
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexey Chubarov | + 854 word(s) | 854 | 2021-01-17 16:47:54 | | | |
| 2 | Catherine Yang | Meta information modification | 854 | 2021-01-19 04:14:36 | | |
Video Upload Options
Human serum albumin (HSA) is the most abundant protein in plasma. It is found at a concentration of roughly 0.7 mM in the vasculature. HSA is a monomeric multidomain biomolecule, representing the main determinant of plasma oncotic pressure and displays an extraordinary ligand binding capacity. HSA represents the main carrier for fatty acids, affects the pharmacokinetics of many drugs, can be a platform for drug discovery, suitable transport for therapy and diagnostics. Here we develop a class of macromolecular constructs from nitroxides conjugated to a human carrier protein as potential Organic radical contrast agents (ORCAs) for magnetic resonance imaging (MRI). MRI is a powerful non-invasive tool for clinics. The MRI specificity can be improved by enhancing by the addition of a contrast agent. The most efficient of the currently-used contrast agents are paramagnetic gadolinium chelates. However, the low stability of some chelates and therefore release toxic metal ions from the chelates provide harmful oxidative stress, mitochondrial membrane dysfunction, changes in gene expression, DNA damage, mutagenicity, etc. Therefore, there is sufficient interest in the production of “metal-free” MRI contrast agents.
1. The Synthesis Process and Structure
Synthesis of nitroxide and albumin conjugates were done using homocysteine thiolactone and nitroxide derivatives under physiological-like conditions (PBS, 37 °C, pH 7.4) (Figure 1). Reagent excess was removed by centrifugal filtration using Centricon concentrators. The yields of HSA-NIT conjugates were 95 %.
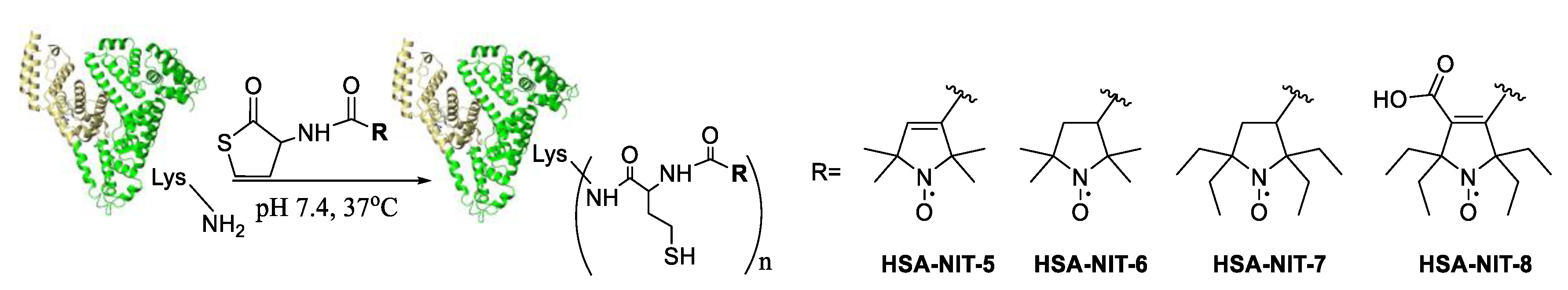 Figure 1. HSA-NIT conjugates synthesis.
Figure 1. HSA-NIT conjugates synthesis.
The incorporation of 2.5-5 Hcy-nitroxide residues into HSA was shown by MALDI-ToF and EPR. Gel electrophoresis (SDS-PAGE) was used to show that no significant changes from the native HSA were observed. Using circular dichroism (CD) spectra were shown that α-helical content of the HSA-NIT conjugates decreased from 55 % to 46-48 % while the β-sheet content was almost unchanged which indicates slight conformational adjustments. The modification sites in HSA were determined using the classical trypsin digestion approach with subsequent peptide digest analysis by MALDI-ToF. Many Lys modification sites were found: Lys-573/564, 564, 560, 525, 519, 475, 466, 444, 439/436, 436, 432, 413, 351, 323/317, 274, 225, 212, 205, 199, 195, 190, 181, 162, 159, 137, 93, 73, 64, 12 and 4. Some of them Lys-525, 212, 205, 159, 137, 12 and 4 were previously found as the major medication sites in vitro and in vivo by homocysteine thiolactone metabolite.[1][2][3] It is practically important that N-homocysteinylation by homocysteine thiolactone derivatives can occur at many previously unreported sites and offers the potential very high loadings of the nitroxides or other residues using this approach.
2. The Activities and Bioactivities
The cytotoxicity of the HSA-NIT conjugates was investigated using breast cancer MCF-7 and human glioblastoma T98G cells and standard MTT test. The HSA–NIT conjugates show the same cell viability as native HSA.
Reduction of nitroxides under human organism condition is a problem of ORCAs, which complicates their use as MRI contrast agents. The nitroxyl radicals stability (reduction constants) in HSA conjugates were measured under pseudo-first-order using great excess of the biological reduction compound as ascorbate and glutathione. For all conjugates, the reduction constants are much lower than for the free nitroxides, which explained protein shielding. The reduction-resistance in HSA-conjugate ORCAs can provide MRI measurements over longer time scales. The best results were obtained for the HSA-NIT-7 and HSA-NIT-8 conjugates, with k = 0.0018 ± 0.0002 M−1s−1 or 0.0022 ± 0.0002 M−1s−1, respectively, which more than an order of magnitude slower than the nitroxides on a dendrimer-based carrier ORCAs for MRI with k = 0.0376 M−1s−1.[4]
3. The Applications
One of the important characteristics of the MRI probes is relaxivities r1 and r2. The relaxivities r1 and r2 of the HSA-NIT conjugates in PBS were measured using a 7 T Bruker Avance III 300 MHz spectrometer at 25 °C. The per-nitroxide r1 values ranged from 0.33 to 0.51 mM−1 s-1 which is comparable to those reported in various nitroxide-bound dendrimers.[4] Per-nitroxide r2 values ranged from 4.7 to 7.2 mM−1s-1, much greater than typical for individual nitroxides in solution. Moreover, using the homocysteine thiolactone modification approach it is possible to increase albumin loading. According, r1 and r2 values the HSA-NIT conjugates may work simultaneously as T1 and T2 contrast agents. Positive and negative contrast enhancements are clearly observed in T1 and T2 MRI images, respectively (Figure 2). The contrast differences among samples correlate principally with their level of loading with nitroxide.
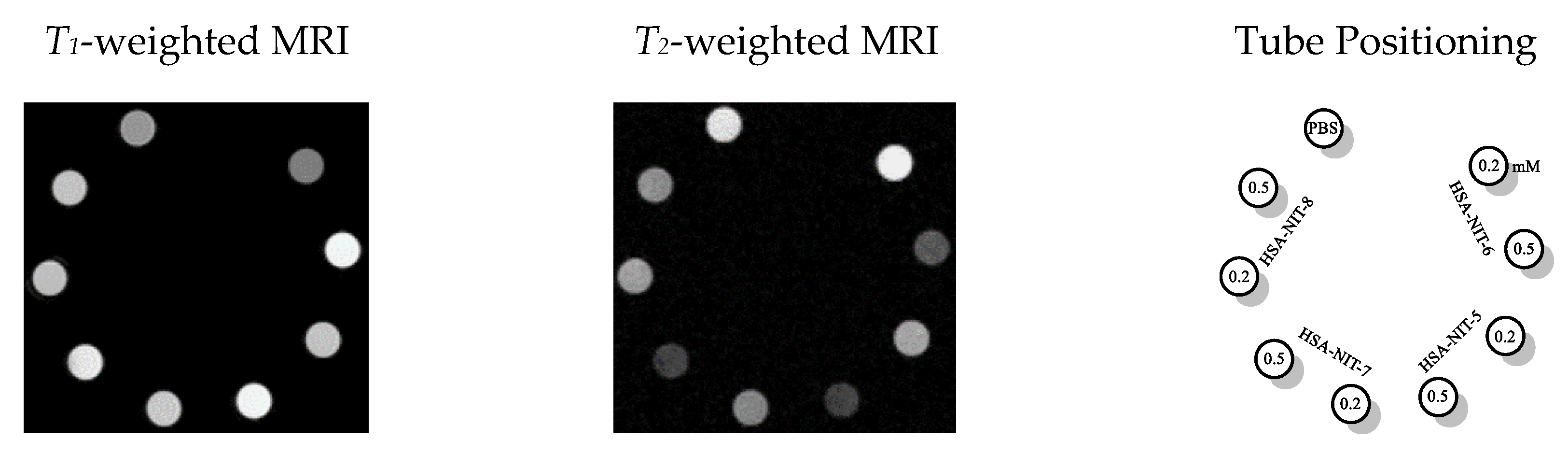 Figure 2. MRI phantoms of HSA-NIT conjugates imaged in a 7 T BioSpec 70/30 USR magnet at RT. T1- and T2-weighted MRI phantoms at HSA-NIT concentrations (0.2 mM and 0.5 mM).
Figure 2. MRI phantoms of HSA-NIT conjugates imaged in a 7 T BioSpec 70/30 USR magnet at RT. T1- and T2-weighted MRI phantoms at HSA-NIT concentrations (0.2 mM and 0.5 mM).
A new family of ORCAs were constructed by the attachment of four different homocysteine thiolactone-based spin labels to HSA. The important physical and biological properties of the HSA-NIT conjugates potentially provide promising contrast media for MRI diagnostics. Previously, such an approach was used by our research group to produce another class of contrast agents for 19F-MRI and theranostics on their basis.[5][6][7][8] N-homocysteinylation reaction using N-substituted homocysteine thiolactone derivatives can provide a new area for site-selective protein modification with preserving protein properties.
The synthesis of homocysteine thiolactone derivatives were synthesized in N.N. Vorozhtsov Institute of Organic Chemistry, SB RAS 630090 Novosibirsk, Russia (Russian Science Foundation project number 19-13-00235 and young scientists scholarship president project СП-514.2021.4). The synthesis and investigation of protein and nitroxide conjugates properties were supported by young scientists scholarship president project СП-4330.2021.4 and the grant from German Academic Exchange Service (DAAD) and Russian Ministry of Education and Science within the program “Mikhail Lomonosov”.
The entry is from 10.3390/molecules25071709
References
- Marta Sikora; Lukasz Marczak; Jolanta Kubalska; Alla Graban; Hieronim Jakubowski; Identification of N-homocysteinylation sites in plasma proteins. Amino Acids 2013, 46, 235-244, 10.1007/s00726-013-1617-7.
- Marta Sikora; Łukasz Marczak; Tomasz Twardowski; Maciej Stobiecki; Hieronim Jakubowski; Direct monitoring of albumin lysine-525 N-homocysteinylation in human serum by liquid chromatography/mass spectrometry. Analytical Biochemistry 2010, 405, 132-134, 10.1016/j.ab.2010.04.034.
- Lukasz Marczak; Marta Sikora; Maciej Stobiecki; Hieronim Jakubowski; Analysis of site-specific N-homocysteinylation of human serum albumin in vitro and in vivo using MALDI-ToF and LC-MS/MS mass spectrometry. Journal of Proteomics 2011, 74, 967-974, 10.1016/j.jprot.2011.01.021.
- Hung V.-T. Nguyen; Qixian Chen; Joseph T. Paletta; Peter Harvey; Yivan Jiang; Hui Zhang; Michael D. Boska; Maria Francesca Ottaviani; Alan Jasanoff; Andrzej Rajca; et al.Jeremiah A. Johnson Nitroxide-Based Macromolecular Contrast Agents with Unprecedented Transverse Relaxivity and Stability for Magnetic Resonance Imaging of Tumors. ACS Central Science 2017, 3, 800-811, 10.1021/acscentsci.7b00253.
- Alexey S. Chubarov; Olga D. Zakharova; Olga A. Koval; Alexander V. Romaschenko; Andrey E. Akulov; Evgenii L. Zavjalov; Ivan A. Razumov; Igor V. Koptyug; Dmitry G. Knorre; Tatyana S. Godovikova; et al. Design of protein homocystamides with enhanced tumor uptake properties for 19F magnetic resonance imaging. Bioorganic & Medicinal Chemistry 2015, 23, 6943-6954, 10.1016/j.bmc.2015.09.043.
- Alexey S. Chubarov; Makhmut M. Shakirov; Igor V. Koptyug; Renad Z. Sagdeev; Dmitry G. Knorre; Tatyana S. Godovikova; Synthesis and characterization of fluorinated homocysteine derivatives as potential molecular probes for 19 F magnetic resonance spectroscopy and imaging. Bioorganic & Medicinal Chemistry Letters 2011, 21, 4050-4053, 10.1016/j.bmcl.2011.04.119.
- Vladimir Lisitskiy; Hamda Khan; Tatyana V. Popova; Alexey S. Chubarov; Olga D. Zakharova; Andrey E. Akulov; Oleg Shevelev; Evgenii L. Zavjalov; Igor V. Koptyug; Mikhail P. Moshkin; et al.Vladimir N. SilnikovSaheem AhmadTatyana S. Godovikova Multifunctional human serum albumin-therapeutic nucleotide conjugate with redox and pH-sensitive drug release mechanism for cancer theranostics. Bioorganic & Medicinal Chemistry Letters 2017, 27, 3925-3930, 10.1016/j.bmcl.2017.05.084.
- Tatyana V. Popova; Hamda Khan; Alexey S. Chubarov; Vladimir A. Lisitskiy; Natalya M. Antonova; Andrey E. Akulov; Oleg B. Shevelev; Evgenii L. Zavjalov; Vladimir N. Silnikov; Saheem Ahmad; et al.Tatyana S. Godovikova Biotin-decorated anti-cancer nucleotide theranostic conjugate of human serum albumin: Where the seed meets the soil?. Bioorganic & Medicinal Chemistry Letters 2018, 28, 260-264, 10.1016/j.bmcl.2017.12.061.




