
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paula Fernández-Palanca | + 3643 word(s) | 3643 | 2021-01-11 13:30:21 | | | |
| 2 | Conner Chen | Meta information modification | 3643 | 2021-10-12 05:38:13 | | |
Video Upload Options
Melatonin (N-acetyl-5-methoxytryptamine) is an indoleamine with beneficial effects in a broad number of tumors, including the primary liver cancers hepatocarcinoma (HCC) and cholangiocarcinoma (CCA). Among them, melatonin has shown to modulate different cancer-associated processes and enhance drug efficacy against HCC and CCA. Therefore, melatonin has a potential role in improving the current therapeutic landscape in these liver tumors.
1. Introduction
Primary liver cancer constitutes the sixth most prevalent type of tumor and is the fourth common cause of cancer-related mortality worldwide [1]. A total of 841,080 new cases were diagnosed in 2018, with an estimated 781,631 deaths and an age-standardized mortality rate of 8.5/100,000. The most frequent types of primary liver cancer in adults are hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA), constituting HCC the 70-85% of cases and CCA the 30-15%, depending on the country [2]. Liver cancer also appears in children and adolescents, accounting hepatoblastoma (HB) and HCC for 67-80% and 20-33% of cases, respectively [2][3].
Unfortunately, most of the liver cancer patients are diagnosed in advanced stages when surgical treatment is not available [4]. Moreover, the high mortality associated with liver cancer is related to its lack of sensitivity and development of resistances to a few treatments that lead to the chemotherapy failure. These drawbacks may be explained, at least in part, by the various phenotypes and histological characteristics of tumor liver cells and its microenvironment [5]. Furthermore, the high refractoriness of liver cancer to treatments has been associated with the interaction of very complex and diverse mechanisms of chemoresistance, which can act synergistically to protect tumor cells from the chemotherapy agents [3].
Melatonin (N-acetyl-5-methoxytryptamine), the main product of the pineal gland, is an indoleamine with antioxidant, chronobiotic and anti-inflammatory properties [6]. A reduction of melatonin levels or a depressed excretion of its main metabolite, 6-sulfatoxymelatonin, have been related to an increased cancer risk, suggesting an anticancer role of this indoleamine [7]. Collectively, the published data strongly support the oncostatic actions of melatonin on different types of tumors, including liver cancer [8][9][10].
The inhibitory role of melatonin in hepatic tumors involves a number of different molecular and cellular processes including reduction of cellular proliferation, cell cycle arrest, limiting angiogenesis and metastasis and promoting cell death [10][11][12][13][14][15][16][17]. Moreover, melatonin reportedly increases the sensitivity of liver cancer cells to currently-available treatments [4][18], and as other natural compounds such as quercetin, curcumin and resveratrol, melatonin properties could ameliorate chemotherapeutic toxicity [19][20][21].
2. Antioxidant and chronobiotic actions of melatonin in liver cancer
Carcinogenetic processes often involve oxidative stress, and administration of antioxidant molecules may reduce the damage to cancerous hepatocytes [22][21][23]. Melatonin acts as an indirect antioxidant and as a direct free radical scavenger, and displays an important role as an immunomodulatory and chronobiotic agent in different tissues, including liver [6][14][24][25]. These effects have been observed in several studies carried out with both in vitro and in vivo models of HCC and CCA, where melatonin administration showed a markedly antioxidant activity by modulating expression and activity of antioxidant enzymes and oxidant agents [26][27][28]. However, under different conditions researches have reported an increase in oxidative stress derived from melatonin treatment in these liver tumors, which could be attributed to the employ of higher doses of this indoleamine [18][29]. Results found suggest that oxidative stress modulation by melatonin could be useful in the HCC and CAA treatment and prevention.
Since circadian clock plays a key role in liver physiology and chronodisruption is known to augment hepatocarcinogenesis [30], different experiments have been carried out to analyze the association between the antioxidant and chronobiotic effects of melatonin in HCC. During hepatocarcinogenesis it has been described the appearance of circadian rhythm perturbations that contribute to the establishment of liver tumors; however, melatonin has also found to be effective in restoring the normal rhythms not only as single treatment but also in combination with several drugs, such as oxaliplatin and α-ketoglutarate. This chronobiotic activity has been associated to the ability of melatonin to regulate the acrophase, mesor and amplitude parameters of lipid peroxidation and components of the antioxidant system, revealing an hepatoprotective action of melatonin derived from circadian rhythms modulation [31][32][33][34].
3. Cell cycle arrest by melatonin in liver cancer
Carcinogenesis is a multistage process usually promoted by a disturbed and uncontrolled cell proliferation, with cell cycle dysregulation, associated with an increase in tumor cell viability. In these events, melatonin involvement has also been described since several studies reported cell cycle arrest after administration of this indoleamine in HCC alone or in combination with several drugs, such as sorafenib and (-)-epigallocatechin-3-gallate (EGCG). Nonetheless, cytostatic effects of melatonin have been identified in different stages of cell cycle, some studies observed an arrest in G0/G1 phase and another in G2/M phase, while induction of p53 and decrease of cyclin D1 expression are mostly found [10][35][36][37][38][39][40]. In this line, melatonin also acts through the melatonin receptors MT1, MT3 and retinoic acid-related orphan receptor alpha (RORα) to arrest cell cycle and inhibit cell proliferation by increasing receptors expression not only in HCC but also in CCA [11][12][41]. Another relevant molecules involved in cell cycle modulation, the brain and muscle arnt-like protein 1 (Bmal1) and the circadian locomotor output cycles protein kaput (Clock) proteins, have been also reported to be modulated by melatonin in HCC [14]. Altogether, these results highlight the potential of melatonin as a cytostatic agent in the treatment of liver tumors.
4. Apoptosis modulation by melatonin in liver cancer
Apoptosis is a cellular mechanism of programmed cell death which is involved in numerous diseases, including cancer. Resistance to apoptosis is one of the main hallmarks of cancer and selective induction of this type of cell death in cancer cells has emerged as an interesting possibility for new antitumor treatments [42]. In liver, apoptotic signaling is transduced mainly via two molecular pathways: the extrinsic or death receptor-mediated pathway and the intrinsic or mitochondria-dependent pathway. Although the extrinsic pathway is induced by death ligands located in the cellular membrane and the intrinsic pathway is initiated as consequence of mitochondria dysfunction, both concur in a common finnal stage [42][43].
Melatonin effects on apoptosis modulation have been broadly studied in liver tumors. In both cellular and animal models, melatonin has demonstrated to be a powerful proapoptotic agent by inducing the extrinsic and the intrinsic pathway not only in HCC but also in CCA [10][14][44][45][29][46]. Moreover, several signaling pathways have been identified as mechanisms responsible for melatonin-derived apoptosis induction, such as promotion of endoplasmic reticulum (ER) stress, activation of NF-κB pathway and reduction of oxidative and nitrosative stress by melatonin [17][45][46][47][48]. Proapoptotic effects of melatonin have been also found when combined with other compounds, such as doxorubicin, cisplatin, sorafenib, regorafenib, 5-fluorouracil and EGCG, where coadministration with melatonin led to a higher induction of apoptosis and chemosensitivity [39][40][49][50][51][52][53][54][55][56].
5. Melatonin regulation of autophagy in liver cancer
The process of autophagy constitutes a bulk degradation system with a key role in the maintenance of cellular homeostasis in order to promote adaptation and cell survival. Nevertheless, when there is a excessive stimulation, programmed cell death is induced instead of survival in a number of different pathophysiological situations including tumorigenesis [57]. Therefore, in cancer cells autophagy can act as a double-edged sword removing malignant cells and damaged mitochondria at the early stages, but inducing survival under hypoxia and ischemia conditions in the later phases, which can be also associated with chemotherapy resistance and tumor progression [18].
As the dual role of autophagy, melatonin effects also depend on the cellular context, finding an autophagy induction in HCC when administered melatonin, which was diminishing the proapoptotic activity of the indoleamine, since disruption of autophagy promoted apoptosis [16][58]. Similarly, melatonin and cisplatin combined administration also induced an autophagy flux, improving antitumor effects of cisplatin [59]. However, coadministration of melatonin with sorafenib led to a reduction of the sorafenib-induced autophagy, promoting death of hepatocytes [60][61]. Furthermore, mitophagy, a selective degradation process of mitochondrias, is also modulated by melatonin. Results showed an increased mitophagy-associated apoptosis when combined with sorafenib under normoxic conditions, while after hypoxic induction melatonin and sorafenib treatment led to the inhibition of a cytoprotective mitophagy [4][18].
6. Inhibitory actions of melatonin on angiogenesis and invasion in liver cancer
Angiogenesis—the process of new blood-vessel growth from the existing vasculature—plays a key role facilitating tumor growth and the metastatic process and has been widely related with progression, invasion and metastasis of liver tumors [62][63][64].
The proangiogenic vascular endothelial growth factor (VEGF) along with the main regulator of hypoxia response, HIF-1α, are two major proteins involved in angiogenesis promotion. Melatonin has shown to impaired angiogenic and invasive abilities of liver cancer cells through inhibition of both VEGF and HIF-1α, as well as other related molecules, including STAT-3, the CBP/p300 co-activator, the forkhead box A2 (FOXA2) transcription factor expression, matrix metalloproteinase 9 (MMP-9) [13][15][65][66][67]. Curiously, long non-coding RNAs (lncRNA) and microRNAs (miRNA) have also been involved in this melatonin-derived antiangiogenic activity, such as lncRNA carbamoyl-phosphate synthase 1 intronic transcript 1 (lncRNA-CPS1-IT1), lncRNA RAD51 antisense RNA 1 (lncRNA RAD51-AS1) and miRNA let7i-p [66][68][69]. Regarding to proangiogenic and angiostatic chemokines, melatonin effects have not fully cleared, since contradictory results where observed in two HCC cell lines susceptible and resistant to amphotericin B (AmB). These findings indicate that clinical application of melatonin in patients with HCC should consider the liver tumor characteristics for the optimization of indole concentrations [70].
7. Immunomodulatory effects of melatonin in liver cancer
As previously indicated, melatonin acts as both antioxidant and chronobiotic but also as an immunomodulatory agent [6][14][24][25]. Some clinical results from case reports have shown that melatonin combination with immunotherapy impaired HCC progression and development of new liver tumors [71]. Additionally, preclinical studies exhibited interesting results where melatonin administration led to an increased immune response in both HCC and CCA models [72][73], in some cases accompanied by an anti-inflammatory activity [73].
All of these findings reporting antitumor actions of melatonin in the HCC and CCA liver tumors are summarized in Table 1.
Table 1. Basic characteristics of experimental studies evaluating antitumor effects of melatonin against liver tumors.
|
First Author, Publication Year, [Reference] |
Country |
Liver Tumor |
Experimental Model (n, Sample Size per Group) |
Administration Strategy |
Treatment Regimen |
Process Alteration |
|
Dakshayani et al. 2005 [26] |
India |
HCC |
In vivo Adult male Wistar rats Intraperitoneal injection of DEN followed by subcutaneous CCl4 (n = 6) |
Intraperitoneal melatonin |
5 mg/kg 20 weeks |
Antioxidant and hepatoprotective activity |
|
Dakshayani et al. 2007 [31] |
India |
HCC |
In vivo Adult male Wistar rats Intraperitoneal injection of DEN followed by subcutaneous CCl4 (n = 6) |
Intraperitoneal melatonin |
5 mg/kg Thrice a week 20 weeks |
Chronobiotic effect Antioxidant effect |
|
Subramanian et al. 2007 [27] |
India |
HCC |
In vivo 3-months-old male Wistar rats Intraperitoneal injection of DEN followed by subcutaneous of CCl4 (n = 6) |
Intraperitoneal melatonin |
5 mg/kg Daily 20 weeks |
Tumor growth inhibition Antioxidant activity |
|
Martín-Renedo et al. 2008 [10] |
Spain |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
0.1–10 mM 4, 6, 8 and 10 days |
Proliferation inhibition Apoptosis induction Cell cycle arrest |
|
Subramanian et al. 2008 [32] |
India |
HCC |
In vivo 3-months-old male Wistar rats Intraperitoneal injection of DEN followed by subcutaneous injection of CCl4 (n = 6) |
Intraperitoneal melatonin |
5 mg/kg Daily 20 weeks |
Chronobiotic effect Antioxidant effect |
|
Carbajo-Pescador et al. 2009 [11] |
Spain |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
1–2.5 mM 2, 4 and 6 days |
Proliferation inhibition Cell cycle arrest |
|
Ozdemir et al. 2009 [35] |
Turkey |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
0.05–1 mM 72 h |
Cell cycle arrest |
|
Fan et al. 2010 [49] |
China |
HCC |
In vitro Human HepG2 and Bel-7402 cell lines |
Melatonin + Doxorubicin |
0.01–10 µM 48 h |
Proliferation inhibition Apoptosis induction |
|
Laothong et al. 2010 [28] |
Thailand |
CCA |
In vivo Male Syrian golden hamsters Oral inoculation of 50 metacercariae of Opisthorchis viverrini (n = 5) |
Oral melatonin |
5, 10, 20 mg/kg Daily 30 days |
Antioxidant and protective activity |
|
Lin and Chuang 2010 [70] |
Taiwan |
HCC |
In vitro Human HCC cell lines: HCC24/KMUH (resistant to AmB-induced oxidative stress) and HCC38/KMUH: (susceptible to AmB-induced oxidative stress) |
Melatonin |
1 and 100 µM 24 h |
Proliferation increase |
|
Melatonin + AmB |
1 and 100 µM 24 h |
Antiangiogenic effect |
||||
|
Carbajo-Pescador et al. 2011 [12] |
Spain |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
1–2.5 mM 12, 24 and 48 h |
Proliferation inhibition |
|
Han et al. 2011 [41] |
New York |
CCA |
In vivo 6-weeks-old male BALB/c nude mice Subcutaneous injection of Mz-ChA-1 cells (n = 4) |
Intraperitoneal melatonin |
4 mg/kg Thrice a week 43 days |
Proliferation inhibition |
|
Carbajo-Pescador et al. 2012 [44] |
Spain |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
50–2000 µM 1, 6, 24 and 48 h |
Apoptosis induction |
|
Cid et al. 2012 [36] |
Spain |
HCC |
In vitro Human HepG2 cell line |
Melatonin + MF exposure |
0.01–1000 nM 4, 5 and 7 days |
Proliferation inhibition |
|
Liu et al. 2012 [58] |
China |
HCC |
In vitro Mouse hepatoma cell line H22 |
Melatonin |
100 µM 24 h |
Apoptosis induction |
|
Melatonin + Beclin-1 RNAi Melatonin + 3-MA |
100 µM 24 h |
Autophagy blockade Apoptosis induction |
||||
|
In vivo 8-weeks-old female BALB/c mice Subcutaneous injection of H22 cells (n = 10) |
Intraperitoneal melatonin |
10 or 20 mg/kg Daily 14 days |
Autophagy induction |
|||
|
Zha et al. 2012 [47] |
China |
HCC |
In vitro Human HCC HepG2 cell line Human hepatocyte HL-7702 cell line |
Melatonin + Tunicamycin |
10−7 µM 24 h |
Proliferation inhibition Apoptosis induction |
|
Carbajo-Pescador et al. 2013 [13] |
Spain |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
1 nM and 1 mM 2, 4, 6, 8, 12 and 24 h or 24 h |
Hypoxia-dependent angiogenesis |
|
Fan et al. 2013 [50] |
China |
HCC |
In vitro Human HepG2 and SMMC-7721 cell lines |
Melatonin + Doxorubicin |
1 mM 24 h |
Apoptosis induction |
|
Melatonin + Doxorubicin + Tunicamycin |
0.1–1000 µM 24 h |
Proliferation inhibition Apoptosis induction |
||||
|
Fan et al. 2013b [51] |
China |
HCC |
In vitro Human HepG2 and SMMC-7721 cell lines |
Melatonin |
0.001–1000 µM 24 and 48 h |
Proliferation inhibition Apoptosis induction |
|
Laothong et al. 2013 [45] |
Thailand |
CCA |
In vivo 4-to-6-weeks-old male Syrian golden hamsters Oral inoculation of 50 metacercariae of Opisthorchis viverrini and 12.5 ppm DEN (n = 15) |
Oral melatonin |
10 or 50 mg/kg Daily 120 days |
Apoptosis induction |
|
Tomov et al. 2013 [71] |
Bulgaria |
HCC |
Case report 67-years-old female |
Intermittent administration of IL-2, BCG and oral melatonin |
20 mg Daily |
Immunomodulation |
|
Bennukul et al. 2014 [59] |
Thailand |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
0.5–5 mM 24 and 48 h |
Autophagy induction |
|
Melatonin + Cisplatin |
||||||
|
Ordóñez et al. 2014 [15] |
Spain |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
1 mM 24 h |
Angiogenesis and invasion inhibition |
|
Melatonin + IL-1β |
||||||
|
Verma et al. 2014 [33] |
Malaysia |
HCC |
In vivo Adult male mice Intraperitoneal injection of DEN (n = 6) |
Intraperitoneal melatonin |
0.5 mg/kg Thrice a week 10 weeks |
Antioxidant activity Modulation of circadian rhythms |
|
Laothong et al. 2015 [29] |
Thailand |
CCA |
In vitro Human KKU-M055 and KKU-M214 cell lines |
Melatonin |
0.5, 1 and 2 mM 48 h |
Oxidative stress activity Apoptosis induction |
|
Moreira et al. 2015 [17] |
Brazil |
HCC |
In vivo Male Wistar rats Intraperitoneal injection of DEN and 2-AAF administration at week 4 (n = 12) |
Oral melatonin |
1 mg/kg Daily 45 and 90 days |
Apoptosis induction |
|
Ordóñez et al. 2015 [16] |
Spain |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
2 mM 0.5–48 h |
Apoptosis induction Autophagy induction |
|
Colombo et al. 2016 [65] |
Brazil |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
1–106 nM 24 h |
Proliferation inhibition |
|
1 mM 24 h |
Inhibition of hypoxia-derived invasion |
|||||
|
Prieto-Domínguez et al. 2016 [18] |
Spain |
HCC |
In vitro Human HepG2, HuH7 and Hep3B cell lines |
Melatonin |
0.1–2 mM |
Proliferation inhibition Pro-oxidant activity Mitophagy induction Apoptosis induction |
|
Melatonin + Sorafenib |
||||||
|
Bu et al. 2017 [48] |
China |
HCC |
In vitro Human HepG2 cell line |
Melatonin + Tunicamycin |
10−6 –1 mM |
Apoptosis induction and ER stress |
|
Cheng et al. 2017 [72] |
China |
HCC |
In vitro Human HepG2 and Bel-7402 cell lines |
Melatonin |
0.1 mM |
Immunomodulation |
|
In vivo 6-weeks-old female BALB/c nude mice Injected with Exo-con or Exo-MT (0.1 mM melatonin) |
Exo-MT |
100 µL Daily 10 days |
||||
|
Hao et al. 2017 [52] |
China |
HCC |
In vitro Human Bel-7402, SNU-449, HepG2 and Hep3B 2.1-7 cell line |
Melatonin |
1 mM 48 h |
Proliferation inhibition Inhibition of cell migration ability Apoptosis induction |
|
Melatonin + CDDP |
||||||
|
Lin et al. 2017 [53] |
China |
HCC |
In vitro Human HuH7 cell line |
Melatonin |
1–5 mM 48 h |
Proliferation inhibition Apoptosis induction |
|
Melatonin + Sorafenib |
||||||
|
Liu et al. 2017 [60] |
China |
HCC |
In vitro Human HepG2 and Bel-7402 cell lines |
Melatonin |
10 µM 48 h |
Apoptosis induction |
|
Melatonin + Sorafenib |
1–100 µM 48 h |
Proliferation inhibition |
||||
|
10 µM 48 h |
Apoptosis induction Autophagy blockage |
|||||
|
Long et al. 2017 [37] |
China |
HCC |
In vitro Human Bel-7402, SMMC-7721 HCC cell lines Human normal liver L02 cell line |
Melatonin |
0.2–2 mM 48–72 h |
Proliferation inhibition |
|
Melatonin + Sorafenib |
1 mM 48 h 2 weeks |
Proliferation inhibition Cell cycle arrest |
||||
|
In vivo 4-weeks-old female BALB/c nude mice Subcutaneous injection of SMMC-7721 cells (n = 4) |
Intraperitoneal melatonin |
25 mg/kg Daily 18 days |
Tumor growth inhibition |
|||
|
Intraperitoneal melatonin + sorafenib |
||||||
|
Prieto-Domínguez et al. 2017 [4] |
Spain |
HCC |
In vitro Human Hep3B cell line |
Melatonin |
1 or 2 mM 24 or 48 h |
Pro-oxidant activity Proliferation inhibition |
|
Melatonin + Sorafenib |
Proliferation inhibition Blockade of sorafenib-induced mitophagy |
|||||
|
Sánchez et al. 2017 [38] |
Spain |
HCC |
In vivo 6-weeks-old male ICR mice Intraperitoneal injection of DEN |
Intraperitoneal melatonin |
5 or 10 mg/kg Daily 10, 20, 30, 40 weeks |
Cell cycle arrest Modulation of sphingolipid metabolism |
|
Wang et al. 2017 [66] |
Taiwan |
HCC |
In vitro Human HepG2 and HuH7 cell lines |
Melatonin |
1 mM 12, 24, 36, 48, 60 and 72 h |
Proliferation inhibition |
|
1 mM 24, 48 and 72 h |
Suppression of cell migration ability |
|||||
|
1 mM 24 h |
EMT inhibition |
|||||
|
In vivo 6-to-8-weeks-old male BALB/c nude mice Subcutaneous injection of HuH7 cells (n = 10) |
Intraperitoneal melatonin |
40 mg/kg Five days per week |
Tumor growth inhibition EMT suppression |
|||
|
Wongsena et al. 2017 [73] |
Thailand |
CCA |
In vivo 6-to-8-weeks-old male Syrian golden hamsters Oral infection with 50 metacercariae of Opisthorchis viverrini and oral administration with DEN (n = 7) |
Oral melatonin |
50 mg/kg Daily 30 days |
Immunomodulation |
|
Chen et al. 2018 [68] |
Taiwan |
HCC |
In vitro Human HuH7 and HepG2 cell lines |
Melatonin |
1 mM 12, 24, 36, 48, 60 and 72 h |
Proliferation inhibition |
|
1 mM 24, 48 and 72 h |
Suppression of migration and invasion abilities |
|||||
|
Melatonin + Etoposide |
1 mM 12, 24, 36, 48, 60, and 72 h |
Proliferation inhibition Apoptosis induction |
||||
|
Melatonin + Camptothecin |
1 mM 24 h |
|||||
|
Chen et al. 2018 [68] |
Taiwan |
HCC |
In vivo 6-weeks-old male BALB/c nude mice Subcutaneous injection of HuH7 cells (n = 6) |
Intraperitoneal melatonin |
40 mg/kg Five days/week 25 days |
Tumor growth inhibition Apoptosis induction |
|
Intraperitoneal melatonin + etoposide |
||||||
|
Colombo et al. 2018 [46] |
Brazil |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
1 mM 24 h |
Increase of NF-κB protein expression |
|
Dauchy et al. 2018 [34] |
USA |
HCC |
In vivo Male Buffalo rats Implantation of Morris 7288CTC hepatomas (control: n = 6; experimental: n = 9) |
Endogenous melatonin |
Increase of endogenous melatonin levels |
Tumor growth inhibition |
|
Sánchez et al. 2018 [14] |
Spain |
HCC |
In vitro Human Hep3B cell line |
Melatonin |
0.5 or 1 mM 1 h |
Proliferation inhibition Apoptosis induction |
|
Melatonin + SR9009 |
Proliferation inhibition |
|||||
|
Melatonin + Bmal1 siRNA |
0.5 or 1 mM 24 h |
Proliferation inhibition Apoptosis induction |
||||
|
In vivo 6-weeks-old male ICR mice Intraperitoneal injection of DEN (n = 4–8) |
Intraperitoneal melatonin |
5 or 10 mg/kg Daily 10, 20, 30, 40 weeks |
Circadian clock modulation Cell cycle arrest Apoptosis induction |
|||
|
Wang et al. 2018 [69] |
Taiwan |
HCC |
In vitro Human HepG2 and HuH7 cell lines |
Melatonin |
1 and 2 mM 12, 24, 36, 48, 60 and 72 h |
Proliferation inhibition |
|
1 and 2 mM 24, 48 and 72 h |
Suppression of migration and invasion abilities |
|||||
|
Melatonin + let-7i-3p inhibitor |
1 and 2 mM 24 and 48 h |
Proliferation inhibition Migration and invasion suppression |
||||
|
In vivo 6–8-weeks-old male BALB/c nude mice Subcutaneous injection of HuH7 cells (n = 6) |
Intraperitoneal melatonin |
40 mg/kg 5 days per week 35 days |
Tumor growth inhibition |
|||
|
El-Magd et al. 2019 [74] |
Egypt |
HCC |
In vivo Adult female rats Intraperitoneal injection of DEN and oral administration of 2-AAF at week 2 (n = 10) |
Intraperitoneal melatonin |
20 mg/kg Twice a week 5 weeks |
Apoptosis induction Antioxidant activity Reduction of angiogenesis and metastasis |
|
Intraperitoneal melatonin + MSCs |
||||||
|
Intraperitoneal injection of MSCs preincubated with melatonin |
5 µM 24 h |
|||||
|
Mohamed et al. 2019 [75] |
Egypt |
HCC |
In vivo Adult female rats Intraperitoneal injection of DEN followed by oral administration of 2-AAF at week 2 (n = 10) |
Intraperitoneal melatonin |
20 mg/kg Twice a week 5 weeks |
Tumor growth inhibition Apoptosis induction |
|
Intraperitoneal injection of MSCs preincubated with melatonin 5 µM for 24 h |
||||||
|
Zhang et al. 2019 [39] |
China |
HCC |
In vitro Human HepG2 cell line |
Melatonin |
3 mM 48 h |
Suppression of migration |
|
1 mM 14 days |
Proliferation inhibition |
|||||
|
Melatonin + EGCG |
3 mM 48 h |
Suppression of migration |
||||
|
1 mM 14 days |
Proliferation inhibition |
|||||
|
Zhou et al. 2019 [61] |
China |
HCC |
In vitro Human HepG2, 7721 and HuH7 HCC cell lines Human liver L02 cell line |
Melatonin |
1–100 µM 48 h |
Apoptosis induction |
|
10 µM 48 h |
Autophagy inhibition |
|||||
|
Melatonin + Sorafenib |
1–100 µM 48 h |
Proliferation inhibition Apoptosis induction |
||||
|
10 µM 48 h |
Autophagy inhibition |
|||||
|
Ao et al. 2020 [54] |
China |
HCC |
In vitro Human HepG2 and HuH7 cell lines |
Melatonin |
2.5 mM 24 h |
Apoptosis induction |
|
Mi and Kuang 2020 [40] |
China |
HCC |
In vitro Human HepG2 and Hep3B cell lines |
Melatonin |
1 or 2 mM 24, 48, 72, 96 h |
Proliferation inhibition |
|
1 or 2 mM 48 h |
Cell cycle arrest |
|||||
|
Melatonin + Cisplatin |
1 or 2 mM 24 and 48 h |
Proliferation inhibition Apoptosis induction |
||||
|
Wang et al. 2020 [55] |
China |
HCC |
In vitro Human SMMC-7721, cell line |
Melatonin + Regorafenib |
50 µM 24 h |
Antioxidant activity Apoptosis induction |
2-AAF, 2-acetylaminofluorene; AmB, amphotericin B; BCG, Bacillus Calmette-Guerin; Bmal1, brain and muscle arnt-like protein 1; CCA, cholangiocarcinoma; CDDP, Cis-dichlorodiamineplatinum; DEN, diethylnitrosamine; EGCG, (–)-epigallocatechin-3-gallate; EMT, epithelial-to-mesenchymal transition; ER, endoplasmic reticulum; Exo-con, exosomes from HepG2 cells; Exo-MT, exosomes from melatonin-treated HepG2 cells; HCC, hepatocarcinoma; MF, magnetic field; IL-1β, interleukin 1 beta; IL-2, interleukin-2; MSCs, mesenchymal stem cells; NF-κB, nuclear factor-kappa B; RNAi, interference RNA; siRNA, small interference RNA.
8. Conclusions
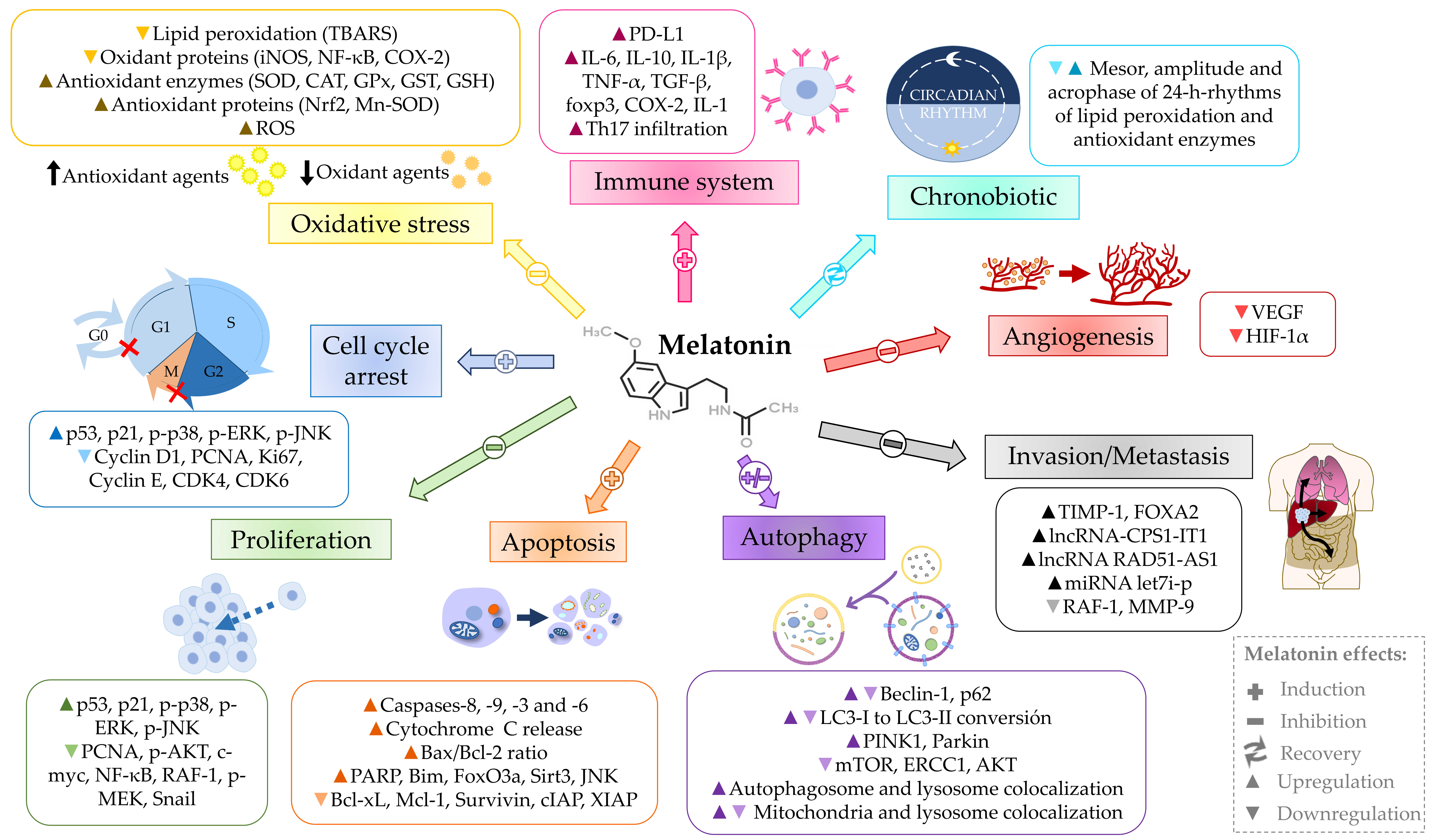
Melatonin has shown to exert antitumor effects in liver tumors, not only in the prevention but also in the treatment of the main primary liver tumors, HCC and CCA, by modulating a wide number of cellular processes, even protecting cells from the hepatotoxicity of other drugs. Among melatonin’s effects, as showed in Figure 1, it improves the immune response, enhances apoptosis, and positively influences the cell cycle and circadian rhythms; it also impairs tumor angiogenesis, invasion and cell proliferation. Considering its different roles on autophagy, melatonin may modulate this process depending on cellular context, always aimed at hindering tumor progression. Finally, combination studies with different molecule types, antitumor drugs, flavonoids and chemotherapeutics, provide evidence of the potential effects of melatonin as a coadjuvant agent to improve current treatments. Overall, research findings support a role for melatonin as a promising drug in the treatment armamentarium of liver tumors.
The entry is from 10.3390/antiox10010103
References
- Freddie Bray; Jacques Ferlay Me; Isabelle Soerjomataram; Rebecca L. Siegel; Lindsey A. Torre; Ahmedin Jemal; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018, 68, 394-424, 10.3322/caac.21492.
- Rajesh Sharma; Descriptive epidemiology of incidence and mortality of primary liver cancer in 185 countries: evidence from GLOBOCAN 2018. Japanese Journal of Clinical Oncology 2020, 50, 1370-1379, 10.1093/jjco/hyaa130.
- Jose J. G. Marin; Oscar Briz; Elisa Herraez; Elisa Lozano; Maitane Asensio; Silvia Di Giacomo; Marta R. Romero; Luis M. Osorio-Padilla; Ana I. Santos-Llamas; Maria A. Serrano; et al.Carolina ArmengolThomas EfferthRocio I.R. Macias Molecular bases of the poor response of liver cancer to chemotherapy. Clinics and Research in Hepatology and Gastroenterology 2018, 42, 182-192, 10.1016/j.clinre.2017.12.006.
- Néstor Prieto-Domínguez; Carolina Méndez-Blanco; Sara Carbajo-Pescador; Flavia Fondevila; Andrés García-Palomo; Javier González-Gallego; José Luis Mauriz; Melatonin enhances sorafenib actions in human hepatocarcinoma cells by inhibiting mTORC1/p70S6K/HIF-1α and hypoxia-mediated mitophagy. Oncotarget 2017, 8, 91402-91414, 10.18632/oncotarget.20592.
- Gang Wang; Qian Wang; Ning Liang; Hongyuan Xue; Tao Yang; Xuguang Chen; Zhaoyan Qiu; Chao Zeng; Tao Sun; Weitang Yuan; et al.Chaoxu LiuZhangqian ChenXianli He Oncogenic driver genes and tumor microenvironment determine the type of liver cancer. Cell Death & Disease 2020, 11, 1-13, 10.1038/s41419-020-2509-x.
- José L. Mauriz; Pilar S. Collado; Christiano Veneroso; Russel J. Reiter; Javier González-Gallego; A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. Journal of Pineal Research 2012, 54, 1-14, 10.1111/j.1600-079x.2012.01014.x.
- Marina Sánchez-Hidalgo; J M Guerrero; I. Villegas; G. Packham; C A De La Lastra; Melatonin, A Natural Programmed Cell Death Inducer in Cancer. Current Medicinal Chemistry 2012, 19, 3805-3821, 10.2174/092986712801661013.
- Russel J. Reiter; Sergio A. Rosales-Corral; Dun-Xian Tan; Darío Acuña-Castroviejo; Lilan Qin; Shun-Fa Yang; Kexin Xu; Melatonin, a Full Service Anti-Cancer Agent: Inhibition of Initiation, Progression and Metastasis. International Journal of Molecular Sciences 2017, 18, 843, 10.3390/ijms18040843.
- Ya Li; Sha Li; Yue Zhou; Xiao Meng; Jiao-Jiao Zhang; Dong-Ping Xu; Hua-Bin Li; Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896-39921, 10.18632/oncotarget.16379.
- Javier Martin-Renedo; José Luis Mauriz; Francisco Jorquera; Olga Ruiz-Andrés; Paquita Gonzalez; Javier González-Gallego; Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. Journal of Pineal Research 2008, 45, 532-540, 10.1111/j.1600-079x.2008.00641.x.
- Sara Carbajo-Pescador; Javier Martin-Renedo; Andres García-Palomo; María Jesús Tuñón; José Luis Mauriz; Javier González-Gallego; Changes in the expression of melatonin receptors induced by melatonin treatment in hepatocarcinoma HepG2 cells. Journal of Pineal Research 2009, 47, 330-338, 10.1111/j.1600-079x.2009.00719.x.
- Sara Carbajo-Pescador; Andrés García-Palomo; Javier Martín-Renedo; Maiara Piva; Javier González-Gallego; José Luis Mauriz; Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: role of the MT1 receptor. Journal of Pineal Research 2011, 51, 463-471, 10.1111/j.1600-079x.2011.00910.x.
- S Carbajo-Pescador; R Ordoñez; M Benet; R Jover; A García-Palomo; J L Mauriz; J González-Gallego; Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. British Journal of Cancer 2013, 109, 83-91, 10.1038/bjc.2013.285.
- Diana I. Sánchez; Bárbara González-Fernández; Irene Crespo; Beatriz San‐Miguel; Marcelino Álvarez; Javier González-Gallego; María Jesús Tuñón; Melatonin modulates dysregulated circadian clocks in mice with diethylnitrosamine-induced hepatocellular carcinoma. Journal of Pineal Research 2018, 65, e12506, 10.1111/jpi.12506.
- Raquel Ordoñez; Sara Carbajo-Pescador; Néstor Prieto-Dominguez; Andrés García-Palomo; Javier González-Gallego; José L. Mauriz; Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. Journal of Pineal Research 2013, 56, 20-30, 10.1111/jpi.12092.
- Raquel Ordoñez; Anna Fernández; Néstor Prieto-Domínguez; Laura Martínez; Carmen García-Ruiz; José C. Fernández-Checa; José Luis Mauriz; Javier González-Gallego; Ceramide metabolism regulates autophagy and apoptotic cell death induced by melatonin in liver cancer cells. Journal of Pineal Research 2015, 59, 178-189, 10.1111/jpi.12249.
- Andrea Janz Moreira; Raquel Ordoñez; Carlos Thadeu Cerski; Jaqueline Nascimento Picada; Andrés García-Palomo; Norma Possa Marroni; Jose L. Mauriz; Javier González-Gallego; Melatonin Activates Endoplasmic Reticulum Stress and Apoptosis in Rats with Diethylnitrosamine-Induced Hepatocarcinogenesis. PLOS ONE 2015, 10, e0144517, 10.1371/journal.pone.0144517.
- Néstor Prieto-Domínguez; Raquel Ordóñez; Anna Fernández; Carolina Méndez-Blanco; Anna Baulies; Carmen Garcia-Ruiz; José C. Fernández-Checa; José L. Mauriz; Javier González-Gallego; Melatonin-induced increase in sensitivity of human hepatocellular carcinoma cells to sorafenib is associated with reactive oxygen species production and mitophagy. Journal of Pineal Research 2016, 61, 396-407, 10.1111/jpi.12358.
- Divya Rawat; Somi Shrivastava; Rayees Ahmad Naik; Saurabh Kumar Chhonker; Aditi Mehrotra; Raj Kumar Koiri; An Overview of Natural Plant Products in the Treatment of Hepatocellular Carcinoma. Anti-Cancer Agents in Medicinal Chemistry 2019, 18, 1838-1859, 10.2174/1871520618666180604085612.
- Quercetin in Hepatocellular Carcinoma . Encyclopedia. Retrieved 2021-1-14
- Paula Fernández-Palanca; Flavia Fondevila; Carolina Méndez-Blanco; María Jesús Tuñón; Javier González-Gallego; José Luis Mauriz; Antitumor Effects of Quercetin in Hepatocarcinoma In Vitro and In Vivo Models: A Systematic Review. Nutrients 2019, 11, 2875, 10.3390/nu11122875.
- Andrea Janz Moreira; Graziella Rodrigues; Silvia Bona; Carlos Thadeu Cerski; Claudio Augusto Marroni; Jose L. Mauriz; Javier González-Gallego; Norma Possa Marroni; Oxidative stress and cell damage in a model of precancerous lesions and advanced hepatocellular carcinoma in rats. Toxicology Reports 2015, 2, 333-340, 10.1016/j.toxrep.2014.11.015.
- Mohammad Hossein Pourhanifeh; Saeed Mehrzadi; Mahboobeh Kamali; Azam Hosseinzadeh; Melatonin and gastrointestinal cancers: Current evidence based on underlying signaling pathways. European Journal of Pharmacology 2020, 886, 173471, 10.1016/j.ejphar.2020.173471.
- Bárbara González-Fernández; Diana I. Sánchez; Irene Crespo; Beatriz San-Miguel; Juan Ortiz De Urbina; Javier González-Gallego; María J. Tuñón; Melatonin Attenuates Dysregulation of the Circadian Clock Pathway in Mice With CCl4-Induced Fibrosis and Human Hepatic Stellate Cells. Frontiers in Pharmacology 2018, 9, 556, 10.3389/fphar.2018.00556.
- Irene Crespo; Paula Fernández-Palanca; Beatriz San‐Miguel; Marcelino Álvarez; Javier González-Gallego; María Jesús Tuñón; Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral‐induced fulminant hepatic failure. Journal of Cellular and Molecular Medicine 2020, 24, 7625-7636, 10.1111/jcmm.15398.
- K B Dakshayani; P Subramanian; T Manivasagam; M Mohamed Essa; S Manoharan; Melatonin modulates the oxidant-antioxidant imbalance during N-nitrosodiethylamine induced hepatocarcinogenesis in rats.. Journal of Pharmacy & Pharmaceutical Sciences 2005, 8, 316-21.
- Perumal Subramanian; Shankaran Mirunalini; Kadiyala Babu Dakshayani; Seithikurippu R. Pandi-Perumal; Ilya Trakht; Daniel P. Cardinali; Prevention by melatonin of hepatocarcinogenesis in rats injected with N-nitrosodiethylamine. Journal of Pineal Research 2007, 43, 305-312, 10.1111/j.1600-079x.2007.00478.x.
- Umawadee Laothong; Porntip Pinlaor; Yusuke Hiraku; Patcharee Boonsiri; Suksanti Prakobwong; Jarinya Khoontawad; Somchai Pinlaor; Protective effect of melatonin against Opisthorchis viverrini-induced oxidative and nitrosative DNA damage and liver injury in hamsters. Journal of Pineal Research 2010, 49, 271-282, 10.1111/j.1600-079x.2010.00792.x.
- Umawadee Laothong; Yusuke Hiraku; Shinji Oikawa; Kitti Intuyod; Mariko Murata; Somchai Pinlaor; Melatonin induces apoptosis in cholangiocarcinoma cell lines by activating the reactive oxygen species-mediated mitochondrial pathway. Oncology Reports 2015, 33, 1443-1449, 10.3892/or.2015.3738.
- Elisabeth Filipski; Perumal Subramanian; Jennyfer Carrière; Catherine Guettier; Hervé Barbason; Francis Lévi; Circadian disruption accelerates liver carcinogenesis in mice. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2009, 680, 95-105, 10.1016/j.mrgentox.2009.10.002.
- K. B. Dakshayani; P. Subramanian; M. Mohamed Essa; Effect Of Melatonin On N-Nitrosodiethylamine-Induced Hepatocarcinogenesis In Rats With Reference To Biochemical Circadian Rhythms. Toxicology Mechanisms and Methods 2007, 17, 67-75, 10.1080/15376520500195798.
- Perumal Subramanian; Kadiyala Babu Dakshayani; Seithikurippu R. Pandi-Perumal; Ilya Trakht; Daniel P. Cardinali; 24-Hour rhythms in oxidative stress during hepatocarcinogenesis in rats: effect of melatonin or α-ketoglutarate. Redox Report 2008, 13, 78-86, 10.1179/135100008x259178.
- Perumal Subramanian; Devi Verma; Onn Haji Hashim; Jaime Jacqueline Jayapalan; Effect of melatonin on antioxidant status and circadian activity rhythm during hepatocarcinogenesis in mice. Journal of Cancer Research and Therapeutics 2014, 10, 1040-4, 10.4103/0973-1482.138227.
- Robert T Dauchy; Melissa A Wren-Dail; Lynell M Dupepe; Steven M Hill; Shulin Xiang; Muralidharan Anbalagan; Victoria P Belancio; Erin M Dauchy; David E Blask; Effect of Daytime Blue-enriched LED Light on the Nighttime Circadian Melatonin Inhibition of Hepatoma 7288CTC Warburg Effect and Progression. Comparative Medicine 2018, 68, 269-279, 10.30802/AALAS-CM-17-000107.
- F Ozdemir; Ozlen Deniz; Kubra Kaynar; Mehmet Arslan; H. Kavgaci; Bulent Yildiz; Fazil Aydin; The effects of melatonin on human hepatoma (Hep G2) cell line.. Bratisl Lek Listy 2009, 110, 276-279.
- María Antonia Cid; Alejandro Úbeda; María Luisa Hernández-Bule; María Antonia Martínez; María Ángeles Trillo; Antagonistic Effects of a 50 Hz Magnetic Field and Melatonin in the Proliferation and Differentiation of Hepatocarcinoma Cells. Cellular Physiology and Biochemistry 2012, 30, 1502-1516, 10.1159/000343338.
- Fei Long; Chengyong Dong; Keqiu Jiang; Yakun Xu; Xinming Chi; Deguang Sun; Rui Liang; Zhenming Gao; Shao Shujuan; Liming Wang; et al. Melatonin enhances the anti-tumor effect of sorafenib via AKT/p27-mediated cell cycle arrest in hepatocarcinoma cell lines. RSC Advances 2017, 7, 21342-21351, 10.1039/c7ra02113e.
- Diana I. Sánchez; Bárbara González-Fernández; Beatriz San-Miguel; Juan Ortiz De Urbina; Irene Crespo; Javier González-Gallego; María J. Tuñón; Melatonin prevents deregulation of the sphingosine kinase/sphingosine 1-phosphate signaling pathway in a mouse model of diethylnitrosamine-induced hepatocellular carcinoma. Journal of Pineal Research 2016, 62, e12369, 10.1111/jpi.12369.
- Lingyun Zhang; Yufeng He; Ximing Wu; Guangshan Zhao; Ke Zhang; Chung S. Yang; Russel J. Reiter; Jinsong Zhang; Melatonin and (-)-Epigallocatechin-3-Gallate: Partners in Fighting Cancer.. Cells 2019, 8, 745, 10.3390/cells8070745.
- Lina Mi; Hongyu Kuang; Melatonin Regulates Cisplatin Resistance and Glucose Metabolism Through Hippo Signaling in Hepatocellular Carcinoma Cells.. Cancer Management and Research 2020, 12, 1863-1874, 10.2147/CMAR.S230466.
- Yuyan Han; Sharon DeMorrow; Pietro Invernizzi; Qing Jing; Shannon Glaser; Anastasia Renzi; Fanyin Meng; Julie Venter; Francesca Bernuzzi; Mellanie White; et al.Heather FrancisAna LleoMarco MarzioniPaolo OnoriMenico AlvaroGuido TorzilliEugenio GaudioGianfranco Alpini Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma; its synthesis is reduced favoring cholangiocarcinoma growth. American Journal of Physiology-Gastrointestinal and Liver Physiology 2011, 301, G623-G633, 10.1152/ajpgi.00118.2011.
- Claire M. Pfeffer; Amareshwar T. K. Singh; Apoptosis: A Target for Anticancer Therapy. International Journal of Molecular Sciences 2018, 19, 448, 10.3390/ijms19020448.
- Bong-Jo Kim; Seung-Wook Ryu; Byoung-Joon Song; JNK- and p38 Kinase-mediated Phosphorylation of Bax Leads to Its Activation and Mitochondrial Translocation and to Apoptosis of Human Hepatoma HepG2 Cells. Journal of Biological Chemistry 2006, 281, 21256-21265, 10.1074/jbc.m510644200.
- S Carbajo-Pescador; C Steinmetz; A Kashyap; S Lorenz; J L Mauriz; M Heise; P R Galle; J González-Gallego; Susanne Strand; Melatonin induces transcriptional regulation of Bim by FoxO3a in HepG2 cells. British Journal of Cancer 2012, 108, 442-449, 10.1038/bjc.2012.563.
- Umawadee Laothong; Porntip Pinlaor; Patcharee Boonsiri; Chawalit Pairojkul; Aroonsri Priprem; Nutjaree Pratheepawanit Johns; Lakhanawan Charoensuk; Kitti Intuyod; Somchai Pinlaor; Melatonin inhibits cholangiocarcinoma and reduces liver injury in Opisthorchis viverrini -infected and N -nitrosodimethylamine-treated hamsters. Journal of Pineal Research 2013, 55, 257-266, 10.1111/jpi.12068.
- Jucimara F Colombo; Bruna V. Jardim-Perassi; Joao Paulo Senna Ferreira; Cristine Zampieri Braga; Nathalia Martins Sonehara; Rubens De Paula Júnior; Marina Gobbe Moschetta; Ana Paula Girol; Debora Aparecida Pires De Campos Zuccari; Melatonin Differentially Modulates NF-кB Expression in Breast and Liver Cancer Cells. Anti-Cancer Agents in Medicinal Chemistry 2019, 18, 1688-1694, 10.2174/1871520618666180131112304.
- Lixia Zha; Lulu Fan; Guoping Sun; Hua Wang; Tai Ma; Fei Zhong; Wei Wei; Melatonin sensitizes human hepatoma cells to endoplasmic reticulum stress-induced apoptosis. Journal of Pineal Research 2012, 52, 322-331, 10.1111/j.1600-079x.2011.00946.x.
- Li-Jia Bu; Han-Qing Yu; Lu-Lu Fan; Xiao-Qiu Li; Fang Wang; Jia-Tao Liu; Fei Zhong; Cong-Jun Zhang; Wei Wei; Hua Wang; et al.Guo-Ping Sun Melatonin, a novel selective ATF-6 inhibitor, induces human hepatoma cell apoptosis through COX-2 downregulation. World Journal of Gastroenterology 2017, 23, 986-998, 10.3748/wjg.v23.i6.986.
- Lu-Lu Fan; Guo-Ping Sun; Wei Wei; Zhang-Gui Wang; Lei Ge; Wei-Zheng Fu; Hua Wang; Melatonin and Doxorubicin synergistically induce cell apoptosis in human hepatoma cell lines. World Journal of Gastroenterology 2010, 16, 1473-1481, 10.3748/wjg.v16.i12.1473.
- Lulu Fan; Guoping Sun; Tai Ma; Fei Zhong; Yu Lei; Xiaoqiu Li; Wei Wei; Melatonin reverses tunicamycin-induced endoplasmic reticulum stress in human hepatocellular carcinoma cells and improves cytotoxic response to doxorubicin by increasing CHOP and decreasing Survivin. Journal of Pineal Research 2013, 55, 184-194, 10.1111/jpi.12061.
- Lulu Fan; Guoping Sun; Tai Ma; Fei Zhong; Wei Wei; Melatonin overcomes apoptosis resistance in human hepatocellular carcinoma by targeting Survivin and XIAP. Journal of Pineal Research 2013, 55, 174-183, 10.1111/jpi.12060.
- Jiaojiao Hao; Zhenglin Li; Changlin Zhang; Wendan Yu; Zhipeng Tang; Yixin Li; Xu Feng; Yue Gao; Quentin Liu; Wenlin Huang; et al.Wei GuoWuguo Deng Targeting NF-κB/AP-2β signaling to enhance antitumor activity of cisplatin by melatonin in hepatocellular carcinoma cells. American journal of cancer research 2017, 7, 13-27.
- Shibo Lin; Katrin Hoffmann; Chao Gao; Marius Petrulionis; Ingrid Herr; P. Schemmer; Melatonin promotes sorafenib-induced apoptosis through synergistic activation of JNK/c-jun pathway in human hepatocellular carcinoma. Journal of Pineal Research 2017, 62, e12398, 10.1111/jpi.12398.
- Lu Ao; Li Li; Huaqin Sun; Huxing Chen; Yawei Li; Haiyan Huang; Xianlong Wang; Zheng Guo; Ruixiang Zhou; Transcriptomic analysis on the effects of melatonin in gastrointestinal carcinomas. BMC Gastroenterology 2020, 20, 1-11, 10.1186/s12876-020-01383-z.
- Ruobing Wang; Yahui Liu; Xuguang Mi; Qingmin Chen; Peiqiang Jiang; Junjie Hou; Yifan Lin; Siqi Li; Bai Ji; Yanqiu Fang; et al. Sirt3 promotes hepatocellular carcinoma cells sensitivity to regorafenib through the acceleration of mitochondrial dysfunction. Archives of Biochemistry and Biophysics 2020, 689, 108415, 10.1016/j.abb.2020.108415.
- Flavia Fondevila; Carolina Méndez-Blanco; Paula Fernández-Palanca; Javier González-Gallego; José Luis Mauriz; Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers. Experimental & Molecular Medicine 2019, 51, 1-15, 10.1038/s12276-019-0308-1.
- Shuo Yang; Liang Yang; Xinyu Li; Bowen Li; Yan Li; Xiaodong Zhang; Yingbo Ma; Xueqiang Peng; Hongyuan Jin; Hangyu Li; et al. New insights into autophagy in hepatocellular carcinoma: mechanisms and therapeutic strategies.. null 2019, 9, 1329-1353.
- Chang Liu; Zhiling Jia; Xia Zhang; Jincai Hou; Li Wang; Shuling Hao; Xinjian Ruan; Zhonghe Yu; Yongqiu Zheng; Involvement of melatonin in autophagy-mediated mouse hepatoma H22 cell survival. International Immunopharmacology 2012, 12, 394-401, 10.1016/j.intimp.2011.12.012.
- Kangsadarn Bennukul; Melatonin attenuates cisplatin-induced HepG2 cell deathviathe regulation of mTOR and ERCC1 expressions. World Journal of Hepatology 2014, 6, 230-42, 10.4254/wjh.v6.i4.230.
- 45. Liu, Y.; Liu, J.; Cheng, L.; Fan, L.; Wang, F.; Yu, H.; Liu, Q.; Li, Y.; Bu, L.; Li, X.; et al.et al Melatonin increases the anti-tumor effects of sorafenib on human hepatoma cell lines via down-regulating autophagy . International Journal of Clinical and Experimental Medicine 2017, 10, 14109-14120.
- Bei Zhou; Qianqian Lu; Jiatao Liu; Lulu Fan; Yu Wang; Wei Wei; Hua Wang; Guoping Sun; Melatonin Increases the Sensitivity of Hepatocellular Carcinoma to Sorafenib through the PERK-ATF4-Beclin1 Pathway. International Journal of Biological Sciences 2019, 15, 1905-1920, 10.7150/ijbs.32550.
- José Luis Mauriz; Javier González-Gallego; Antiangiogenic Drugs: Current Knowledge and New Approaches to Cancer Therapy. Journal of Pharmaceutical Sciences 2008, 97, 4129-4154, 10.1002/jps.21286.
- Michael A. Morse; Weijing Sun; Richard Kim; Aiwu Ruth He; Paolo B. Abada; Michelle Mynderse; Richard S. Finn; The Role of Angiogenesis in Hepatocellular Carcinoma. Clinical Cancer Research 2018, 25, 912-920, 10.1158/1078-0432.ccr-18-1254.
- Romina Mancinelli; Caterina Loredana Mammola; Roberta Sferra; Simona Pompili; Antonella Vetuschi; Luigi Pannarale; Role of the Angiogenic Factors in Cholangiocarcinoma. Applied Sciences 2019, 9, 1393, 10.3390/app9071393.
- Jucimara Colombo; João Marcos Wolf Maciel; Lívia Carvalho Ferreira; Renato Ferreira Da Silva; Debora Aparecida Pires De Campos Zuccari; Effects of melatonin on HIF-1α and VEGF expression and on the invasive properties of hepatocarcinoma cells. Oncology Letters 2016, 12, 231-237, 10.3892/ol.2016.4605.
- Tong-Hong Wang; Chi-Hao Wu; Chau-Ting Yeh; Shih-Chi Su; Shih-Min Hsia; Kung-Hao Liang; Chin-Chuan Chen; Chuen Hsueh; Chi-Yuan Chen; Melatonin suppresses hepatocellular carcinoma progression via lncRNA-CPS1-IT-mediated HIF-1α inactivation. Oncotarget 2017, 8, 82280-82293, 10.18632/oncotarget.19316.
- Joo Eun Jung; Hyun‐Gyu Lee; Ik-Hyun Cho; Doo Hyun Chung; Sun‐Hee Yoon; Young Mok Yang; Jung Weon Lee; Seongwon Choi; Jong‐Wan Park; Sang‐Kyu Ye; et al.Myung-Hee Chung STAT3 is a potential modulator of HIF‐1‐mediated VEGF expression in human renal carcinoma cells. The FASEB Journal 2005, 19, 1296-1298, 10.1096/fj.04-3099fje.
- Chin-Chuan Chen; Chi-Yuan Chen; Shu-Huei Wang; Chau-Ting Yeh; Shih-Chi Su; Shir-Hwa Ueng; W.-Y. Chuang; Chuen Hsueh; Tong-Hong Wang; Melatonin Sensitizes Hepatocellular Carcinoma Cells to Chemotherapy Through Long Non-Coding RNA RAD51-AS1-Mediated Suppression of DNA Repair. Cancers 2018, 10, 320, 10.3390/cancers10090320.
- Tong-Hong Wang; Chuen Hsueh; Chin-Chuan Chen; Wan-Syuan Li; Chau-Ting Yeh; Jang-Hau Lian; Junn-Liang Chang; Chi-Yuan Chen; Melatonin Inhibits the Progression of Hepatocellular Carcinoma through MicroRNA Let7i-3p Mediated RAF1 Reduction. International Journal of Molecular Sciences 2018, 19, 2687, 10.3390/ijms19092687.
- Zu-Yau Lin; Wan-Long Chuang; Pharmacologic concentrations of melatonin have diverse influence on differential expressions of angiogenic chemokine genes in different hepatocellular carcinoma cell lines. Biomedicine & Pharmacotherapy 2010, 64, 659-662, 10.1016/j.biopha.2010.09.006.
- Bojidar Tomov; Dimitar Popov; Radosveta Tomova; Nicola Vladov; Willem Den Otter; Zachary Krastev; Therapeutic response of untreatable hepatocellular carcinoma after application of the immune modulators IL-2, BCG and melatonin.. Anticancer Research 2013, 33, 4531-4536.
- Liang Cheng; Jiatao Liu; Qingqing Liu; Yu Liu; Lulu Fan; Fang Wang; Hanqing Yu; Yuhuan Li; Lijia Bu; Xiaoqiu Li; et al.Wei WeiHua WangGuoping Sun Exosomes from Melatonin Treated Hepatocellularcarcinoma Cells Alter the Immunosupression Status through STAT3 Pathway in Macrophages. International Journal of Biological Sciences 2017, 13, 723-734, 10.7150/ijbs.19642.
- Wachanan Wongsena; Lakhanawan Charoensuk; Rungtiwa Dangtakot; Porntip Pinlaor; Kitti Intuyod; Somchai Pinlaor; Melatonin suppresses eosinophils and Th17 cells in hamsters treated with a combination of human liver fluke infection and a chemical carcinogen. Pharmacological Reports 2018, 70, 98-105, 10.1016/j.pharep.2017.07.017.
- Mohammed A. El-Magd; Yasser Mohamed; Eman S. El-Shetry; Shafika A. Elsayed; Maha Abo Gazia; Ghada A. Abdel-Aleem; Noha M. Shafik; Walied S. Abdo; Nabila I. El-Desouki; Mohamed A. Basyony; et al. Melatonin maximizes the therapeutic potential of non-preconditioned MSCs in a DEN-induced rat model of HCC. Biomedicine & Pharmacotherapy 2019, 114, 108732, 10.1016/j.biopha.2019.108732.
- Yasser Mohamed; Mohamed A. Basyony; Nabila I. El-Desouki; Walied S. Abdo; Mohammed A. El-Magd; The potential therapeutic effect for melatonin and mesenchymal stem cells on hepatocellular carcinoma. BioMedicine 2019, 9, 24, 10.1051/bmdcn/2019090424.




