
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Martin Stoddart | + 1177 word(s) | 1177 | 2021-01-13 07:30:28 | | | |
| 2 | Vicky Zhou | Meta information modification | 1177 | 2021-01-19 06:39:52 | | |
Video Upload Options
Articular cartilage injury and repair is an issue of growing importance. Although common, defects of articular cartilage present a unique clinical challenge due to its poor self-healing capacity, which is largely due to its avascular nature. There is a critical need to better study and understand cellular healing mechanisms to achieve more effective therapies for cartilage regeneration. This article aims to describe the key features of cartilage which is being modelled using tissue engineered cartilage constructs and ex vivo systems. These models have been used to investigate chondrogenic differentiation and to study the mechanisms of cartilage integration into the surrounding tissue.
1. Introduction
Articular cartilage, which covers the osseous ends in diarthrodial joints, is an anisotropic tissue with a complex structure. Mature tissue is constructed of four layers: surface zone, middle zone, deep zone and calcified zone [1][2][3]. Each zone has a well-defined structural, functional and mechanical property that responds to different stimuli and is populated by a distinct cell phenotype that secretes different proteins and generates a well-defined organization of collagen fibers in each zone [4][5][6][7][8] (Figure 1). The main cell type resident in articular cartilage tissue is the chondrocyte and its main function is to maintain the extracellular matrix. The extracellular matrix in healthy cartilage consists predominantly of type II collagen fibers (>90%) with lesser amounts of type VI, IX and XI collagen [1]. In addition, to the collagenous molecules that provide a mesh-like framework responsible for the tensile properties, there are the non-collagenous molecules represented by a proteoglycan component. These confer the shock-absorbing properties of the matrix, due to the highly sulfated aggrecan monomers attached to the hyaluronic acid and to the link protein. The cartilage environment, therefore, is a hydrophilic environment that absorbs and retains large amounts of water. For this reason, 60–85% of cartilage tissue is made up of water. Moreover, as the weight bearing material of diarthrodial joints, the main function of articular cartilage is to produce a low friction surface capable of withstanding in vivo load in the mega Pascal range [9].
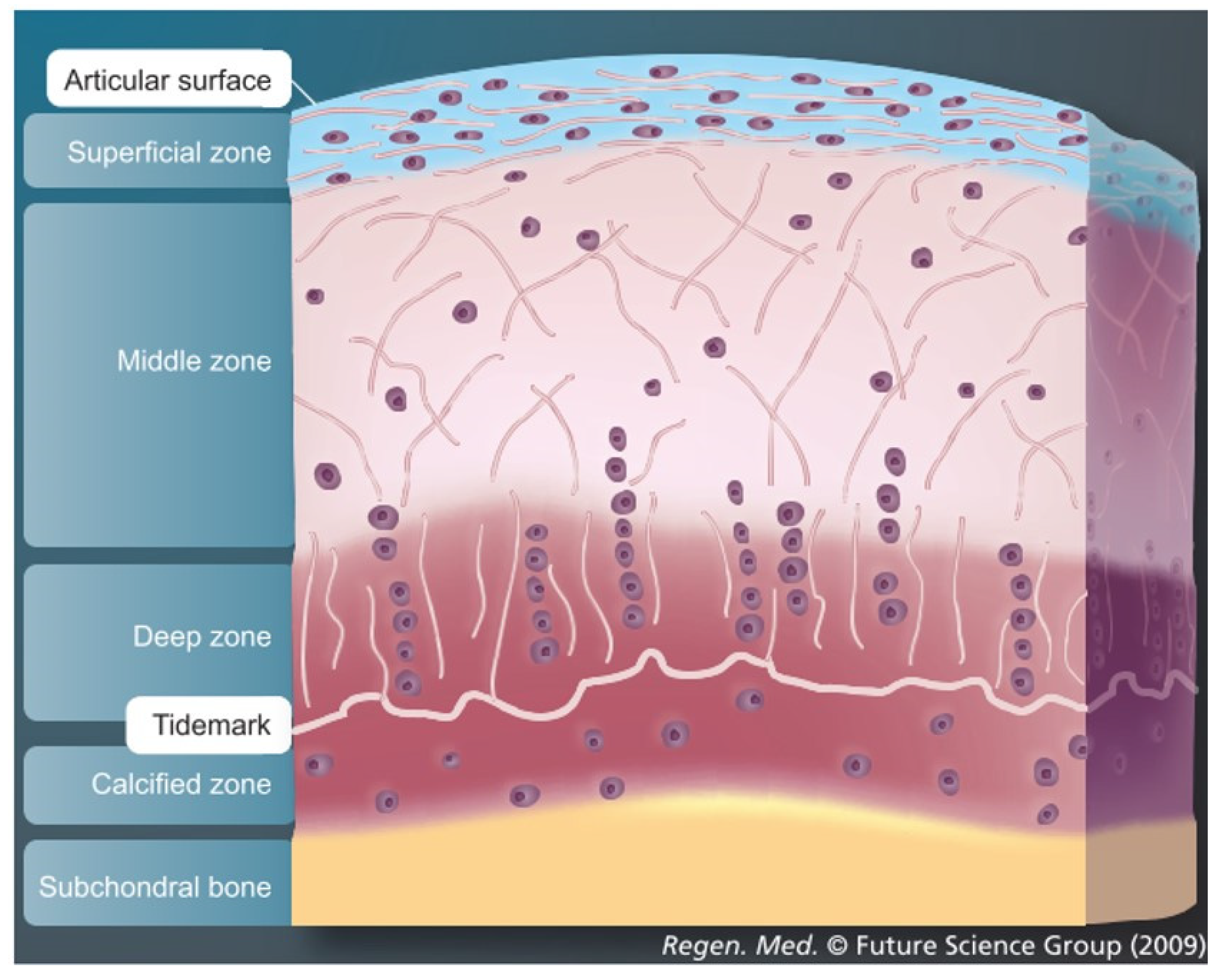
As articular cartilage is an avascular, aneural and alymphatic tissue, it is highly suited to dissipate and absorb load. However, the low metabolic activity of the mature tissue exerts a detrimental effect on its regeneration. Therefore, articular cartilage once damaged through trauma or disease has limited repair capacity.
Cartilage Defects and Healing Response
Cartilage defects can be divided into three major classes: partial thickness defects, full thickness defects and osteochondral defects depending on the depth of the damage [10]. In partial or full thickness defects, the damage is restricted entirely to the cartilaginous tissue and does not penetrate the subchondral bone. Partial thickness defects differ from full thickness defects because they do not span the whole depth of the articular cartilage, while full thickness defects, although affecting the whole thickness of the articular cartilage, do not penetrate the subchondral bone [11]. Cartilage defects present a unique clinical challenge due to its poor self-healing capacity, largely dependent on its’ avascular nature that impede the blood cells and bone marrow MSCs from the surrounding environment reaching the defect and contributing to the healing response which normally occurs in vascularized tissue [12][13]. Moreover, cartilage tissue has a very low cell number content and the main cellular component, the chondrocyte, has a limited metabolic activity, proliferation and biosynthesis [14]. If cartilage damage is left untreated, not only the surrounding cartilage will be pathologically affected, but also the subchondral bone [15].
In osteochondral defects, the damage penetrates the subchondral bone and this event enables a rudimentary healing response. Blood first enters the lesion from damaged vasculature or from bone marrow and a fibrin clot is formed [16][17]. Platelets are trapped and release bioactive factors such as platelet derived growth factor (PDGF) and transforming growth factor beta (TGF-β). These factors then attract vessels and mesenchymal progenitors into the defect [17]. Unfortunately, this repair response often leads to the generation of a mechanically inferior fibrocartilage-like repair tissue, which is unable to withstand normal joint load and ultimately degenerates further [18]. Untreated defects, or failed treatments, can progress to osteoarthritis, which ultimately can lead to total joint replacement.
2. Ex Vivo Systems to Study Chondrogenic Differentiation and Cartilage Integration
The most advanced ex vivo models include the co-culture of at least two different cell types combined with proper tissue engineering strategies and loading motions aiming to reproducing the complex structure and function of the native tissue by using biomimetic scaffolds and suitable biological cues. These models represent reliable systems to reduce the gap existing between the complexity of the in vivo environment and the simplicity of in vitro condition thereby decreasing the needs of animal studies. As such, the ex vivo systems are useful tools to investigate, in a more controlled environment, the complexity of the in vivo physiological and pathological processes and in so doing, they allow to better prevent in vitro artefacts and to achieve more truthful results if compared with previous simpler models.
The further development of new ex vivo models approximating an in vivo environment, is a promising approach to improve our knowledge of the biological mechanisms underlying cartilage regeneration process. Future studies should aim to better elucidate the crosstalk mechanisms between the different cell types involved in osteochondral repair and should consider that several cytokines secreted by bone cells can lead to chondrocyte differentiation [19]. Understanding the dialogue between cartilage and underlying bone might be the key to shed light on the molecular signaling pathways of physio-pathological conditions and may help to restore the healthy situation. The dialogue between the different cell types might also be affected and regulated by the location of the cells within the tissue and the distance between the source of the stimulus. Thus, a specific stimulus might direct cell behavior as a zonal-dependent cell response (Figure 2).
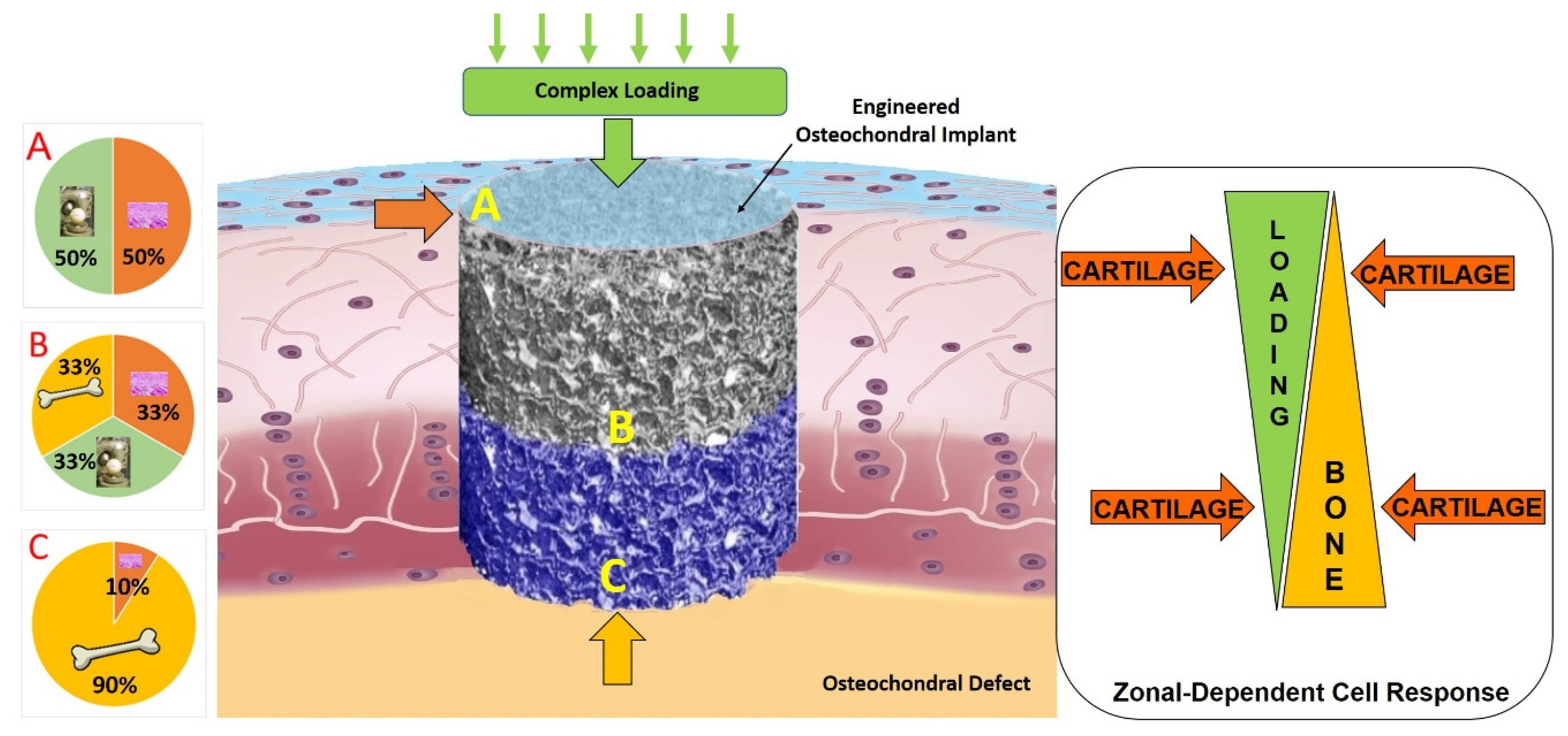
Indeed, it is also noteworthy to highlight that biomechanical factors profoundly influence the processes of tissue growth, development, maintenance, degeneration, and repair. Therefore, the ability to apply joint kinematic motion through the appropriate bioreactors allows for a more physiological system to study in-vitro or ex-vivo cartilage regeneration mechanisms, to create living tissue replacements and to test new potential cartilage repair therapies.
References
- Stoddart, M.J.; Grad, S.; Eglin, D.; Alini, M. Cells and biomaterials in cartilage tissue engineering. Regen. Med. 2009, 4, 81–98.
- Klein, T.J.; Malda, J.; Sah, R.L.; Hutmacher, D.W. Tissue Engineering of Articular Cartilage with Biomimetic Zones. Tissue Eng. Part B Rev. 2009, 15, 143–157.
- Baumann, C.A.; Hinckel, B.B.; Bozynski, C.C.; Farr, J. Articular Cartilage: Structure and Restoration; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–24.
- Darling, E.M.; Athanasiou, K.A. Retaining Zonal Chondrocyte Phenotype by Means of Novel Growth Environments. Tissue Eng. 2005, 11, 395–403.
- Darling, E.M.; Hu, J.C.Y.; Athanasiou, K.A. Zonal and topographical differences in articular cartilage gene expression. J. Orthop. Res. 2004, 22, 1182–1187.
- Klein, T.J.; Schumacher, B.L.; Schmidt, T.A.; Li, K.W.; Voegtline, M.S.; Masuda, K.; Thonar, E.-M.; Sah, R.L. Tissue engineering of stratified articular cartilage from chondrocyte subpopulations. Osteoarthr. Cartil. 2003, 11, 595–602.
- Aydelotte, M.B.; Kuettner, K.E. Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect. Tissue Res. 1988, 18, 205–222.
- Aydelotte, M.B.; Greenhill, R.R.; Kuettner, K.E. Differences between sub-populations of cultured bovine articular chondro-cytes. II. Proteoglycan metabolism. Connect. Tissue Res. 1998, 18, 223–234.
- Hodge, W.A.; Fijan, R.S.; Carlson, K.L.; Burgess, R.G.; Harris, W.H.; Mann, R.W. Contact pressures in the human hip joint measured in vivo. Proc. Natl. Acad. Sci. USA 1986, 83, 2879–2883.
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721.
- Hunziker, E.B. Biologic repair of articular cartilage. Defect models in experimental animals and matrix requirements. Clin. Orthop. Relat. Res. 1999, 367, S135–S146.
- Hunziker, E.B.; Rosenberg, L.C. Repair of partial-thickness defects in articular cartilage: Cell recruitment from the synovial membrane. J. Bone Jt. Surg. Am. 1996, 78, 721–733.
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage repair and transplantation. Arthritis Rheum. 1998, 41, 1331–1342.
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and structure of articular cartilage: A template for tissue repair. Clin. Orthop. Relat. Res. 2001, 391, S26–S33.
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237.
- Buckwalter, J.A. Articular Cartilage: Injuries and Potential for Healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202.
- Steinert, A.F.; Ghivizzani, S.C.; Rethwilm, A.; Tuan, R.S.; Evans, C.H.; Nöth, U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res. Ther. 2007, 9, 213.
- Hunziker, E. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463.
- Funck-Brentano, T.; Cohen-Solal, M. Crosstalk between cartilage and bone: When bone cytokines matter. Cytokine Growth Factor Rev. 2011, 22, 91–97.




