| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jay Meegoda | + 1321 word(s) | 1321 | 2020-12-02 11:39:27 |
Video Upload Options
Per- and polyfluoroalkyl substances (PFAS) are a family of synthetic fluorinated organic compounds whose widespread use and resistance to biodegradation have led to their accumulation in the environment, causing growing concerns over their impact on humans.
1. Introduction
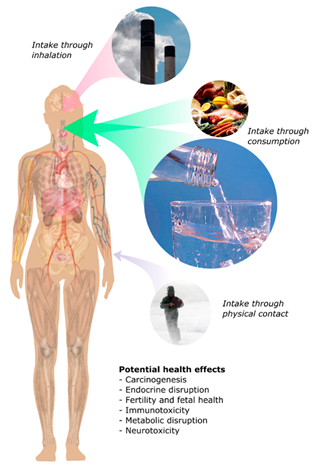
PFAS are versatile family of chemicals used in many diverse applications such as fire suppressants, paints and coatings, adhesive sealants, recovery of oil and gas, agrochemical adjuvants, repellant cookware coatings, waterproofing, among others [1][2][3][4][5][6][7]. Their commercial success and widespread use can be attributed to physical and chemical properties, such as a high degree of thermal and chemical stability of the F-C bonds compared to traditional C-H bonds in their alkyl chains [7][8][9][10] and the ability of flurosurfactants to reduce surface tension in water to about 15 mNm; in contrast, traditional hydrocarbon surfactants (HS) that can only reduce surface tension to about 28 mNm. This is a double-edged sword; these very same properties cause bio-accumulative PFAS to contaminate and persist in numerous environmental media such as groundwater, causing them to be nicknamed “forever chemicals” [11][12][13]. Global studies have identified significant concentrations of PFAS at urban and rural sites on all six continents, with many sites located at far distances from potential sources, such as in the Antarctic [14][15][16][17][18][19]. Throughout these sites, PFAS have been detected to be present in the local streams soil, plants, and animal tissues [20][21][22]. The presence of PFAS in animal tissues and food packaging has been cited as an important pathway for human exposure to PFAS, along with other forms of environmental exposure [23][24]. Figure 1 shows schematic pathways of human exposure.
2. Classification of PFAS
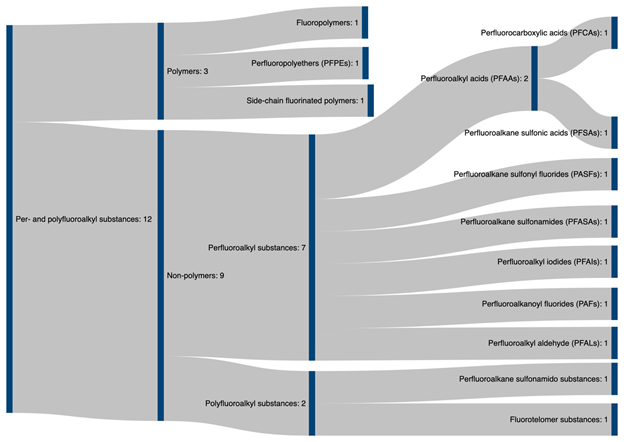
PFAS can be broadly divided into two main classes: fluoropolymers and non-polymeric flurosurfactants. While the class of fluropolymers include well known substances such as PTFE (Teflon) and ethylene tetrafluoroethylene (ETFE, trade name Tefzel), most research into PFAS focuses on fluorosurfactants (FS) , as they are widely used in industry and as such frequently detected in the environment [7][25] and as such have been subjected to increased regulatory scrutiny. The FS, depending upon the degree of fluorination of their alkyl chains, are classified into two classes: perfluoroalkyl and polyfluoroalkyl surfactants (PFAS). Perfluoroalkyl surfactants contain fully fluorinated alkyl chains where all possible hydrogen atoms in the alkyl chain have been replaced by fluorine. On the other hand, polyfluoroalkyl substances are only partially fluorinated, leaving some carbon-hydrogen bonds intact. The hydrophilic end groups of these two classes can be carboxylic acid (PFCA), quaternary ammonium, or sulfonic acid (PFSA) functionalities . Among these the perflurooctanoic carboxyic acid (PFOA) and perflurooctanoic sulfonate (PFOS) are the most frequently detected FS in the environment and are known to be toxic to humans. The majority of the fluoropolymers in the environment as well are ultimately transformed to perfluoroalkyl acids (PFAA) via biotic and abiotic transformation [7][26][27]. Figure 2 illustrates a family tree of PFAS.
3. Physical and Chemical Properties of PFAS
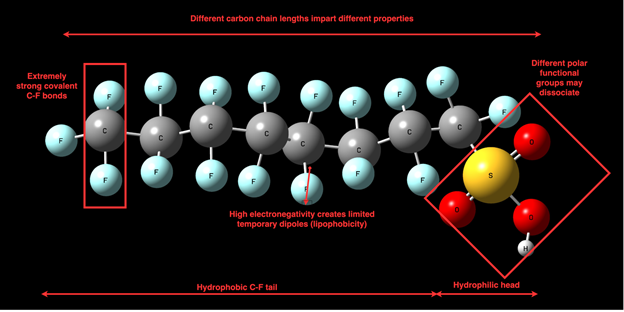
A thorough comprehension of the physical and chemical properties of PFAS is vital for understanding their release into the environment and the difficulties related to their removal from the environment [28]. These unique properties are primarily associated with the fluorine atoms that replace hydrogen along PFAS carbon chains. The high electronegativity and small size of fluorine atom makes the C-F bond one of the strongest covalent bonds in nature. The large amount of energy required to break this bond is responsible for the chemical stability of PFAS in the presence of oxidants and high temperature environments as well as their resistance to biological degradation by microbes [10][28]. Additionally, the high degree polarizability of fluorine imparts the signature property of PFAS having both hydrophobicity and lipophobicity within the same molecule [29]. The structure of a prototypical FS molecule, in this case, PFOS, is outlined in Figure 3.
Figure 1. Potential intake pathways of PFAS
While the C-F bond in their alkyl chain is common to all PFAS, there is significant structural variation across these compounds. Alkyl chain length and its degree of fluorination is often used in the classification of PFAS, as they have a strong impact on surface and performance properties. Increasing the alkyl chain length and the degree of fluorination is associated with increasing lipophilicity and hydrophobicity [28][29]. PFAS are found to accumulate in surface water, ground water and biosolid/sediment/soil substrates, although longer-chain PFAS due to their poor solubility in water are more likely to adsorb onto soil particles These differences in aqueous solubility and absorptivity cause disparities in mobility and environmental transport of these molecules [30]. Therefore, the distribution of long-chain and short-chain PFAS in the environment is highly dependent on the alkyl chain length.
Figure 2. Classification tree of PFAS family compounds adapted from Buck et al. [3][10] and ITRC PFAS Team [10].
Where industrial applications are considered, shorter alkylated FS (<C6) , as expected, have much lower surface activity and considerably much poor performance compared to longer chain FS in many of the applications. In the early 2000 traditional long chain FS (>C6) came under scrutiny by regulatory bodies due to severity of bioaccumulation of FS in the environment and their toxicity to humans. The accumulation of long-chain and short-chain PFAS in the environment is highly dependent on the alkyl chain length among other factors including (but not limited to) charge type of the hydrophilic functional group: carboxylates , sulfonates, and amines.
4. Synthesis and Environmental Detection of PFAS
Electrochemical fluorination (ECF) and fluorotelomerization are two major processes that are used to manufacture fluorinated polymers, and flurosurfactants Among FS, PFSA are produced using the ECF process, whereas PFCA are produced by both ECF and fluorotelomerization [7]. During the ECF process, the corresponding hydrocarbon compound is fluorinated using anhydrous HF [25]. By this process, short-chain sulfonyl fluorides as precursor for the synthesis of PFAS can be obtained in excellent yields; however, the yield of this fluorination decreases steadily with increasing alkyl chain-length (>C6 ). The fluorotelomerization process involves the reaction of perfluoroethyl iodide (CF3CF2-I) and tetrafluoroethylene (TFE, CF2=CF2) to manufacture fluorotelomer-based surfactants as well as fluoropolymers. When compared to EFC, the fluorotelomarization process is considered safer due to the generation of less toxic byproducts. Table 1 shows the major suppliers of FS.
Figure 3. Generic structure of a PFAS molecule (PFOS)
Table 1 Key Suppliers of PFAS
|
Supplier |
Market |
Trade name |
CF chain length and ST reduction |
|
DuPont (US) |
Paints, coatings, wax, polishers, cleaners, films, adhesives, stain guard |
Capstone |
Wide range of FS, Fluroalky, perfluroethers ,etc. C4F and C6 F ST reduction 20 mNm |
|
3M (US) |
Stain resistant Paints, coatings, wax, polishers, cleaners, films, adhesives, |
Novec |
Fluropolymers and surfactants CF4 ST reduction 20 mNm |
|
Advanced Polymer Inc. (US) |
Architectural finishes, paints, inks, coatings, water repellents, waxes, polishers |
APFS |
Both FC surfactants and polymers CF8 4 and up to 8 DT reduction of 15 mNm for CF 8 |
|
Chem Guard (US) |
Firefighting foams; electroplating ,paints, coatings, wax, stain guard and adhesives |
Lodyne |
Telomerization FS, 4, C4F,C&F,ST 19 mNm |
|
Omnova Solutions (US) |
coatings, wax, wax emulsions, |
Polyfox |
Mostly polymers No ST reduction |
|
Dynex Corporation (US) |
Fire suppressants; coatings, paints, adhesives, printing inks |
Dynax DX |
CF 6 flurotelomer process ST reduction not reported |
|
Innovative Chemical Technologies- ICT (US) |
Hydrocarbon Foamers and ST reducers, coatings waxes, electroplating /metal finishing |
Flexiwet |
No information available |
|
AOC Seimi Chemical Co (Japan) |
Inks, coatings , floor waxes , fire extinguishing, electroplating |
Surflon |
6 and < C^F |
|
Merck KGaA (Germany) EMD |
Adheisves, coatings |
Trivida |
Shortest chain FS claimed fastest dynamic ST reduction 20 mNm |
Currently available commercial analytical techniques in detecting and characterizing PFAS are very limited. PFAS detection is currently done using high resolution liquid chromatography with mass spectroscopy (LC/MS/MS). However, LC/MS/MS is unable to identify most PFAS compounds and their byproducts generated after remediation. To overcome these limitations there are other advanced analytical techniques that are currently being developed (e.g., total oxidizable precursor (TOP) assays, particle-induced gamma-ray emission (PIGE) spectroscopy, adsorbable organic fluorine (AOF) paired with combustion ion chromatography (CIC), and high-resolution mass spectrometry techniques such as quadrupole time-of-flight (qTOF)). Those emerging analytical methods still remain at the research and development stage and are not commercially available [31][32][33][34][35][36][37][38].
References
- S. Ebnesajjad, ed., Discovery and History of Fluoropolymers, in: Introd. to Fluoropolymers, Elsevier, Oxford, 2013: pp. 17–35. https://doi.org/10.1016/B978-1-4557-7442-5.00003-6.
- OECD Environment Directorate, Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs), Organisation for Economic Co-operation and Development (OECD), Paris, France, 2018.
- X. Dauchy, V. Boiteux, C. Bach, C. Rosin, J.F. Munoz, Per- and Polyfluoroalkyl Substances in Firefighting Foam Concentrates and Water Samples Collected Near Sites Impacted by the Use of these Foams, Chemosphere. 183 (2017) 53–61. https://doi.org/10.1016/j.chemosphere.2017.05.056.
- H.P. Tannenbaum, Non-Stick Coating System with PTFE-FEP for Concentration Gradient, 5,230,961. https://patents.google.com/patent/US5230961A/en.
- M.H. Whittaker, L. Heine, Toxicological and Environmental Issues Associated with Waterproofing and Water Repellent Formulations, in: J. Williams (Ed.), Waterproof Water Repel. Text. Cloth., Elsevier, Oxford, UK, 2018: pp. 89–120. https://doi.org/10.1016/B978-0-08-101212-3.00004-6.
- W.S. Dean, H.A. Adejumo, A. Caiati, P.M. Garay, A.S. Harmata, L. Li, E.E. Rodriguez, S. Sundar, A Framework for Regulation of New and Existing PFAS by EPA, J. Sci. Policy Gov. 16 (2020) 14.
- ITRC PFAS Team, PFAS Fact Sheets: Naming Conventions and Use, Interstate Technology Regulatory Council, Washington, DC, 2020. https://pfas-1.itrcweb.org/2-pfas-chemistry-and-naming-conventions-history-and-use-of-pfas-and-sources-of-pfas-releases-to-the-environment-overview/.
- Z. Wang, I.T. Cousins, M. Scheringer, K. Hungerbuehler, Hazard Assessment of Fluorinated Alternatives to Long-Chain Perfluoroalkyl Acids (PFAAs) and their Precursors: Status Quo, ongoing Challenges and Possible Solutions, Environ. Int. 75 (2015) 172–179. https://doi.org/10.1016/j.envint.2014.11.013.
- F.M. Hekster, R.W.P.M. Laane, P. de Voogt, Environmental and Toxicity Effects of Perfluoroalkylated Substances, in: Rev. Environ. Contam. Toxicol., Springer, New York, 2003: pp. 99–121. https://doi.org/10.1007/0-387-21731-2_4.
- E. Kissa, Fluorinated Surfactant Repellents, Marcel Dekker, New York, 2001.
- M.P. Krafft, J.G. Riess, Per- and Polyfluorinated Substances (PFASs): Environmental Challenges, Curr. Opin. Colloid Interface Sci. 20 (2015) 192–212. https://doi.org/10.1016/j.cocis.2015.07.004.
- F. Suja, B.K. Pramanik, S.M. Zain, Contamination, Bioaccumulation and Toxic Effects of Perfluorinated Chemicals (PFCs) in the Water Environment: a Review Paper, Water Sci. Technol. 60 (2009) 1533–1544. https://doi.org/10.2166/wst.2009.504.
- T.D. Appleman, C.P. Higgins, O. Quiñones, B.J. Vanderford, C. Kolstad, J.C. Zeigler-Holady, E.R.V. Dickenson, Treatment of Poly- and Perfluoroalkyl Substances in U.S. Full-Scale Water Treatment Systems, Water Res. 51 (2014) 246–255. https://doi.org/10.1016/j.watres.2013.10.067.
- J.P. Giesy, K. Kannan, Global Distribution of Perfluorooctane Sulfonate in Wildlife, Environ. Sci. Technol. 35 (2001) 1339–1342. https://doi.org/10.1021/es001834k.
- M.L. Brusseau, R.H. Anderson, B. Guo, PFAS Concentrations in Soils: Background Levels Versus Contaminated Sites, Sci. Total Environ. 740 (2020) 140017. https://doi.org/10.1016/j.scitotenv.2020.140017.
- K. Rankin, S.A. Mabury, T.M. Jenkins, J.W. Washington, A North American and global survey of perfluoroalkyl substances in surface soils: Distribution patterns and mode of occurrence, Chemosphere. 161 (2016) 333–341. https://doi.org/10.1016/j.chemosphere.2016.06.109.
- L. Ahrens, Z. Xie, R. Ebinghaus, Distribution of Perfluoroalkyl Compounds in Seawater from Northern Europe, Atlantic Ocean, and Southern Ocean, Chemosphere. 78 (2010) 1011–1016. https://doi.org/10.1016/j.chemosphere.2009.11.038.
- K.Y. Kwok, E. Yamazaki, N. Yamashita, S. Taniyasu, M.B. Murphy, Y. Horii, G. Petrick, R. Kallerborn, K. Kannan, K. Murano, P.K.S. Lam, Transport of Perfluoroalkyl Substances (PFAS) from an Arctic Glacier to Downstream Locations: Implications for sources, Sci. Total Environ. 447 (2013) 46–55. https://doi.org/10.1016/j.scitotenv.2012.10.091.
- J.L. Domingo, M. Nadal, Human Exposure to Per- and Polyfluoroalkyl Substances (PFAS) Through Drinking Water: A Review of the Recent Scientific Literature, Environ. Res. 177 (2019) 108648. https://doi.org/10.1016/j.envres.2019.108648.
- T. Stoiber, S. Evans, O. V. Naidenko, Disposal of Products and Materials Containing Per- and Polyfluoroalkyl Substances (PFAS): A Cyclical Problem, Chemosphere. 260 (2020) 127659. https://doi.org/10.1016/j.chemosphere.2020.127659.
- B.C. Kelly, M.G. Ikonomou, J.D. Blair, B. Surridge, D. Hoover, R. Grace, F.A.P.C. Gobas, Perfluoroalkyl Contaminants in an Arctic Marine Food Web: Trophic Magnification and Wildlife Exposure, Environ. Sci. Technol. 43 (2009) 4037–4043. https://doi.org/10.1021/es9003894.
- ITRC PFAS Team, PFAS Fact Sheet: Fate and Trasnport, Interstate Technology Regulatory Council, Washington, DC, 2020. https://pfas-1.itrcweb.org/5-environmental-fate-and-transport-processes/.
- J.L. Domingo, Health risks of Dietary Exposure to Perfluorinated Compounds, Environ. Int. 40 (2012) 187–195. https://doi.org/10.1016/j.envint.2011.08.001.
- S.A. Tittlemier, K. Pepper, C. Seymour, J. Moisey, R. Bronson, X.-L. Cao, R.W. Dabeka, Dietary Exposure of Canadians to Perfluorinated Carboxylates and Perfluorooctane Sulfonate via Consumption of Meat, Fish, Fast Foods, and Food Items Prepared in Their Packaging, J. Agric. Food Chem. 55 (2007) 3203–3210. https://doi.org/10.1021/jf0634045.
- R.C. Buck, J. Franklin, U. Berger, J.M. Conder, I.T. Cousins, P. de Voogt, A.A. Jensen, K. Kannan, S.A. Mabury, S.P.J. van Leeuwen, Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins, Integr. Environ. Assess. Manag. 7 (2011) 513–541. https://doi.org/10.1002/ieam.258.
- K. Prevedouros, I.T. Cousins, R.C. Buck, S.H. Korzeniowski, Sources, Fate and Transport of Perfluorocarboxylates, Environ. Sci. Technol. 40 (2006) 32–44. https://doi.org/10.1021/es0512475.
- J.W. Washington, K. Rankin, E.L. Libelo, D.G. Lynch, M. Cyterski, Determining Global Background Soil PFAS Loads and the Fluorotelomer-Based Polymer Degradation Rates That can Account for These Loads, Sci. Total Environ. 651 (2019) 2444–2449. https://doi.org/https://doi.org/10.1016/j.scitotenv.2018.10.071.
- ITRC PFAS Team, PFAS Fact sheets: Physical and Chemical Properties, Interstate Technology Regulatory Council, Washington, DC, 2020. https://pfas-1.itrcweb.org/4-physical-and-chemical-properties/#4_1.
- E. Gagliano, M. Sgroi, P.P. Falciglia, F.G.A. Vagliasindi, P. Roccaro, Removal of Poly-and Perfluoroalkyl Substances (PFAS) from Water by Adsorption: Role of PFAS Chain Length, Effect of Organic Matter and Challenges in Adsorbent Regeneration, Water Res. 171 (2020) 115381. https://doi.org/10.1016/j.watres.2019.115381.
- S. Brendel, É. Fetter, C. Staude, L. Vierke, A. Biegel-Engler, Short-Chain Perfluoroalkyl Acids: Environmental Concerns and a Regulatory Strategy under REACH, Environ. Sci. Eur. 30 (2018) 9. https://doi.org/10.1186/s12302-018-0134-4.
- A.M. Trautmann, H. Schell, K.R. Schmidt, K.-M. Mangold, A. Tiehm, Electrochemical Degradation of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Groundwater, Water Sci. Technol. 71 (2015) 1569–1575. https://doi.org/10.2166/wst.2015.143.
- B.J. Place, J.A. Field, Identification of Novel Fluorochemicals in Aqueous Film-Forming Foams Used by the US Military, Environ. Sci. Technol. 46 (2012) 7120–7127. https://doi.org/10.1021/es301465n.
- K.A. Barzen-Hanson, S.E. Davis, M. Kleber, J.A. Field, Sorption of Fluorotelomer Sulfonates, Fluorotelomer Sulfonamido Betaines, and a Fluorotelomer Sulfonamido Amine in National Foam Aqueous Film-Forming Foam to Soil, Environ. Sci. Technol. 51 (2017) 12394–12404. https://doi.org/10.1021/acs.est.7b03452.
- H. Lee, S.A. Mabury, Sorption of Perfluoroalkyl Phosphonates and Perfluoroalkyl Phosphinates in Soils, Environ. Sci. Technol. 51 (2017) 3197–3205. https://doi.org/10.1021/acs.est.6b04395.
- J.A. Field, PFASs in Leachate and Wastewater, in: Environ. Res. Educ. Summit, Ann Arbor, MI, n.d.
- S. Mejia-Avendaño, G. Munoz, S. Vo Duy, M. Desrosiers, P. Benoı̂t, S. Sauvé, J. Liu, Novel Fluoroalkylated Surfactants in Soils Following Firefighting Foam Deployment During the Lac-Mégantic Railway Accident, Environ. Sci. Technol. 51 (2017) 8313–8323. https://doi.org/10.1021/acs.est.7b02028.
- F. Xiao, S.A. Golovko, M.Y. Golovko, Identification of novel non-ionic, cationic, zwitterionic, and anionic polyfluoroalkyl substances using UPLC–TOF–MSE high-resolution parent ion search, Anal. Chim. Acta. 988 (2017) 41–49. https://doi.org/https://doi.org/10.1016/j.aca.2017.08.016.
- C.A. McDonough, J.L. Guelfo, C.P. Higgins, Measuring total PFASs in water: The tradeoff between selectivity and inclusivity, Curr. Opin. Environ. Sci. Heal. 7 (2019) 13–18. https://doi.org/https://doi.org/10.1016/j.coesh.2018.08.005.