
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Silvana Alfei | + 3713 word(s) | 3713 | 2021-01-14 13:24:25 | | | |
| 2 | Karina Chen | Meta information modification | 3713 | 2021-01-19 04:32:02 | | |
Video Upload Options
Antimicrobial resistance, based on the built-in abilities of bacteria to nullify the activity of current antibiotics, leaves a growing number of bacterial infections untreatable. The development of novel agents able to interact with the external layers of bacteria, causing irreparable damage, without the need to interact with processes or substances vital for the bacterium but subjected to mutation, is a recent approach to address this issue. In this contest, natural cationic antimicrobial peptides (CAMPs), able to kill bacteria by non-specific detrimental interaction with the negative bacterial membranes, have inspired the syntesis of cationic polymers which proved to be promising solutions. Only recently, also dendrimers were considered suitable macromolecules for the preparation of more advanced cationic biomimetic nanoparticles, able to harmonize the typical properties of dendrimers, including nanosize, mono-dispersion, long-term stability, high functionality, and the non-specific mechanism of action of CAMPs. As reviewed in an article available on Nanomaterials at https://www.mdpi.com/2079-4991/10/10/2022/htm#, different types of cationic dendrimers which showed considerable antibacterial effects have been synthetized and studied in the last decade, but only few structures, such as PAMAM and peptides-based ones have been extensively investigated. Moreover, despite the very promising results deriving from the evaluations of the different cationic dendrimers, these devices require further studies and more rationalized investigations. In this new entry, we have reviewed the cationic dendrimers and their antibacterial effects, which until now have only been minimally studied. In addition, we have allowed ourselves to express our considerations on how the case studies reported in the last decade regarding the different types of cationic dendrimers should be improved.
Introduction
Gram-negative bacteria such as Klebsiella pneumoniae, Acinetobacter baumannii, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, Burkholderia cepacia, and Escherichia coli pose a major threat to human health, since they are the most critically resistant and rapidly spreading bacteria. In particular, aerobic non-fermenting Gram-negative bacilli such as A. baumannii, P. aeruginosa, and S. maltophilia, are emerging as clinically relevant superbugs, contributing significantly, with their alarming resistance levels, to numerous therapeutic failures. Given this situation, the medical research community must develop new antimicrobial agents active on current resistant strains of Gram-positive and Gram-negative bacteria. Natural cationic antimicrobial peptides (CAMPs), which are molecules killing bacteria without the need to enter the bacterium cell and to interfere with specific metabolic processes, which can eventually be modified—by genetic mutation—providing resistance, have inspired the synthesis of cationic macromolecular structures with high molecular weight, including in the last decade also dendrimers (Ds). The available case studies have concerned mainly cationic poly(amidoamine) (PAMAM), polypropylenimine (PPI), peptide dendrimer structures and ammonium terminated dendrimers. Minor consideration has been reserved for other categories of cationic dendrimers, including amino acid-modified, polyester-based dendrimer scaffolds, which should be the most attractive, also because of their good biodegradability [1][2]. In this entry, the state of the art of the cationic dendrimer structures till now only minimally investigated as antibacterial agents has been reviewed. The few case studies for each dendrimer class have been also included. In addition, we have allowed ourselves to express our considerations on how the case studies reported in the last decade regarding the different types of cationic dendrimers should be improved. In addition, we have allowed ourselves to express our considerations on how the case studies reported in the last decade regarding the different types of cationic dendrimers should be improved.
PEI-Based Dendrimers (PEI-Ds)
As far as our knowledge is concerned, the application of water-soluble PEI derivatives, containing quaternized ammonium salt groups with long alkyl or aromatic groups, such as antimicrobial polymers, and of water-insoluble hydrophobic modified PEIs, such as antimicrobial coatings have been widely documented[3]. On the contrary, the application of PEI-Ds as antimicrobial agents has been reported in a few studies. Concerning their proposed mechanism of action, the protonated ammonium-terminated groups of the arms of PEI-Ds are the positive part serving for electrostatic interactions with bacteria membranes, while the non-protonated amine groups and ethylene backbone serve as hydrophobic groups, helpful for PEIs diffusion through bacterial membranes. Overall, the repeated cationic amphiphilic structures, along the dendrimer backbone of unmodified PEI-Ds, by providing the necessary cationic amphiphilic structures, mimic of CAMPs, are capable of inducing membrane permeabilization, disruption, and bacterial death. In this regard, a systematic investigation of the antimicrobial activity and toxicity of PEI-Ds which differ in generation number and molecular weight has been reported by Gibney and colleagues (2012)[3]. The authors focused on the structure-activity relationship responsible for antimicrobial activity against E. coli and S. aureus as well as on the toxicity against human red blood cells (hemolysis) and human epithelial carcinoma HEp-2 cells. The polymer-induced permeabilization of bacterial cell membranes of E. coli and S. aureus was also evaluated. The PEI-Ds under study exhibited considerable antimicrobial activity (MIC values = 16–32 μg/mL) and selectivity against S. aureus, whereas they demonstrated poor activity against E. coli, with MIC values up to >1000 μg/mL). All compounds proved low hemolytic toxicity, while considerable cytotoxicity was observed mainly for PEI-Ds with high MW and after 24 h of exposure. Lately, considering the wide range of applications of oxadiazole compounds in biomedicine and the multivalent PEI-Ds, which provide many branches such as –NH2 functional groups exploitable for modifications with several bioactive heterocyclic derivatives, the synthesis of seven PEI-based oxidiazole-modified Ds (PEI-dend-4[N[(Ts)(2-(methyl)-5-aryl-1,3,4 oxadiazole)]] was reported[4]. Prepared from PEIs and 2-aryl-1,3,4 oxadiazole derivatives differently substituted on the phenyl group, the achieved Ds were investigated for their in vitro antimicrobial activities by MICs (μg/mL) determination. Results indicated that although four compounds manifested from moderate to very poor antimicrobial activity, two of them exhibited broad-spectrum antimicrobial activity against bacteria and fungi, highlighting that the presence of more electron donating groups at para position in phenyl ring bearing oxadiazole influences antimicrobial activity positively.
Cationic Organometallic Ds
Organometallic Ds, are a class of saline macromolecules, turned cationic due to the presence of a metal, with a plethora of applications. In this regard, a new class of organometallic Ds, containing iron and different types of counteranions, with tunable activity against MDR Gram-positive bacteria including MRSA and Enterococcus faecium (VRE), were synthetized by Abd-El-Aziz et al. (2015) [5]. Interestingly, these Ds were non-cytotoxic to human epidermal keratinocytes cells (HEka), to human foreskin BJ fibroblast cells, and to human breast adenocarcinoma cells (HTB-26). Furthermore, they were not hemolytic for mammalian red blood cells, even at the highest tested concentration of 128 μg/mL. For these reasons, such Ds can be considered to be potential antimicrobial platforms for topical applications. The cationic organometallic Ds prepared by Abd-El-Aziz, are both cationic and redox-active macromolecules and therefore can exert a dual-mode antibacterial activity, by a disruptive action on the microbial membrane and by an oxidative effect causing oxidative stress on bacteria. Actually, nine Ds were prepared (DEN1-DEN9) and their antimicrobial activity was essayed against a broad spectrum of infection-causing pathogens, including Gram-positive and Gram-negative bacteria, i.e., S. aureus ATCC 33591 (MRSA), S. warneri ATCC 17917, E. faecium EF379 (VRE), P. aeruginosa ATCC 14210, P. vulgaris ATCC 12454, and mycetes as Candida albicans ATCC 14035. At the tested concentrations (<128 μg/mL), all Ds were inactive against the Gram-negative bacteria and Candida albicans, whereas all Ds, except for DEN 9, showed MIC values in the ranges of 1.8–15 μM, 2.2–22 μM, 2.1–12 μM against MRSA, VRE, and S. warneri respectively. DEN 2 was the most active dendrimer against MRSA and VRE strains (MIC values of 1.8 and 2.2 μM, respectively) with an IC50 value against HTB-26 of 20 μM, while DEN7, DEN8 were the most active against S. warneri (MIC values of 2.2 and 2.1 μM, respectively) and did not present cytotoxic activity on HTB-26 cells.
Entirely Polyester-Based Uncharged Scaffolds Peripherally Modified with Cationic Amino Acids
Despite being considered very attractive for biomedical applications because of their lower density of cationic charge when compared to PAMAM Ds, PEI-Ds, and PPI Ds, generally associated with low cytotoxicity[6][7][8][9], only very recently uncharged dendrimer matrices, decorated with amino acid residues, were considered to be less toxic antimicrobial alternatives. Within this category, peripherally amino acid-modified, polyester-based dendrimer scaffolds, obtained starting from 2,2-bis(hydroxymethyl)propanoic acid (bis-HMPA), as AB2 monomer, have several features that make them particularly suitable as novel antimicrobial agents. These structures are characterized by good biodegradability, due to the easy physiological hydrolysis, a favorable hydrophilic lipophilic balance (HLB) due to the presence of an uncharged matrix and a cationic periphery, and can be esterified with a high number of amino acids, given the high multivalence of Ds. For these reasons, amino acid-modified polyester-based Ds could mimic synthetic dendrimer peptides which, as reported in the previous section, have provided remarkable results as antimicrobial agents, together with low levels of toxicity to eukaryotic cells. Research in this field is, however, still very limited and, as far as our knowledge is concerned, the literature presents only two studies. A further study not dealing with Ds but only dendrons, reporting the synthesis of low-generation polyester dendrons, based on bis-HMPA, peripherally cationic for the presence of β-alanine, has been recently published by Chen and colleagues (2020)[10].
In this regard, probably inspired by previous studies (2017)[6][7], hydroxyl functional Ds of one to five generations based on bis-HMPA and endowed with a tri-functional core corresponding to the International Union of Pure and Applied Chemistry (IUPAC) name of 2,2-bis-hydoxymethyl-1-butonol, and an analogous fourth-generation dendrimer, with a disulfide core, have been synthetized[2]. Both uncharged matrices were modified to bear from 6 to 96 peripheral amino groups, through esterification reactions with the non-natural amino acid β-alanine, achieving two types of cationic Ds which differ from one another in the inner core. In particular, the achieved biodegradable Ds without a disulfide core (TMP-Gx-NH3+TFA) were tested for cytotoxicity against human skin cells (HDF), monocytes (RAW 264.7), and glioblastoma cells (U87) for 24 h with concentrations of 0.1–50 μM and with the extreme concentration of 100 μM. According to the results, G1 and G2 Ds were non-toxic at most concentrations for all three cell lines, while they were slightly toxic at 100 μM. The G3 D was tolerated only at concentrations <1 μM by RAW 264.7 and U87 cells and concentrations « to 5 μM by HDF cells. The G4 and G5 Ds were toxic at very low concentrations (0.1 μM). Cationic Ds were also functionalized via carbamate coupling with monobenzylated tetraethylene glycol (BnTEG) and via amidation with the same species modified with a carboxylic acid linker (BnTEGCOOH), obtaining OH-Ds. Neurotoxicity of the prepared Ds was also evaluated on SH-SY5Y neuroblastoma cells. A comparison was made on the cytotoxic effects of TMP-G4-NH3+TFA− and TMP-G4-OH, those one of a PAMAM-D (SS-G3-PAMAM-NH3+TFA−) and of the SS bis-MPA dendrimer, with an equal number of amino end groups (SS-G4-NH3+TFA−). Concerning G3 and G4 Ds, OH-Ds were, in general, less toxic than NH3+, which instead caused only a slight reduction in neurotoxicity, when compared to commercially available PAMAM Ds. Overall, all NH3+ Ds proved considerable cytotoxicity and with regard to their antimicrobial activity against E. coli, only the TMP-G3-NH3+TFA dendrimer, while being non-toxic toward human cells, presented excessively high MIC values (MIC = 203 μg/mL). A second study on dendrimer-like polyester-based molecules, prepared using bis-HMPA as a building block and peripherally functionalized with an amino acid (β-alanine) in order to confer the cationic character essential for exerting the antibacterial activity on contact, was recently reported by Chen et al. (2020). A combinatorial library of first- to third-generation (G1–G3) dendrons, which juxtapose a cluster of cationic charges with a hydrophobic alkyl chain of different length (from C2 to C14), were synthetized by the authors, using the so-called “molecular umbrella” design approach. Eighteen amphiphilic cationic dendrons (G1–G3/C2–C14) were obtained and their antimicrobial activity was first evaluated against E. coli and S. aureus bacterial species. Among the compounds of each generation, the most active were those bearing the longest C14 chain, confirming that a greater hydrophobicity could help dendrons diffuse from OM toward the CM (in the case of Gram-negative bacteria) and/or through the CM destroying it and inactivating the bacterium. These compounds, tested for their hemolytic toxicity, showed that HC50 values increased with the generation of the devices (from 10 to 5000 μg/mL), thus affirming the absence of hemolytic toxicity for G3/C14 (HC50 = 5000 μg/mL), associated with a potent antimicrobial activity against both the bacteria species (MIC values = 7.8 and 3.9 μg/mL, respectively)[10]. The greater hemolytic activity of the lower-generation dendrons is probably due to their greater hydrophobicity, deriving from the presence of the same C14 chain, and a lower number of cationic charges, which helps such molecules diffuse through cells membranes, thus improving their non-specific lytic activity. The killing activity of the third-generation dendron G3/C14, considered to be the leading candidate as a novel antimicrobial agent, since it is endowed with high efficacy and no hemolytic toxicity, was evaluated against E. coli at a concentration of 2× MIC (16 μg/mL). The results showed that the number of viable E. coli cells decreased immediately, and that within the first 30 min, the number of E. coli viable cells was reduced by approximately 2-logs (99.7% killing). Furthermore, after 1 and 2 h of incubation, reductions of 3.8 and 4.5-logs (99.9985 and 99.9997% killing) respectively were observed. Considerably, the rate of bactericidal action was more rapid than the rate at which these polyester bis-HMPA-based dendrons typically are degraded by hydrolysis (37 °C, pH 7)[2]. Further investigations into G3/C14 antimicrobial activity against a large panel of bacterial strains showed a potent broad-spectrum antibacterial potency against several pathogenic microorganisms, including MRSA and A. baumannii. Investigations into the mechanism of action on representative examples of dendrons from the library developed by Chen’s group, revealed that cationic dendrons indeed exerted a potent lytic activity against E. coli membranes, probably supported by the presence of the hydrophobic chain pendant from the dendron structure. Cytotoxicity essays on HeLa cell lines revealed that within the library, G2/C14 was cytotoxic (LC50 = 32 μg/mL), G3/C14 moderately cytotoxic (LC50 = 85 μg/mL), whereas G3/C2 was completely non-toxic, up to the highest concentration tested (LC50 > 250 μg/mL), but also completely inactive against the pathogen. Finally, very recently, three fifth-generation polyester-based Ds (G5Ds), characterized by a biodegradable inner matrix and a surface decorated with amino acids, were selected among the library of 15 polycationic homo- and hetero-Ds[7] that probably inspired the uncharged scaffolds reported later by Stenström et al. Those selected were of fifth generation, contain L-lysine (G5K), L-histidine (G5H) or a 50/50 mixture of both amino acids (G5KH) and all of them possessed 192 peripheral cationic groups and the ability to create electrostatic interactions with phosphate anionic charges[7]. These Ds, formerly synthesized for gene therapy applications, unlike compounds previously studied as antimicrobial agents, possessed a polycationic character in the presence of natural L-amino acids and, besides the potent G3/C14 previously discussed and described as moderately cytotoxic, totally lack toxicity in human HeLa in vitro cell lines. G5Ds were investigated as novel antimicrobials agents against different clinical bacterial strains, including multidrug-resistant Gram-positive and Gram-negative bacteria[1]. Interestingly, all G5Ds (MICs = 0.5–33.2 μM), and particularly G5K (0.5–2.1 μM), displayed remarkable activity against non-fermenting Gram-negative species such as P. aeruginosa, S. maltophilia and A. baumannii, irrespective of their antibiotic resistance. G5K activity was comparable to that of the previously described 3G/C14 amphiphilic dendron against P. aeruginosa (MIC = 2.1 μM vs. 2.2 μM) and greater than the activity showed against A. baumannii (MIC = 1.1–2.1 μM vs. 4.4 μM). As can be observed, G5K proved to be more active than the potent colistin (2.1 vs. 3.19 μM) against P. aeruginosa, responsible for alarming healthcare-associated infections. Moreover, in time-killing experiments and turbidimetric studies, G5K displayed an unexpected rapid non-lytic bactericidal activity on P. aeruginosa, probably due to the absence of strongly hydrophobic residues, such as alkyl chains of the cationic umbrella molecules developed by Chen et al., which instead proved to possess lytic behavior. In this study, conducted by Schito and Alfei, E. coli, along with P. mirabilis, K. pneumoniae and several Gram-positive species were considered refractory to G5Ds, since the MIC values obtained by the devices were >32–33 μM, a much higher value than that of the different antibiotics active today. On the contrary, the second generation of polyester-based alanine-modified cationic Ds reported by Stenström et al., and similar to G5Ds, was considered active against E. coli even though it showed a MIC value of 100 μM.
Considerations of the Authors Concerning the Research on Antibacterial Cationic Dendrimers
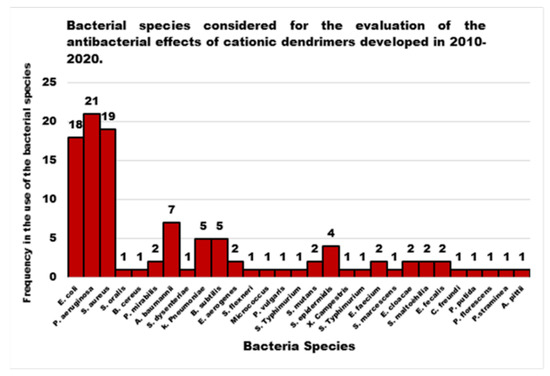
Figure 1, shows as the antibacterial activity of the cationic Ds achieved through a nanobiotechnology approach in the last decade has been evaluated on 30 bacterial species. Among these species, P. aeruginosa, E. coli and S. aureus were the most studied bacterial ones, followed by A. baumannii, K. pneumoniae, B. subtilis, S. epidermidis and other minor species.
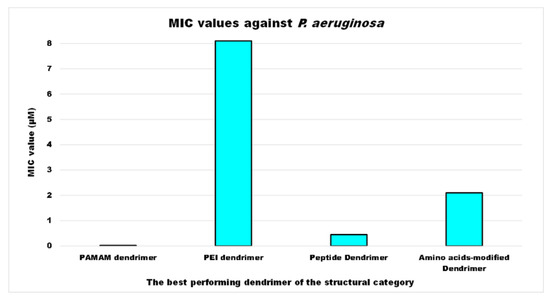
As for P. aeruginosa, the most studied bacterial species, according to available data expressed in μM, among the structural categories of antibacterial cationic dendrimers developed, the compound with the strongest activity was a PAMAM-D, followed by a peptide dendrimer (PD), by an amino acid-modified polyester-based dendrimer (PEAAM-D) and, finally, by a PEI-D, which showed a MIC value higher than that of PAMAM-D, PD and PEAAM-D of 405, 18, and 3.9 times respectively (Figure 2).
In general, the scenario obtained by examining the progress made in the development of new CADs and, in particular, of cationic ones, highlights that nanobiotechnology offers the possibility to engineer different structures of promising Ds, biomimetics of known cationic antimicrobials, which act as membrane disrupters, usually with better performance, mainly due to their multivalence. However, despite the very promising results deriving from the evaluations of the different CADs, developed and tested on representatives of Gram-positive and Gram-negative bacteria, since they were born and raised only in the last decade, these devices require further studies and more rationalized investigations. In this regard, analyzing the structural classes of Ds mainly studied, the investigations carried out, the strains of bacteria taken into consideration (Figure 1), the reported antibacterial activities, and the presentation of the results, major discrepancies and imbalances occur. Although many dendrimer structures, per se cationic or cationic yieldable through post-synthesis functionalization have been developed in the last 30 years, only a few categories have been taken into consideration as antimicrobial agents in the last ten years. Moreover, noticeably in Figure 3, while there are dendrimer structures that have been extensively investigated by modifying native structures with several different approaches, by researching for possible synergistic co-operations with conventional antibiotics, by searching for different types of bioactive residues, and by studying structure-activity relationships etc., other Ds have been considered only marginally, despite the first promising results.
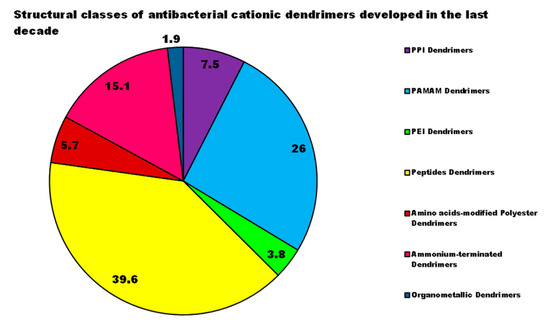
Although generally, PAMAM Ds and PPI Ds were and are the most extensively studied devices for applications in multiple sectors, including nanomedicine, until now peptide dendrimers are the cationic dendrimer structures investigated more as antibacterial devices, perhaps due to their close relationship with natural and synthetic CAMPs, whose activity has been improved due to the multivalence allowed by the generational structure of the Ds. On the contrary, organometallic cationic Ds (OMC-Ds) received very poor consideration and, to the best of our knowledge, only one study reporting their antimicrobial activity has been found. Considering that, even if ineffective against Gram-negative bacteria, they proved considerable activity against Gram-positive species, this class of CADs deserve further investigation in the future. Amino acid-modified polyester-based Ds, extensively studied for drug delivery, gene therapy, or as solubilizing devices, have only very recently attracted researchers’ interest as antibacterial agents, with very appealing results at least in two cases out of three. These types of Ds, by merging an uncharged ester-type inner matrix with the presence of amino acids as cationic moieties, could mimic peptide Ds and CAMPs. In addition, due to the high generations easily achievable, they could provide very high multivalence and density of positive charges, helpful for exerting high antibacterial activity on contact, maintaining low levels of toxicity, due to their high biodegradability. For these reasons, more in-depth investigations, concerning hydrophilic or amphiphilic Ds based on polyester structures modified with amino acids, are suggested. Regarding the study of bioactivity of the developed Ds, in order to allow a critical comparison, the types of investigations to be performed should be standardized, e.g., by following the typical microbiologic path that applies during the preliminary evaluation of the antibacterial potency of a new substance. This path should include the determination of the minimum inhibitory concentration of the growth of bacteria (MIC), then the determination of the concentration capable of killing the bacterium (MBC) confirmed by the set-up of the time-killing experiments, which monitor the logarithmic reduction of bacterial inoculos exposed to MBC values of the test compound over 24 h. By reviewing the studies performed, and as can be observed in Figure 4, the MIC values have been reported in the 43% of cases, the MBC ones in 24%, and the time-killing experiments were performed only in 6% of the case studies; and in one case only they were representative of a 24 h experiment. Histogram plots have frequently been reported, showing the reduction in bacteria cell viability after exposure to different concentrations of Ds and, sometimes, without a known antibiotic as a reference.
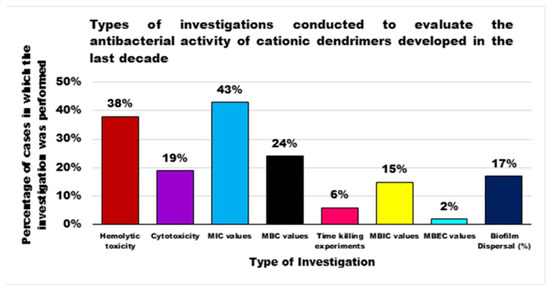
Furthermore, considering the harmful infections caused by drug resistant bacteria, also supported by the formation of the biofilm, further experiments are suggested to evaluate the potency of the developed Ds in inhibiting biofilm formation (MBIC), killing bacterial cells under biofilm-forming conditions (MBEC) and dispersing established detrimental biofilm. Furthermore, investigations concerning both the assessment of hemolytic toxicity, a major concern of cationic devices acting on the bacterial membrane, and the cytotoxicity of CADs to eukaryotic cells should be improved. Hemolytic toxicity and cytotoxicity were determined in only 38% and 19% of cases respectively, while they should always be determined to judge the real clinical applicability of the synthesized materials. Finally, to allow the scientific community interested in the field to easily compare the antibacterial activity of developed Ds, the way results are presented (MIC, MBC values) should also be standardized. In this regard, a debate could be opened, relating to the expression of MIC/MBC values as μg/mL, usually adopted by microbiologists, or as μM, perhaps preferred by chemists dealing with high MW macromolecules. Although as reported earlier in the main text of this review, the MIC values expressed in μM, which considers the MW of the Ds and which provides the actual equivalents of D able to exert some effect, could indeed provide comparable values, a suggestion to resolve the debate could be to express the results in both ways.
References
- Schito, A.M.; Alfei, S. Antibacterial activity of non-cytotoxic, amino acid-modified polycationic dendrimers against Pseudomonas aeruginosa and other non-fermenting Gram-negative bacteria. Polymers 2020, 12, 1818.
- Stenström, P.; Hjorth, E.; Zhang, Y.; Andrén, O.C.J.; Guette-Marquet, S.; Schultzberg, M.; Malkoch, M. Synthesis and in Vitro Evaluation of Monodisperse Amino-Functional Polyester Dendrimers with Rapid Degradability and Antibacterial Properties. Biomacromolecules 2017, 18, 4323–4330.
- Gibney, K.A.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly(ethylene imine)s as antimicrobial agents with selective activity. Macromol. Biosci. 2012, 12, 1279–1289.
- Avval, M.M.; Murthy, S.V.; Shashikanth, S. Synthesis and antimicrobial activity evaluation of poly ethylene imine (PEI) dendrimer modified with 1,2,4-oxadiazole derivatives. Int. J. Chem. Pharm. Sci. 2014, 2, 678–683.
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N.; Overy, D.P.; Lanteigne, M.; McQuillan, K.; Kerr, R.G. Antimicrobial Organometallic Dendrimers with Tunable Activity against Multidrug-Resistant Bacteria. Biomacromolecules 2015, 16, 3694–3703.
- Alfei, S.; Castellaro, S. Synthesis and Characterization of Polyester-Based Dendrimers Containing Peripheral Arginine or Mixed Amino Acids as Potential Vectors for Gene and Drug Delivery. Macromol. Res. 2017, 25, 1172–1186.
- Alfei, S.; Castellaro, S.; Taptue, G.B. Synthesis and NMR characterization of dendrimers based on 2, 2-bis-(hydroxymethyl)-propanoic acid (bis-HMPA) containing peripheral amino acid residues for gene transfection. Org. Commun. 2017, 10, 144–177.
- Alfei, S.; Catena, S. Synthesis and characterization of versatile amphiphilic dendrimers peripherally decorated with positive charged amino acids. Polym. Int. 2018, 67, 1572–1584.
- Alfei, S.; Catena, S. Synthesis and characterization of fourth generation polyester-based dendrimers with cationic amino acids-modified crown as promising water soluble biomedical devices. Polym. Adv. Technol. 2018, 29, 2735–2749.
- Chen, A.; Karanastasis, A.; Casey, K.R.; Necelis, M.; Carone, B.R.; Caputo, G.A.; Palermo, E.F. Cationic Molecular Umbrellas as Antibacterial Agents with Remarkable Cell-Type Selectivity. ACS Appl. Mater. Interfaces 2020, 12, 21270–21282.




