
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jeong Heo | + 2136 word(s) | 2136 | 2021-01-14 09:36:22 | | | |
| 2 | Vivi Li | Meta information modification | 2136 | 2021-01-15 03:21:49 | | | | |
| 3 | Conner Chen | Meta information modification | 2136 | 2021-10-12 05:38:33 | | |
Video Upload Options
Peretinoin, an retinoid acid (RA), a metabolite of vitamin A and its related analogues (termed retinoids) has been suggested as a promising chemotherapeutic agent in cancer treatment. The synthetic oral retinoid peretinoin is the only agent for the secondary chemoprevention of HCC after curative therapy that is currently well applied into clinical development.
1. Introduction
Liver cancer is the fifth most common neoplasm in men and the seventh most common neoplasm in women worldwide [1][2]. The incidence and mortality rates of liver cancer are particularly high in Asia, with over 75% of patients with liver cancer being from Asian countries [1][3]. Hepatocellular carcinoma (HCC) is the most common type of liver cancer. The prognosis of patients with HCC has improved recently owing to developments in various therapeutic methods, including surgical resection, ablation therapy [mostly radiofrequency ablation (RFA), percutaneous-ethanol-injection (PEI)], transarterial chemoembolization (TACE), and molecularly targeted agents such as sorafenib, lenvatinib, and regorafenib, liver transplantation [4][5][6][7][8][9]. Despite successful treatment, however, HCC has a high risk of recurrence. The mode of recurrence is identical in patients with HCC caused by hepatitis B virus (HBV) and hepatitis C virus (HCV) and is thought to be due to manifestations of intrahepatic metastases and metachronous multicentric carcinogenesis. Moreover, although it is difficult to determine the mode of recurrence of individual lesions, the timing of recurrence is believed to differ. For example, recurrence from intrahepatic metastases is predominant within 2 years after radical treatment of the primary tumor, whereas metachronous multicentric recurrence occurs predominantly after 2 years [10][11]. Most patients ultimately die of HCC, due to the occurrence of non-responding lesions, such as intraportal tumor thrombi, diffuse multiple cancers, and distant metastases.
In addition to early detection and early treatment, the prognosis of patients with HCC may be improved by aggressively suppressing HCC recurrence. Antiviral therapy, mainly with nucleic acid analogs and Direct acting antivirals (DAAs), may inhibit the recurrence of HBV- and HCV-positive HCC after radical treatment of patients [12][13]. However, there is no established non-viral strategy to prevent recurrence of virus associated HCC after curative therapy. The incidence of HCC in patients with non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome has recently increased [14]. Because HCC can develop in patients with NAFLD, even in the absence of established cirrhosis, to prevent recurrence of non-virus associated HCC after curative therapy, there is an urgent unmet need for secondary chemoprevention of HCC recurrence after curative therapy. This review investigated the current status and prospects of peretinoin, an acyclic retinoid (ACR) compound developed for secondary prevention of HCC.
2. Peretinoin
Peretinoin is a synthetic polyprenoic acid that binds to cellular retinoic acid-binding protein [15] and has retinoid-like properties. Initially, it was developed under the name E-5166 (Eisai Co., Ltd.) to treat dermal diseases. A randomized placebo controlled study showed that oral administration of peretinoin (600 mg/day, twice daily) for one year to patients who had undergone curative resection or percutaneous ethanol injection therapy for viral and non-viral HCC showed good tolerability, inhibited HCC recurrence, and improved patient survival rate [16][17]. Based on these promising results, peretinoin was developed under the name NIK 333 (Kowa Company, Ltd.) in 1997, clinical studies were started in February 2012, and the name of the compound was changed to K-333. The mechanism of action of peretinoin includes transcriptional activation via the retinoic acid receptor (RAR) and retinoid X receptor (RXR), promoting, along with other transcriptional complexes, the transcription of target genes. Peretinoin was found to modulate genes involved in the regulation of cellular proliferation, cellular differentiation and apoptosis in HCC cells [18][19][20]. Several pharmacologic studies have indicated that peretinoin inhibits the recurrence of HCC by inhibiting carcinogenesis of precancerous lesions in the liver and/or by inhibiting the growth of subclinical cancers [19][21].
2.1. Chemistry
The chemical name of peretinoin is (2E,4E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,4,6,10,14-pentaenoic acid (Scheme 1). Peretinoin consists of crystals or crystalline powder, pale yellow to yellow in color, with a molecular formula of C20H30O2 and a molecular weight of 302.45 g/mol. Peretinoin was highly soluble in dimethylsulfoxide, soluble in ethanol (99.5%) and acetone, sparingly soluble in methanol, slightly soluble in acetonitrile and hexane, and practically insoluble in water.
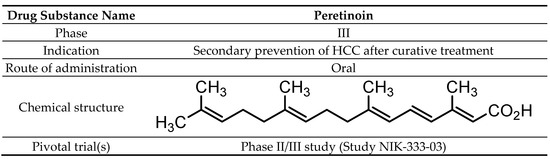
Scheme 1. Drug substance summary.
2.2. Pharmacodynamics
Peretinoin was found to activate transcription through retinoic acid receptor (RAR) and retinoid X receptor (RXR), and could activate the differentiation of human acute promyelocytic leukemia in HL-60 cells [22][23]. Peretinoin significantly inhibited hepatocarcinogenesis in rat chemical carcinogenesis models using 3′-methyl-4-(dimethylamino)azobenzene (3′-MeDAB) and diethylnitrosamine (DEN) [24][25], suggesting that peretinoin was also involved in suppressing hepatocarcinogenesis. Furthermore, peretinoin was reported to reduce the numbers of oval cells with stem cell-like properties that appear during early stages of 3′-MeDAB-induced hepatocarcinogenesis in rats [26][27]. All-trans-RA was found to induce the differentiation of hepatic precursor cells derived from mouse fetal liver cells [28], and peretinoin is thought to act similarly. Peretinoin treatment also prevented obesity-related liver carcinogenesis and attenuated liver steatosis and inflammation [29], thereby being expected to reduce HCC incidence in patients with liver cirrhosis. These results suggest that, due to its retinoid-dependent and -independent effects, peretinoin prevents the recurrence of HCC by inhibiting oncogenesis of precancerous lesions in the liver and/or by inhibiting the growth of occult hepatic cancer. In addition to preclinical study, the effect of peretinoin in human was investigated through clinical study (NIK-333-02) [18]. In this study, the change of gene expression profile after 8 weeks of peretinoin treatment was examined in liver biopsy for patients who had received curative treatment for HCC. The levels of expression of genes encoding interferon, tumor suppressors, negative regulators of Wnt and insulin-like growth factor (IGF) signaling, hepatocyte differentiation and retinoid-induced genes were higher after 8 weeks of peretinoin treatment than before treatment. In contrast, genes related to mammalian target of rapamycin (mTOR), tumor progression, the cell cycle and metastasis/angiogenesis were downregulated. Through retinoid target gene, peretinoin inhibit HCC proliferation, suppress tumor growth or induce tumor apoptosis [30][31][32][33].
It is well known that abnormalities in the genes regulating Wnt signaling, IGF signaling, interferon, mTOR, and the cell cycle have been indicated to play a crucial role in the development of HCC [34][35]. Gene expression profiles in the liver identified candidate drug-response genes among the genes which exhibited changes in expression before and after peretinoin administration. These genes included Wnt signal-related, IGF signal-related, interferon-related, mTOR-related, and cell cycle-related genes known to be involved in the process of carcinogenesis of hepatocellular carcinoma, in addition to the retinoid-related genes which are the targets of peretinoin, suggesting that peretinoin directly or indirectly regulate many signal transduction systems in the carcinogenic process. Furthermore, with a hierarchical cluster analysis showing the feasibility of differentiating within-2-year-recurrence and non-recurrence groups, a comparative analysis of the two groups was performed. The results showed that, in the non-recurrence group, differentiation of hepatocytes and expression of the genes involved in tumor suppression were accelerated, while expression of the genes involved in promotion of liver fibrosis and lipidation, as well as the genes which can become markers for the stem cells of hepatic cancer was reduced. Changes in the expression of these genes may not reflect the direct action of peretinoin, but they were assumed to become eventual candidates for the genes related to drug efficacy. Furthermore, genes with a potential to distinguish the two doses were extracted by a comparison of the two dose groups of 300 mg and 600 mg of peretinoin. These genes included retinoid-related genes as well as genes that play a significant role in hepatic cancer and hepatic cirrhosis. The exact modes of action are not certain. Through these mechanisms, peretinoin suppresses HCC cell proliferation, and therefore could prevent HCC recurrence by modulating multiple signaling cascades involved in carcinogenesis, either directly or indirectly (Figure 1) [18][20].
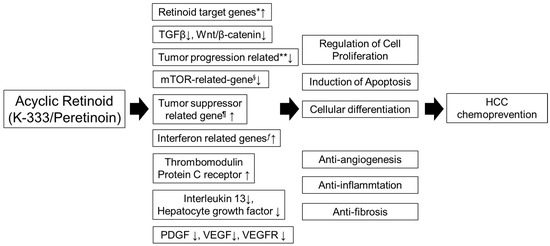
Figure 1. Molecular pathway of peretinoin. Peretinoin enhances the expression of multiple retinoid target genes. Through the retinoid target gene, peretinoin inhibits HCC proliferation, suppresses tumor growth and induces tumor apoptosis. In addition to enhancing retinoid target gene expression, peretinoin induces changes in the expression levels of a variety of genes involved in hepatocarcinogenesis, such as those related to Wnt signaling, IGF signaling, interferon, mTOR, and cell cycle regulation. Therefore, peretinoin modulates multiple signaling cascades involved in carcinogenesis, either directly or indirectly. HCC, hepatocellular carcinoma; IGF, insulin-like growth factor; mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor. * cytochrome P450, family 26, subfamily B, polypeptide 1, insulin-like growth factor binding protein 6, regulatory factor X-associated ankyrin-containing protein, putative lymphocyte G0/G1 switch gene, retinol binding protein 1, retinol binding protein 4, retinoic acid induced 3, transglutaminase 2, CCAAT/enhancer binding protein (C/EBP). ** junctional adhesion molecule 3, V-myc myelocytomatosis viral oncogene homolog, Src-like-adaptor, cell division cycle 2, G1 to S and G2 to M, BCL2-associated athanogene, chemokine (C-C motif) receptor 9, pre-B-cell leukemia transcription factor 1. § FK506 binding protein 12-rapamycin associated protein 1. ¶ Jumonji domain containing 3, jumping translocation breakpoint, protein kinase, AMP-activated, alpha 2 catalytic subunit. ƒ guanylate binding protein 1, interferon-inducible, 67kDa, interferon-induced protein 44, chemokine (C-X-C motif) ligand 9.
The Wnt/β-catenin carcinogenesis pathway is frequently activated in HCC associated with hepatitis virus, especially HCV. As peretinoin has been shown to upregulate genes associated with the negative regulation of Wnt/β-catenin signaling, studies have investigated the chemopreventive activities of peretinoin in HCV- and HBV-associated HCCs [18][36]. In contrast, the β-catenin pathway is not activated in metabolic syndrome associated HCCs. Rather, carcinogenic mediators include insulin, lipid peroxidation and oxidative stress induced by free radicals, all of which stimulate cellular proliferation, activate hepatic progenitor cells and induce p53 mutations and epigenetic aberrations [37][38]. The non-activation of the Wnt/β-catenin pathway in metabolic syndrome associated HCCs suggested that peretinoin may not be effective as a chemopreventive agent in these patients. However, peretinoin also downregulates the expression of genes related to inflammation [18]. In a rat model, peretinoin targeted platelet-derived growth factor (PDGF) signaling, preventing hepatic fibrosis, steatosis and HCC development [20] and suggesting that peretinoin may be useful in the chemoprevention of metabolic syndrome related HCCs. It was reported that peretinoin activates the autophagy pathway by increasing Atg16L1 expression to prevent the progression of HCC in NAFLD mouse model [39]. Indeed, a recent preclinical study in obese and diabetic db/db mice with DEN-induced HCC showed that peretinoin significantly reduced the incidence of obesity related HCC [29]. Thus, peretinoin may be useful in preventing metabolic syndrome related HCC in humans, a hypothesis requiring testing in clinical trials.
2.3. Pharmacokinetics and Metabolism
Administration of single doses (300, 600 and 900 mg) of peretinoin to human subjects confirmed dose proportionality, with the lipid form of peretinoin reaching saturation at high doses. The route of drug administration is per oral. At all dose levels, urinary excretion of peretinoin was not observed [40]. Results of 24-week repeated-dose administration (150 mg × 2/day, 300 mg × 2/day, and 450 mg × 2/day) confirmed that peretinoin and its lipid form reached and maintained steady state concentrations in plasma for up to 24 weeks. Assessments of the elimination phase showed that peretinoin concentrations near the quantitation limit were present in some subjects after completion of administration at each dose. However, the level was lower than 1% of the Cmax after a single dose at each dose level. In most subjects, the plasma concentrations of peretinoin and peretinoin lipids were lower after completion of administration than the quantitation limit at 12 weeks. These results suggested that peretinoin does not accumulate in plasma, and that the steady state can be maintained for up to 24 weeks after administration. Moreover, food intake had no effect on plasma peretinoin concentration, as these concentrations did not differ in subjects administered a single of 300 mg peretinoin in the presence or absence of food [40]. A study of its clinical pharmacology showed dose-dependent increases in plasma peretinoin concentrations following 8-week repeated administration at doses of 300 mg/day and 600 mg/day. Peretinoin concentrations in the liver were below the lower quantitation limit (0.050 μg/g) in all six subjects administered 300 mg/day peretinoin and in four of the six subjects administered 600 mg/day peretinoin. The other two subjects administered 600 mg/day peretinoin had plasma peretinoin concentrations of 0.052 μg/g and 0.059 μg/g, respectively. Repeated-dose administration for up to 96 weeks showed no evidence of drug accumulation in the liver [18].
3. Conclusions
Peretinoin, a synthetic oral ACR discovered in 1980, has been specifically developed for secondary chemoprevention in patients who had undergone curative surgical or local ablative treatment for HCC. Clinical trials on the tolerability and efficacy of peretinoin indicated that twice daily administration of 600 mg/day peretinoin would be effective in preventing HCC recurrence and that peretinoin was safe and tolerable. Its long-term safety and marginal efficacy with hepatitis C virus- or hepatitis B virus-associated HCC for its clinical use as adjuvant therapy in patients is of concern. Subsequent placebo-controlled phase III clinical trials are underway. The outcome of these phase III trials will determine whether peretinoin is approved for clinical use. Therefore, the dose of peretinoin (2 × 600 mg/day) might be considered high as for the anticancer drug under clinical development. To exert more effective and satisfactory outcomes of ACR to enable HCC chemoprevention, the combination therapy of ACR with more potent anticancer agents would be considered [41][42].
References
- International Agency for Research on Cancer. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; International Agency for Research on Cancer: Lyon, France, 2010; Volume 93.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604.
- El–Serag, H.B.; Marrero, J.A.; Rudolph, L.; Reddy, K.R. Diagnosis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2008, 134, 1752–1763.
- Ebara, M.; Okabe, S.; Kita, K.; Sugiura, N.; Fukuda, H.; Yoshikawa, M.; Kondo, F.; Saisho, H. Percutaneous ethanol injection for small hepatocellular carcinoma: Therapeutic efficacy based on 20-year observation. J. Hepatol. 2005, 43, 458–464.
- Matsui, O.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Arai, K.; Demachi, H.; Miyayama, S.; Takashima, T.; Unoura, M.; Kogayashi, K. Small hepatocellular carcinoma: Treatment with subsegmental transcatheter arterial embolization. Radiolohy 1993, 188, 79–83.
- Yamasaki, T.; Kurokawa, F.; Shirahashi, H.; Kusano, N.; Hironaka, K.; Okita, K. Percutaneous radiofrequency ablation therapy with combined angiography and computed tomography assistance for patients with hepatocellular carcinoma. Cancer 2001, 91, 1342–1348.
- Todo, S.; Furukawa, H. Living donor liver transplantation for adult patients with hepatocellular carcinoma: Experience in Japan. Ann. Surg. 2004, 240, 451–461.
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390.
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003, 38, 200–207.
- Mazzaferro, V.; Romito, R.; Schiavo, M.; Mariani, L.; Camerini, T.; Bhoori, S.; Capussotti, L.; Calise, F.; Pellicci, R.; Belli, G.; et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 2006, 44, 1543–1554.
- Kuzuya, T.; Katano, Y.; Kumada, T.; Toyoda, H.; Nakano, I.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Hayashi, K.; Honda, T.; et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2007, 22, 1929–1935.
- Breitenstein, S.; Dimitroulis, D.; Petrowsky, H.; Puhan, M.A.; Müllhaupt, B.; Clavien, P. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. BJS 2009, 96, 975–981.
- Loria, P.; Lonardo, A.; Anania, F. Liver and diabetes. A vicious circle. Hepatol. Res. 2013, 43, 51–64.
- Muto, Y.; Moriwaki, H.; Omori, M. In vitro binding affinity of novel synthetic polyprenoids (polyprenoic acids) to cellular retinoid-binding proteins. Gan 1981, 72, 974–977.
- Muto, Y.; Moriwaki, H.; Ninomiya, M.; Adachi, S.; Saito, A.; Takasaki, K.T.; Tanaka, T.; Tsurumi, K.; Okuno, M.; Tomita, E.; et al. Prevention of Second Primary Tumors by an Acyclic Retinoid, Polyprenoic Acid, in Patients with Hepatocellular Carcinoma. N. Engl. J. Med. 1996, 334, 1561–1568.
- Muto, Y.; Moriwaki, H.; Saito, A.M. Prevention of Second Primary Tumors by an Acyclic Retinoid in Patients with Hepatocellular Carcinoma. N. Engl. J. Med. 1999, 340, 1046–1047.
- Honda, M.; Yamashita, T.; Yamashita, T.; Arai, K.; Sakai, Y.; Sakai, A.; Nakamura, M.; Mizukoshi, E.; Kaneko, S. Peretinoin, an acyclic retinoid, improves the hepatic gene signature of chronic hepatitis C following curative therapy of hepatocellular carcinoma. BMC Cancer 2013, 13, 191.
- Funaki, M.; Kitabayashi, J.; Shimakami, T.; Nagata, N.; Kai, T.; Takegoshi, K.; Okada, H.; Murai, K.; Shirasaki, T.; Oyama, T.; et al. Peretinoin, an acyclic retinoid, inhibits hepatocarcinogenesis by suppressing sphingosine kinase 1 expression in vitro and in vivo. Sci. Rep. 2017, 7, 16978.
- Okada, H.; Honda, M.; Campbell, J.S.; Sakai, Y.; Yamashita, T.; Takebuchi, Y.; Hada, K.; Shirasaki, T.; Takabatake, R.; Nakamura, M.; et al. Acyclic Retinoid Targets Platelet-Derived Growth Factor Signaling in the Prevention of Hepatic Fibrosis and Hepatocellular Carcinoma Development. Cancer Res. 2012, 72, 4459–4471.
- Tan, C.-K. Peretinoin as an adjuvant therapy for hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 1201–1210.
- di Martino, O.; Welch, J.S. Retinoic acid receptors in acute myeloid leukemia therapy. Cancers 2019, 11, 1915.
- Uray, I.P.; Dmitrovsky, E.; Brown, P.H. Retinoids and rexinoids in cancer prevention: From laboratory to clinic. Semin. Oncol. 2016, 43, 49–64.
- Kagawa, M.; Sano, T.; Ishibashi, N.; Hashimoto, M.; Okuno, M.; Moriwaki, H.; Suzuki, R.; Kohno, H.; Tanaka, T. An acyclic retinoid, nik-333, inhibits n-diethylnitrosamine-induced rat hepatocarcinogenesis through suppression of tgf-alpha expression and cell proliferation. Carcinogenesis 2004, 25, 979–985.
- Hoshida, Y.; Fuchs, B.C.; Tanabe, K.K. Prevention of hepatocellular carcinoma: Potential targets, experimental models, and clinical challenges. Curr. Cancer Drug Targets. 2012, 12, 1129–1159.
- Sano, T.; Kagawa, M.; Okuno, M.; Ishibashi, N.; Hashimoto, M.; Yamamoto, M.; Suzuki, R.; Kohno, H.; Matsushima-Nishiwaki, R.; Takano, Y.; et al. Prevention of Rat Hepatocarcinogenesis by Acyclic Retinoid Is Accompanied by Reduction in Emergence of Both TGF-α-Expressing Oval-Like Cells and Activated Hepatic Stellate Cells. Nutr. Cancer 2005, 51, 197–206.
- Xu, L.-B.; Liu, C. Role of liver stem cells in hepatocarcinogenesis. World J. Stem Cells 2014, 6, 579–590.
- Huang, J.; Bi, Y.; Zhu, G.-H.; He, Y.; Su, Y.; He, B.-C.; Wang, Y.; Kang, Q.; Chen, L.; Zuo, G.-W.; et al. Retinoic acid signalling induces the differentiation of mouse fetal liver-derived hepatic progenitor cells. Liver Int. 2009, 29, 1569–1581.
- Shimizu, M.; Sakai, H.; Shirakami, Y.; Iwasa, J.; Yasuda, Y.; Kubota, M.; Takai, K.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Acyclic retinoid inhibits diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BLKS/J- +Leprdb/+Leprdb mice. Cancer Prev. Res. 2011, 4, 128.
- Tomaru, Y.; Nakanishi, M.; Miura, H.; Kimura, Y.; Ohkawa, H.; Ohta, Y.; Hayashizaki, Y.; Suzuki, M. Identification of an inter-transcription factor regulatory network in human hepatoma cells by Matrix RNAi. Nucleic Acids Res. 2009, 37, 1049–1060.
- Nakanishi, M.; Tomaru, Y.; Miura, H.; Hayashizaki, Y.; Suzuki, M. Identification of transcriptional regulatory cascades in retinoic acid-induced growth arrest of HepG2 cells. Nucleic Acids Res. 2008, 36, 3443–3454.
- Uray, I.P.; Shen, Q.; Seo, H.S.; Kim, H.; Lamph, W.W.; Bissonnette, R.P.; Brown, P.H. Rexinoid-induced expression of IGFBP-6 requires RARbeta-dependent permissive cooperation of retinoid receptors and ap-1. J. Biol. Chem. 2009, 284, 345–353.
- Ma, Y.; Koza-Taylor, P.H.; DiMattia, D.A.; Hames, L.; Fu, H.; Dragnev, K.H.; Turi, T.; Beebe, J.S.; Freemantle, S.J.; Dmitrovsky, E. Microarray analysis uncovers retinoid targets in human bronchial epithelial cells. Oncogene 2003, 22, 4924–4932.
- Llovet, J.M.; Bruix, J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008, 48, 1312–1327.
- Zender, L.; Villanueva, A.; Tovar, V.; Sia, D.; Chiang, D.Y.; Llovet, J.M. Cancer gene discovery in hepatocellular carcinoma. J. Hepatol. 2010, 52, 921–929.
- Huang, H.; Fujii, H.; Sankila, A.; Mahler-Araujo, B.M.; Matsuda, M.; Cathomas, G.; Ohgaki, H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis c virus infection. Am. J. Pathol. 1999, 155, 1795–1801.
- Roskams, T.; Yang, S.Q.; Koteish, A.; Durnez, A.; Devos, R.; Huang, X.; Achten, R.; Verslype, C.; Diehl, A.M. Oxidative Stress and Oval Cell Accumulation in Mice and Humans with Alcoholic and Nonalcoholic Fatty Liver Disease. Am. J. Pathol. 2003, 163, 1301–1311.
- Zhaohui, F.; Feng, Z.; Eveleigh, J.; Iyer, G.; Pan, J.; Amin, S.; Chung, F.-L.; Tang, M.-S. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis 2002, 23, 1781–1789.
- Okada, H.; Takabatake, R.; Honda, M.; Takegoshi, K.; Yamashita, T.; Nakamura, M.; Shirasaki, T.; Sakai, Y.; Shimakami, T.; Nagata, N.; et al. Peretinoin, an acyclic retinoid, suppresses steatohepatitis and tumorigenesis by activating autophagy in mice fed an atherogenic high-fat diet. Oncotarget 2017, 8, 39978–39993.
- Okusaka, T.; Ueno, H.; Ikeda, M.; Morizane, C. Phase I and pharmacokinetic clinical trial of oral administration of the acyclic retinoid NIK-333. Hepatol. Res. 2011, 41, 542–552.
- Shimizu, M.; Imai, K.; Moriwaki, H.; Shirakami, Y.; Takai, K. Acyclic retinoid in chemoprevention of hepatocellular carcinoma: Targeting phosphorylated retinoid X receptor-α for prevention of liver carcinogenesis. J. Carcinog. 2012, 11, 11.
- Nishimura, N.; Kaji, K.; Kitade, M.; Aihara, Y.; Sato, S.; Seki, K.; Sawada, Y.; Takaya, H.; Okura, Y.; Kawaratani, H.; et al. Acyclic retinoid and angiotensin-II receptor blocker exert a combined protective effect against diethylnitrosamine-induced hepatocarcinogenesis in diabetic OLETF rats. BMC Cancer 2018, 18, 1164.




