
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elicia Wong | + 2512 word(s) | 2512 | 2021-01-18 07:34:22 | | | |
| 2 | Camila Xu | Meta information modification | 2512 | 2021-01-19 08:48:15 | | | | |
| 3 | Camila Xu | Meta information modification | 2512 | 2021-01-19 08:48:56 | | |
Video Upload Options
Nanozymes are advanced nanomaterials which possess unique physicochemical properties with the precise structural fabrication capability to mimic intrinsic biologically relevant reactions.
1. General Introduction to Nanozymes
Nanozymes are advanced nanomaterials which possess unique physicochemical properties with the precise structural fabrication capability to mimic intrinsic biologically relevant reactions. Specifically, nanozymes mimic natural enzymes and exhibit enzyme-like properties. The enzymatic catalytic reactions are highly effective, with reactions occurring rapidly even under mild conditions, and more importantly, such reactions are also highly selective. The high efficiency and selectivity are immensely desirable properties for sensing and monitoring applications. However, natural enzymes, including proteins, suffer from limitations such as low thermostability and narrow pH window, which will denature the enzymes and greatly reduce and inhibit their enzymatic activities. Low thermostability also places stringent requirements on the storage, transportation and handling of natural enzymes, which can be labour- and infrastructure-intensive for the users. Susceptible denaturation adds complexity to the interpretation of sensing and monitoring outputs, which may yield a false positive/negative outcome. From this perspective, nanozymes address these limitations by offering high structural durability and stability while maintaining desirable catalytic activities.
By incorporating the unique physicochemical properties and enzyme-like activities, nanozymes exhibit promising applications in different fields such as the biomedical sector (in vivo diagnostics/and therapeutics) and the environmental sector (detection and remediation of inorganic and organic pollutants). The biomedical and clinical translation of nanozymes have been extensively reviewed [1][2][3][4], from their applications in immunoassays [5][6][7][8][9][10] to cancer diagnostics and therapeutics [11][12][13][14][15]. As nanozymes offer better structural stability over their respective natural enzymes, with wider physical (e.g., temperature) and chemical (e.g., pH) operational windows, they are ideal candidates for real-time and/or remote environmental monitoring and remediation. This is especially so given the challenging and unpredictable nature of the outdoor environment (compared to a more physiologically stable in vivo or in vitro environment with a more defined and narrower operational window).
2. Types of Nanozymes
Over the last few decades, various types of nanomaterials have been reported to have intrinsic enzyme-like activities [16][17]. Natural enzymes exhibit intrinsic catalytic ability, usually at a single active site, to catalyse a specific chemical transformation [18][19]. Since nanozymes lack such an active site, different strategies have been devised to enhance the catalytic properties of these nanomaterials, enabling them to selectively and effectively react with target molecules. In this review, we categorise nanozymes into four types based on their mode of natural enzyme-mimicking behaviour (Figure 1).
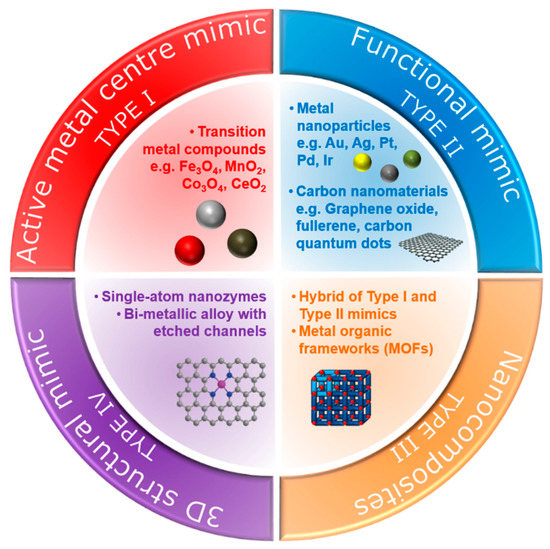
Figure 1. Types of nanozymes based on their mode of natural enzyme-mimicking behaviour.
One strategy utilises constructed metal sites (type I nanozymes), such as metal oxides or metal sulphides, to mimic the metal catalytic active site found in metalloenzymes [20]. An early example of such nanozymes is iron oxide (Fe3O4) nanoparticles, as reported by Gao et al. [6], which exhibit peroxidase-like activity similar to the natural horseradish peroxidase (HRP) enzyme. Peroxidases catalyse the oxidation of a chromogenic substrate, such as 3,3′,5,5′-tetramethylbenzidine (TMB), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), o-phenylenediamine dihydrochloride (OPD), in the presence of hydrogen peroxide, H2O2. The smallest Fe3O4 nanoparticles (out of 30, 150 and 300 nm) were found to have the highest catalytic activity, which showed that the high surface area of the Fe3O4 nanoparticles was responsible for the peroxidase-like activity. Gao et al. [6] postulated that the active surface ferrous and ferric ions in the nanoparticles were the key components that enabled this catalytic activity, mimicking the iron–heme binding site in HRP. Cerium oxide (CeO2) nanoparticles also utilise their metal as a nanozyme due to its similarity in structure and biochemistry to the iron ion, particularly in binding to proteins [21]. In fact, CeO2 nanoparticles are multifunctional catalysts whereby they exhibit catalase-like (breakdown of H2O2 into O2 and H2O) and superoxidase-like (dismutation of O2•− into O2 and H2O2) activities in addition to bearing peroxidase-like activity. The multifunctional catalytic behaviour arises from the coexistence of Ce(III) and Ce(IV) oxidation states. The switch between the III/IV valence resembles the mechanism of redox enzymes, which use metals as cofactors to catalyse a range of reversible redox reactions [16]. As such, reactions comprising redox cycles between Ce(III) and Ce(IV) oxidation states make it possible for CeO2 nanoparticles to react catalytically with oxygen radicals and hydrogen peroxide, thus mimicking the function of two key antioxidant enzymes, namely superoxide and catalase [22]. Similar to the Fe3O4 nanoparticles, the enzyme-mimicking behaviours of CeO2 are also size-dependent, whereby the smaller CeO2 (5 nm) have a superior catalytic activity to that of larger (28 nm) nanoparticles [23]. This is further exemplification that the catalytic activities of the nanozymes are dependent on the amount of catalytically active atoms exposed on the surface (where the catalytic mechanism is related to changes in metal valence), which is usually inversely proportional to the diameter of the nanoparticles.
Other common metal–heme centres found in metalloenzymes include copper, cobalt and manganese ions (Table 1). As such, nanoparticles synthesised from cobalt oxide (Co3O4) [24], cobalt sulphide (Co9S8) [25], copper oxide (CuO) [26] and manganese dioxide (MnO2) [27][28] are also known to possess either peroxidase, oxidase and/or catalase-mimicking activities.
Table 1. Examples of metalloenzymes.
| Metal Centre | Enzymes |
|---|---|
| Zinc | Carbonic anhydrase, alcohol dehydrogenase, organophosphate hydrolase |
| Iron | Catalase, peroxidase, cytochrome oxidase |
| Manganese | Enolase, hexokinase |
| Copper | Tyrosinase, lysyl oxidase, laccase |
| Cobalt | Dipeptidase |
While the type I metal compound nanoparticles mimic the metal–heme redox centre of metalloenzymes, other metal nanoparticles that catalyse the same reactions as natural enzymes are classified as type II nanozymes. These metal nanoparticles are synthesised from metals that exhibit intrinsic catalytic behaviour for various heterogeneous reactions. Such metals include gold, silver, platinum, palladium and iridium [29][30][31]. Rossi and co-workers reported that gold nanoparticles, under controlled conditions (unprotected “naked” nanoparticles, 3.6 nm diameter, in the presence of excess glucose) could initially catalyse reactions similar to glucose oxidase and thus serve as a mimic for glucose oxidase [32]. Moreover, gold nanoparticles also showed peroxidase-mimicking activity [33][34]. Chen and co-workers [33] demonstrated that unmodified gold nanoparticles had significantly higher catalytic behaviours towards peroxidase substrates, which indicated that the superficial gold atoms were the key component to the observed peroxidase-like activity. Modified gold nanoparticles with different surface charges (positive or negative) also exhibited peroxidase-mimicking activity [34]. In fact, it was found that their enzyme-mimetic activities could be modulated by changing the pH of the environment (i.e., pH-switchable). Gao and co-workers [35] demonstrated that gold, silver, platinum and palladium nanomaterials exhibited peroxidase-like activities at acidic pH and catalase-like activities at basic pH [35]. The pH-switchable phenomenon was further investigated by Nie and co-workers using 1–2-nm platinum nanoparticles, which showed that the catalase-like activity was evident under basic conditions while the peroxidase-like activity was more dominant under acidic conditions [36]. The catalytic mechanism of the metal nanoparticles is different from the metal compound-based nanozymes and is related to the adsorption, activation and electron transfer of substrate (e.g., TMB, ABTS or OPD) on metal surfaces rather than a change in the metal valence of the nanomaterial.
Another intriguing aspect of metal-based nanozymes is that they can form alloys with different elemental compositions [37]. By combining the independent electronic characteristics of two metals, bimetallic nanoparticles can further exhibit unique properties through the synergetic effect of the two metals [38]. Thus, this makes it feasible to tailor the enzyme-mimicking activities by adjusting alloy compositions, classified here as type III nanozymes. In one example, He et al. showed that for Ag-M (M = Au, Pd, Pt) bimetallic alloy-based peroxidase nanozymes, the efficiency of the catalytic activity could be tuned by gradually changing the ratio of the two metals [39]. They suggested that the composition-dependent activity was from the electronic structure due to alloying. In another example, further enhancement of the multi-enzymatic activities was demonstrated by Yin, Wu and co-workers [40] using Au-Pt bimetallic nanoparticles by controlling the Pt and Au molar ratio to exhibit oxidase, peroxidase and catalase-like activities. The enhanced peroxidase-like activity of Ir-Pd nanocubes was obtained by depositing an Ir atomic layer on the surface of Pd nanocubes [41]. It was postulated that the adsorption energy of the Ir-Pd(100) surface was larger than that of the Pd(100), making it more energy-efficient to dissociate hydrogen peroxides into hydroxyl radicals.
Non-metallic nanozymes such as carbon-based nanomaterials, including fullerene and their derivatives, carbon quantum dots, carbon nanotubes, and graphene oxide, show great promise enzyme-mimicking capability owing to their intrinsic catalytic properties. The peroxidase, catalase and oxidase-like activities have all been reported [42][43][44][45][46][47]. In one example, Shi et al. [44] reported that carbon quantum dots exhibited peroxidase-like catalytic activity. It was concluded that the catalytic mechanism came from an increase in the electron density and mobility in the carbon quantum dots acting as effective catalytically active sites. Qu and co-workers [45] also reported that carboxyl-modified graphene oxide exhibited peroxidase-like activity, with electron transfer occurring from the top of the valence band of graphene to the lowest unoccupied molecular orbital of hydrogen peroxide. To further lower this bandgap and improve the peroxidase-like behaviour, Kim et al. [46] co-doped the graphene oxide with nitrogen and boron and demonstrated a much higher catalytic behaviour than undoped graphene oxide. Besides peroxidase-like behaviour, the catalase-like behaviour was reported by Ren et al. [47] using graphene oxide quantum dots.
Metal–carbon nanocomposites have also been investigated as a strategy to further improve the catalytic activities of carbon nanozymes [48][49][50][51]. An oxidase-like nanozyme, catalysing an oxidation-reduction reaction involving oxygen as an electron acceptor, was constructed using a metal-carbon nanocomposite hybrid through doping a N-rich porous carbon with Fe nanoparticles [51]. The group suggested that the N-doped porous carbon acted as the binding sites to trap and transfer O2 molecules to catalytic sites and subsequently catalysed their redox reaction with the Fe nanoparticles. In another example, Guo, Zhang and co-workers [48] integrated graphene quantum dots with Fe3O4 nanoparticles and demonstrated superior peroxidase-like activities compared to individual graphene quantum dots and Fe3O4 nanoparticles. This superiority was attained from the synergistic interactions between graphene quantum dots and the Fe3O4 nanoparticles. Compared to the native HRP, this nanocomposite showed comparable, if not better, removal efficiencies for some phenolic compounds from aqueous solution, rendering it useful for industrial wastewater treatment [48].
All metal-, metal-compound- and carbon-based nanozymes rely on the high surface area, enabled either through small particle size (of the order of tens of nanometres for metal- or metal-compound-based nanozymes) or porous structure (carbon-based nanozymes) to maximise the exposure of the catalytically active atoms. Metal-organic frameworks (MOFs) which consist of metal ions as nodes and organic ligands as linkers also have highly porous structures that can be utilised as nanozymes. In this construct, the transition metal nodes containing the MOFs themselves can act as biomimetic catalysts, while the high porosity structure created by the metal-organic linkers can serve as the binding sites for the substrates. Their tuneable pore sizes, highly specific surface areas and exposed active sites provide MOFs with high catalytic efficiency [52]. In one example, Li and co-workers [53] demonstrated the use of a nanosized MOF, Fe-MIL-88NH2, as a peroxidase mimic. The catalytic mechanism was proposed as follows: hydrogen peroxide was adsorbed onto the surface or into the mesopores of Fe-MIL-88NH2, and the hydrogen peroxide was decomposed into hydroxyl radicals by iron. Other than the Fe-MOF, Cu-MOF [54], Ni-MOF [55], Pt-MOF [56] and Co/2Fe MOF [57] are also known to exhibit peroxidase-like behaviours. The bimetallic-MOF, Co/2Fe-MOF, exhibited dual enzymatic activities, peroxidase and oxidase. Additionally, Min, Chu and co-workers showed that CeO2-MOF acted as a hydrolase mimic (breakage of a chemical bond using water) to remove a phosphate group, PO43−, from phosphopeptides [58]. Prussian Blue nanoparticles are an analogue of MOFs which can simultaneously behave as multienzyme mimics (peroxidase, superoxide dismutase and catalase-like activities) and were used effectively as a scavenger for reactive oxygen species [59].
Although the aforementioned metal-compound-, metal-, carbon-, nanocomposite- and MOF-based nanomaterials show promising enzyme-mimicking abilities, achieving the same level of binding affinity and specificity as natural enzymes remains a challenge. The limiting factors include (i) the density of the catalytically active surface ions (such as the metal ions) and functional groups (such as the carboxyl group in the carbon nanomaterials), and (ii) the efficiency of the catalytic mechanism. It has been demonstrated that nanozymes with a low density of active sites show much lower catalytic activities [60]. Additionally, the elemental composition and facet structure of these nanozymes cause the catalytic mechanism of nanozymes to be different and are usually more intricate than natural enzymes [37][61]. These limitations constrain the extensive applications of these standard nanozymes. Consequently, new strategies have emerged to mitigate these constraints through spatial or three-dimensional structural mimicking of the active sites of natural enzymes [62][63]. These structural mimics can be achieved by mimicking the geometry of pre-existing metal binding centres, the binding sites at the peripheral or the confined and empty space at the centre of natural enzymes (type IV nanozymes).
Single-atom nanozymes resemble spatial structures to mimic the electronic, geometric and chemical structure of the pre-existing metal binding centre of metalloenzymes. For example, the FeN4 in iron-based single-atom nanozymes mimic the active sites of oxymyoglobin, HRP and cytochrome P450 enzymes which contain a single heme Fe with a proximal ligand (Figure 2) [64][65]. In particular, Huang, Zhu and co-workers reported that densely isolated FeN4 single-atom nanozymes exhibited outstanding peroxidase-like activities [66]. Both their experimental and theoretical analyses showed that FeN4 led to the strong adsorption of hydrogen peroxide, weakened the bonding between the single Fe atom and the two adsorbed hydroxyl groups and lowered the energy barrier for the formation of hydroxyl radicals to boost the peroxidase-like activities. Additionally, other metals such as cobalt and zinc could also be used to create CoN4, and ZnN4 single-atom nanozymes that exhibited peroxidase-like activities [66]. The addition of an axial N coordination to form FeN5 single-atom nanozymes enhanced the oxidase-like behaviour of the Fe based single-atom nanozymes [62]. The FeN5 structure had the most adsorption energy, by promoting strong oxygen adsorption that led to weakening of the O–O bond. Wang, Dong and co-workers [62] postulated that the weakening of the O–O bond was a result of the electron donor via the electron push effect of the axial-coordinated N in the FeN5 single-atom nanozyme.
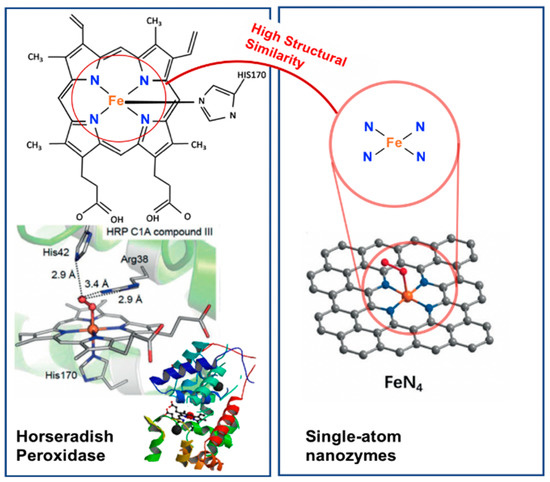
Figure 2. A comparison of the HRP enzyme and FeN4 single-atom nanozyme showing the high structural similarity of the single-atom nanozyme to the active centre (iron-heme group) of HRP. (Adapted with permission from [64]. Copyright © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany).
Other structure-mimicking strategies have been achieved through the creation of binding sites, such as nano-channels at the nanozymes, by resembling the binding sites of natural enzymes either at the periphery or in the centre (usually a confined empty cavity); see Figure 3. In a pioneering example, Schuhmann, Tilley, Gooding and co-workers designed a nanoparticle that mimicked the 3D architecture of a natural enzyme by using surfactant-covered PtNi bimetallic nanoparticles [63][67]. In their design, the surfactant-covered PtNi particles were selectively etched to create nano-channels that were specific for catalysing the reduction reaction of oxygen. The group reported that the oxygen reduction reaction activity normalised by the electrochemically active surface area was enhanced by a factor of 3.3 for the nanozymes compared to the unetched PtNi nanoparticles.
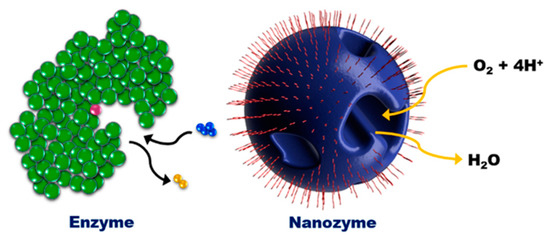
Figure 3. Illustration of a nanozyme as a 3D geometric architectural mimic of an enzyme. (Reprinted with permission from [63]. Copyright © 2018, American Chemical Society).
References
- Jiang, D.W.; Ni, D.L.; Rosenkrans, Z.T.; Huang, P.; Yan, X.Y.; Cai, W.B. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704, doi:10.1039/c8cs00718g.
- Wang, Q.Q.; Wei, H.; Zhang, Z.Q.; Wang, E.K.; Dong, S.J. Nanozyme: An emerging alternative to natural enzyme for bio-sensing and immunoassay. Trends Anal. Chem. 2018, 105, 218–224, doi:10.1016/j.trac.2018.05.012.
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412, doi:10.1021/acs.chemrev.8b00672.
- Wang, X.Y.; Hu, Y.H.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2016, 3, 41–60, doi:10.1039/c5qi00240k.
- Gao, Y.; Zhou, Y.Z.; Chandrawati, R. Metal and metal oxide nanoparticles to enhance the performance of enzyme-linked immunosorbent assay (ELISA). ACS Appl. Nano Mater. 2020, 3, 1–21, doi:10.1021/acsanm.9b02003.
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583, doi:10.1038/nnano.2007.260.
- Gao, Z.Q.; Xu, M.D.; Hou, L.; Chen, G.N.; Tang, D.P. Magnetic bead-based reverse colorimetric immunoassay strategy for sensing biomolecules. Anal. Chem. 2013, 85, 6945–6952, doi:10.1021/ac401433p.
- Wang, Z.F.; Zheng, S.; Cai, J.; Wang, P.; Feng, J.; Yang, X.; Zhang, L.M.; Ji, M.; Wu, F.G.; He, N.Y.; et al. Fluorescent artificial enzyme-linked immunoassay system based on Pd/C nanocatalyst and fluorescent chemodosimeter. Anal. Chem. 2013, 85, 11602–11609, doi:10.1021/ac403001y.
- Dong, J.L.; Song, L.N.; Yin, J.J.; He, W.W.; Wu, Y.H.; Gu, N.; Zhang, Y. Co3O4 nanoparticles with multi-enzyme activities and their application in immunohistochemical assay. ACS Appl. Mater. Interfaces 2014, 6, 1959–1970, doi:10.1021/am405009f.
- Gao, Z.Q.; Xu, M.D.; Lu, M.H.; Chen, G.N.; Tang, D.P. Urchin-like (gold core)@(platinum shell) nanohybrids: A highly effi-cient peroxidase-mimetic system for in situ amplified colorimetric immunoassay. Biosens. Bioelectron. 2015, 70, 194–201, doi:10.1016/j.bios.2015.03.039.
- Huo, M.F.; Wang, L.Y.; Chen, Y.; Shi, J.L. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017, 8, 357, doi:10.1038/s41467-017-00424-8.
- Wang, G.L.; Xu, X.F.; Qiu, L.; Dong, Y.M.; Li, Z.J.; Zhang, C. Dual responsive enzyme mimicking activity of AgX (X = Cl, Br, I) nanoparticles and its application for cancer cell detection. ACS Appl. Mater. Interfaces 2014, 6, 6434–6442, doi:10.1021/am501830v.
- Maji, S.K.; Mandal, A.K.; Nguyen, K.T.; Borah, P.; Zhao, Y.L. Cancer cell detection and therapeutics using peroxidase-active nanohybrid of gold nanoparticle-loaded mesoporous silica-coated graphene. ACS Appl. Mater. Interfaces 2015, 7, 9807–9816, doi:10.1021/acsami.5b01758.
- Tian, Z.M.; Li, J.; Zhang, Z.Y.; Gao, W.; Zhou, X.M.; Qu, Y.Q. Highly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detection. Biomaterials 2015, 59, 116–124, doi:10.1016/j.biomaterials.2015.04.039.
- Asati, A.; Kaittanis, C.; Santra, S.; Perez, J.M. pH-tunable oxidase-like activity of cerium oxide nanoparticles achieving sensi-tive fluorigenic detection of cancer biomarkers at neutral pH. Anal. Chem. 2011, 83, 2547–2553, doi:10.1021/ac102826k.
- Wei, H.; Wang, E.K. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093, doi:10.1039/c3cs35486e.
- Wu, J.J.X.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteris-tics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076, doi:10.1039/c8cs00457a.
- Kazlauskas, R.J. Enhancing catalytic promiscuity for biocatalysis. Curr. Opin. Chem. Biol. 2005, 9, 195–201, doi:10.1016/j.cbpa.2005.02.008.
- Khersonsky, O.; Roodveldt, C.; Tawfik, D.S. Enzyme promiscuity: Evolutionary and mechanistic aspects. Curr. Opin. Chem. Biol. 2006, 10, 498–508, doi:10.1016/j.cbpa.2006.08.011.
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and functional aspects of metal sites in biology. Chem. Rev. 1996, 96, 2239–2314, doi:10.1021/cr9500390.
- Palizban, A.A.; Sadeghi-Aliabadi, H.; Abdollahpour, F. Effect of cerium lanthanide on Hela and MCF-7 cancer cell growth in the presence of transferrin. Res. Pharm. Sci. 2010, 5, 119–125. doi.
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420, doi:10.1039/c0nr00875c.
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 2018, 10, 6971–6980, doi:10.1039/c8nr00325d.
- Mu, J.S.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540–2542, doi:10.1039/c2cc17013b.
- Lin, S.B.; Wang, Y.Y.; Chen, Z.Z.; Li, L.B.; Zeng, J.F.; Dong, Q.R.; Wang, Y.; Chai, Z.F. Biomineralized enzyme-like cobalt sulfide nanodots for synergetic phototherapy with tumor multimodal imaging navigation. ACS Sustain. Chem. Eng. 2018, 6, 12061–12069, doi:10.1021/acssuschemeng.8b02386.
- Chen, W.; Chen, J.; Liu, A.L.; Wang, L.M.; Li, G.W.; Lin, X.H. Peroxidase-like activity of cupric oxide nanoparticle. Chem-CatChem. 2011, 3, 1151–1154, doi:10.1002/cctc.201100064.
- Wan, Y.; Qi, P.; Zhang, D.; Wu, J.J.; Wang, Y. Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2012, 33, 69–74, doi:10.1016/j.bios.2011.12.033.
- Pijpers, I.A.B.; Cao, S.; Llopis-Lorente, A.; Zhu, J.; Song, S.; Joosten, R.R.M.; Meng, F.; Friedrich, H.; Williams, D.S.; Sánchez, S.; et al. Hybrid biodegradable nanomotors through compartmentalized synthesis. Nano Lett. 2020, 20, 4472–4480, doi:10.1021/acs.nanolett.0c01268.
- He, W.W.; Zhou, Y.T.; Warner, W.G.; Hu, X.N.; Wu, X.C.; Zheng, Z.; Boudreau, M.D.; Yin, J.J. Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials 2013, 34, 765–773, doi:10.1016/j.biomaterials.2012.10.010.
- Liu, C.P.; Wu, T.H.; Lin, Y.L.; Liu, C.Y.; Wang, S.; Lin, S.Y. Tailoring enzyme-like activities of gold nanoclusters by polymeric tertiary amines for protecting neurons against oxidative stress. Small 2016, 12, 4127–4135, doi:10.1002/smll.201503919.
- Long, R.; Huang, H.; Li, Y.P.; Song, L.; Xiong, Y.J. Palladium-based nanomaterials: A platform to produce reactive oxygen species for catalyzing oxidation reactions. Adv. Mater. 2015, 27, 7025–7042, doi:10.1002/adma.201502068.
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The catalytic activity of "naked" gold particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815, doi:10.1002/anie.200460446.
- Wang, S.; Chen, W.; Liu, A.L.; Hong, L.; Deng, H.H.; Lin, X.H. Comparison of the peroxidase-like activity of unmodified, amino-modified, and citrate-capped gold nanoparticles. ChemPhysChem. 2012, 13, 1199–1204, doi:10.1002/cphc.201100906.
- Jv, Y.; Li, B.X.; Cao, R. Positively-charged gold nanoparticles as peroxidiase mimic and their application in hydrogen peroxide and glucose detection. Chem. Commun. 2010, 46, 8017–8019, doi:10.1039/c0cc02698k.
- Li, J.N.; Liu, W.Q.; Wu, X.C.; Gao, X.F. Mechanism of pH-switchable peroxidase and catalase-like activities of gold, silver, platinum and palladium. Biomaterials 2015, 48, 37–44, doi:10.1016/j.biomaterials.2015.01.012.
- Fan, J.; Yin, J.J.; Ning, B.; Wu, X.C.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.Y.; Zhao, Y.L.; Nie, G.J. Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomaterials 2011, 32, 1611–1618, doi:10.1016/j.biomaterials.2010.11.004.
- Shen, X.M.; Liu, W.Q.; Gao, X.J.; Lu, Z.H.; Wu, X.C.; Gao, X.F. Mechanisms of oxidase and superoxide dismutation-like activ-ities of gold, silver, platinum, and palladium, and their alloys: A general way to the activation of molecular oxygen. J. Am. Chem. Soc. 2015, 137, 15882–15891, doi:10.1021/jacs.5b10346.
- Xu, Y.; Chen, L.; Wang, X.C.; Yao, W.T.; Zhang, Q. Recent advances in noble metal based composite nanocatalysts: Colloidal synthesis, properties, and catalytic applications. Nanoscale 2015, 7, 10559–10583, doi:10.1039/c5nr02216a.
- He, W.W.; Wu, X.C.; Liu, J.B.; Hu, X.N.; Zhang, K.; Hou, S.A.; Zhou, W.Y.; Xie, S.S. Design of AgM bimetallic alloy nanostructures (M = Au, Pd, Pt) with tunable morphology and peroxidase-like activity. Chem. Mater. 2010, 22, 2988–2994, doi:10.1021/cm100393v.
- He, W.W.; Liu, Y.; Yuan, J.S.; Yin, J.J.; Wu, X.C.; Hu, X.N.; Zhang, K.; Liu, J.B.; Chen, C.Y.; Ji, Y.L.; et al. Au@Pt nanostruc-tures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials 2011, 32, 1139–1147, doi:10.1016/j.biomaterials.2010.09.040.
- Xia, X.H.; Zhang, J.T.; Lu, N.; Kim, M.J.; Ghale, K.; Xu, Y.; McKenzie, E.; Liu, J.B.; Yet, H.H. Pd-Ir core-shell nanocubes: A type of highly efficient and versatile peroxidase mimic. ACS Nano 2015, 9, 9994–10004, doi:10.1021/acsnano.5b03525.
- Garg, B.; Bisht, T. Carbon nanodots as peroxidase nanozymes for biosensing. Molecules 2016, 21, 1653, doi:10.3390/molecules21121653.
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon nanozymes: Enzymatic properties, catalytic mechanism, and applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237, doi:10.1002/anie.201712469.
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697, doi:10.1039/C1CC11943E.
- Song, Y.J.; Qu, K.G.; Zhao, C.; Ren, J.S.; Qu, X.G. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210, doi:10.1002/adma.200903783.
- Kim, M.S.; Cho, S.; Joo, S.H.; Lee, J.; Kwak, S.K.; Kim, M.I. N- and B-codoped graphene: A strong candidate to replace natu-ral peroxidase in sensitive and selective bioassays. ACS Nano 2019, 13, 4312–4321, doi:10.1021/acsnano.8b09519.
- Ren, C.X.; Hu, X.G.; Zhou, Q.X. Graphene oxide quantum dots reduce oxidative stress and inhibit neurotoxicity in vitro and in vivo through catalase-like activity and metabolic regulation. Adv. Sci. 2018, 5, 1700595, doi:10.1002/advs.201700595.
- Wu, X.C.; Zhang, Y.; Han, T.; Wu, H.X.; Guo, S.W.; Zhang, J.Y. Composite of graphene quantum dots and Fe3O4 nanoparti-cles: Peroxidase activity and application in phenolic compound removal. RSC Adv. 2014, 4, 3299–3305, doi:10.1039/c3ra44709j.
- Dong, Y.M.; Zhang, J.J.; Jiang, P.P.; Wang, G.L.; Wu, X.M.; Zhao, H.; Zhang, C. Superior peroxidase mimetic activity of car-bon dots-Pt nanocomposites relies on synergistic effects. New J. Chem. 2015, 39, 4141–4146, doi:10.1039/c5nj00012b.
- Zheng, C.; Ke, W.J.; Yin, T.X.; An, X.Q. Intrinsic peroxidase-like activity and the catalytic mechanism of gold@carbon dots nanocomposites. RSC Adv. 2016, 6, 35280–35286, doi:10.1039/c6ra01917j.
- Chen, Q.M.; Liang, C.H.; Zhang, X.D.; Huang, Y.M. High oxidase-mimic activity of Fe nanoparticles embedded in an N-rich porous carbon and their application for sensing of dopamine. Talanta 2018, 182, 476–483, doi:10.1016/j.talanta.2018.02.032.
- Farha, O.K.; Shultz, A.M.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Active-site-accessible, porphyrinic metal-organic frame-work materials. J. Am. Chem. Soc. 2011, 133, 5652–5655, doi:10.1021/ja111042f.
- Liu, Y.L.; Zhao, X.J.; Yang, X.X.; Li, Y.F. A nanosized metal-organic framework of Fe-MIL-88NH2 as a novel peroxidase mimic used for colorimetric detection of glucose. Analyst 2013, 138, 4526–4531, doi:10.1039/c3an00560g.
- Wang, C.H.; Gao, J.; Cao, Y.L.; Tan, H.L. Colorimetric logic gate for alkaline phosphatase based on copper (II)-based met-al-organic frameworks with peroxidase-like activity. Anal. Chim. Acta 2018, 1004, 74–81, doi:10.1016/j.aca.2017.11.078.
- Chen, J.Y.; Shu, Y.; Li, H.L.; Xu, Q.; Hu, X.Y. Nickel metal-organic framework 2D nanosheets with enhanced peroxidase nanozyme activity for colorimetric detection of H2O2. Talanta 2018, 189, 254–261, doi:10.1016/j.talanta.2018.06.075.
- Li, H.P.; Liu, H.F.; Zhang, J.D.; Cheng, Y.X.; Zhang, C.L.; Fei, X.Y.; Xian, Y.Z. Platinum nanoparticle encapsulated met-al-organic frameworks for colorimetric measurement and facile removal of mercury(II). ACS Appl. Mater. Inter. 2017, 9, 40716–40725, doi:10.1021/acsami.7b13695.
- Yang, H.G.; Yang, R.T.; Zhang, P.; Qin, Y.M.; Chen, T.; Ye, F.G. A bimetallic (Co/2Fe) metal-organic framework with oxidase and peroxidase mimicking activity for colorimetric detection of hydrogen peroxide. Microchim. Acta 2017, 184, 4629–4635, doi:10.1007/s00604-017-2509-4.
- Xu, H.M.; Liu, M.; Huang, X.D.; Min, Q.H.; Zhu, J.J. Multiplexed quantitative MALDI MS approach for assessing activity and inhibition of protein kinases based on postenrichment dephosphorylation of phosphopeptides by metal-organic frame-work-templated porous CeO2. Anal. Chem. 2018, 90, 9859–9867, doi:10.1021/acs.analchem.8b01938.
- Zhang, W.; Hu, S.L.; Yin, J.J.; He, W.W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian blue nanoparticles as multienzyme mi-metics and reactive oxygen species scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865, doi:10.1021/jacs.5b12070.
- Lin, Y.H.; Ren, J.S.; Qu, X.G. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105, doi:10.1021/ar400250z.
- Ghosh, S.; Roy, P.; Karmodak, N.; Jemmis, E.D.; Mugesh, G. Nanoisozymes: Crystal-facet-dependent enzyme-mimetic activi-ty of V2O5 nanomaterials. Angew. Chem. Int. Edit. 2018, 57, 4510–4515, doi:10.1002/anie.201800681.
- Huang, L.; Chen, J.X.; Gan, L.F.; Wang, J.; Dong, S.J. Single-atom nanozymes. Sci. Adv. 2019, 5, eaav5490, doi:10.1126/sciadv.aav5490.
- Benedetti, T.M.; Andronescu, C.; Cheong, S.; Wilde, P.; Wordsworth, J.; Kientz, M.; Tilley, R.D.; Schuhmann, W.; Gooding, J.J. Electrocatalytic nanoparticles that mimic the three-dimensional geometric architecture of enzymes: Nanozymes. J. Am. Chem. Soc. 2018, 140, 13449–13455, doi:10.1021/jacs.8b08664.
- Chen, Y.J.; Ji, S.F.; Wang, Y.G.; Dong, J.C.; Chen, W.X.; Li, Z.; Shen, R.A.; Zheng, L.R.; Zhuang, Z.B.; Wang, D.S.; et al. Iso-lated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2017, 56, 6937–6941, doi:10.1002/anie.201702473.
- Huang, X.Y.; Groves, J.T. Oxygen activation and radical transformations in heme proteins and metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553, doi:10.1021/acs.chemrev.7b00373.
- Jiao, L.; Wu, J.B.; Zhong, H.; Zhang, Y.; Xu, W.Q.; Wu, Y.; Chen, Y.F.; Yan, H.Y.; Zhang, Q.H.; Gu, W.L.; et al. Densely iso-lated FeN4 sites for peroxidase mimicking. ACS Catal. 2020, 10, 6422–6429, doi:10.1021/acscatal.0c01647.
- Wordsworth, J.; Benedetti, T.M.; Alinezhad, A.; Tilley, R.D.; Edwards, M.A.; Schuhmann, W.; Gooding, J.J. The importance of nanoscale confinement to electrocatalytic performance. Chem. Sci. 2020, 11, 1233–1240, doi:10.1039/c9sc05611d.





