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 2010, 121, 1113–1123.
- Hu, G.W.; Li, Q.; Niu, X.; Hu, B.; Liu, J.; Zhou, S.M.; Guo, S.C.; Lang, H.L.; Zhang, C.Q.; Wang, Y.; et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res. Ther. 2015, 6, 10.
- Sun, Y.Q.; Deng, M.X.; He, J.; Zeng, Q.X.; Wen, W.; Wong, D.S.; Tse, H.F.; Xu, G.; Lian, Q.; Shi, J.; et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 2012, 30, 2692–2699.