| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leslie Chavez-Galan | + 1639 word(s) | 1639 | 2021-01-13 07:49:01 |
Video Upload Options
Flow cytometry (FCM) arises with the design of the cell spectrophotometer, which makes it possible to measure both the content of nucleic acids and the size of the analyzed cells. The subject of the study is not limited to humans, other animal species and bacterias can also be studied. Moreover, FCM allows identify expression of molecules in the membrane, cytoplasm or nucleus, beside soluble proteins (cytokines, chemokines, etc), extracellular vesicles, antibodies, etc.
1. Introduction
Flow cytometry (FCM) arises with the design of the cell spectrophotometer, which makes it possible to measure both the content of nucleic acids and the size of the analyzed cells [1]. The use of FCM in clinical practice required the development of (1) monoclonal antibodies (mAbs), attributed to Cesar Milstein, Georges Köhler and Niels Jerne and, (2) molecules with intrinsic excitation and emission capacity at different wavelengths (fluorophores); since the former is required to be chemically coupled to fluorescent molecules to be detected by FCM [2][3][4].
Currently, the “International Society for Advancement of Cytometry (ISAC)” and the “International Clinical Cytometry Society (ICCS)”, are the institutions that dictate the correct standardization of techniques and equipment for FCM, to generate effective and reproducible results. FCM has established itself as one of the main tools to support the diagnosis and clinical monitoring of diseases since it allows the monitoring of the expression of specific cell markers (Figure 1A).
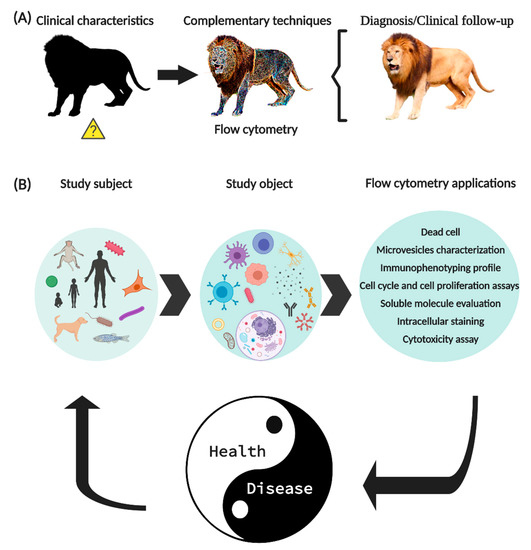
Figure 1. New generation flow cytometry applications. (A) FCM is a tool widely used in the clinic to support the diagnosis and clinical follow-up of patients. (B) The use of FCM is not limited to humans, its application allows to identify molecules expressed on the surface or intracellular of circulating cells, soluble molecules, signaling pathways, intracellular organelles, genetic material, and even microorganisms. In summary, FCM has allowed important improvements in basic research, as well as established parameters that help to diagnose and monitor various diseases.
2. Basic Systems for the Operation of the Flow Cytometer
The expression “flow cytometry” is composed of the Greek ‘κντοζ (cell)’, ‘μετρον (measurement)’ and from the English word ‘flow’. FCM is a technique that allows evaluating events (commonly cells) in suspension, through the use of mAb coupled to fluorochromes (mAb-F). In FCM, the subject of the study is not limited to humans, since other animal species can also be studied, including bacteria. The use of FCM is diverse, it allows to identify cell’s subpopulation utilizing the expression of molecules in the cell membrane, cytoplasm or nucleus, besides soluble proteins (cytokines, chemokines, growth factors, etc.), extracellular vesicles, antibodies, etc. (Figure 1).
The proper functioning of FCM is the conjunction of six fundamental systems, which involve different areas of science such as fluid physics, optics, electronics, and computing [5] (Figure 2).
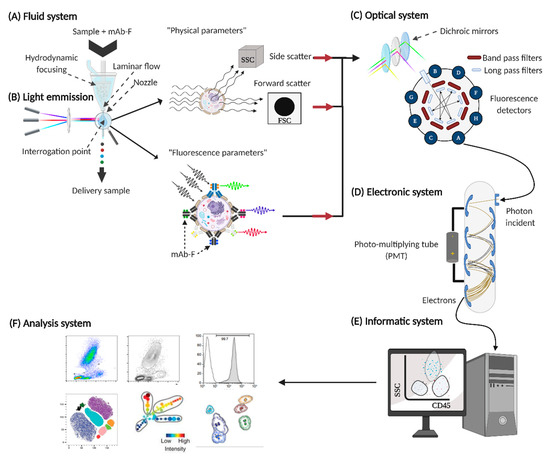
Figure 2. Fundamental systems in flow cytometers. For the proper functioning of the flow cytometer, six basic systems are required: (A) fluid system, (B) source of light emission, (C) optical system, (D) electronic system, (E) informatic system, and (F) analysis system.
3. Basic Considerations to Perform Flow Cytometry
To date, more than 400 mAbs have been standardized and approved for use in research and clinical diagnosis in human leukocytes. When considering FCM as a work tool, we must start from basic questions to establish an adequate experimental design that suits our interests, for example:
-
Related to the object of study: Will cells, soluble components, functional processes (in vitro activation) be evaluated?
-
Related to the staining protocol: What type of sample is it? Do I need to identify surface marks, intracellular, phosphoproteins, etc? Will the acquisition of the samples be immediate or do I need to preserve them?
-
Equipment related: What equipment is available? How many types of lasers do you have attached? What detectors does it have?
-
Related to fluorescence: How much do the emission signals of the selected fluorochromes overlap?
-
Related to the analysis system: What analysis program is available?
It is recommended that for the selection of the fluorochrome that will be coupled to the mAb, the intensity of expression of the molecule to be evaluated is considered; thus, with regard to molecules with abundant expression, it is preferable to use low-bright fluorochromes such as those of the blue laser (488 nm) and red (633 nm), while for molecules with minimal or unknown expression, it is preferable to use bright fluorochromes such as those excited by the violet laser (405 nm) [6]. Another consideration is to avoid selecting those that fluoresce at similar wavelengths, for example, the emission spectrum of fluorochrome called phycoerythrin cyanine 5 spans ~637–705 with a maximum emission at 667 nm; because the fluorochrome called allophycocyanin (APC) emits 660 nm wavelength, the detector for APC can recognize the signal and quantify false positives.
4. Quality management in Flow Cytometry
The applications of FCM are aimed at evaluating the expression pattern of molecules in health and disease, based on the biological principle that there are both phenotypic and functional cellular alterations in the immunopathogenesis of disease [7].
Currently, FCM serves as a tool for the diagnosis and clinical monitoring of pathologies of various origins; including oncological, immunodeficiencies or infectious, it is essential not to compromise the obtained results for the proper processing of the samples, for example, it is recommended that peripheral blood samples from patients with sepsis be subjected to bi-hemolysis processes, to avoid problems of specificity of mAb-F [8][9].
5. Use of Flow Cytometry inthe diagnosis of respiratory diseases
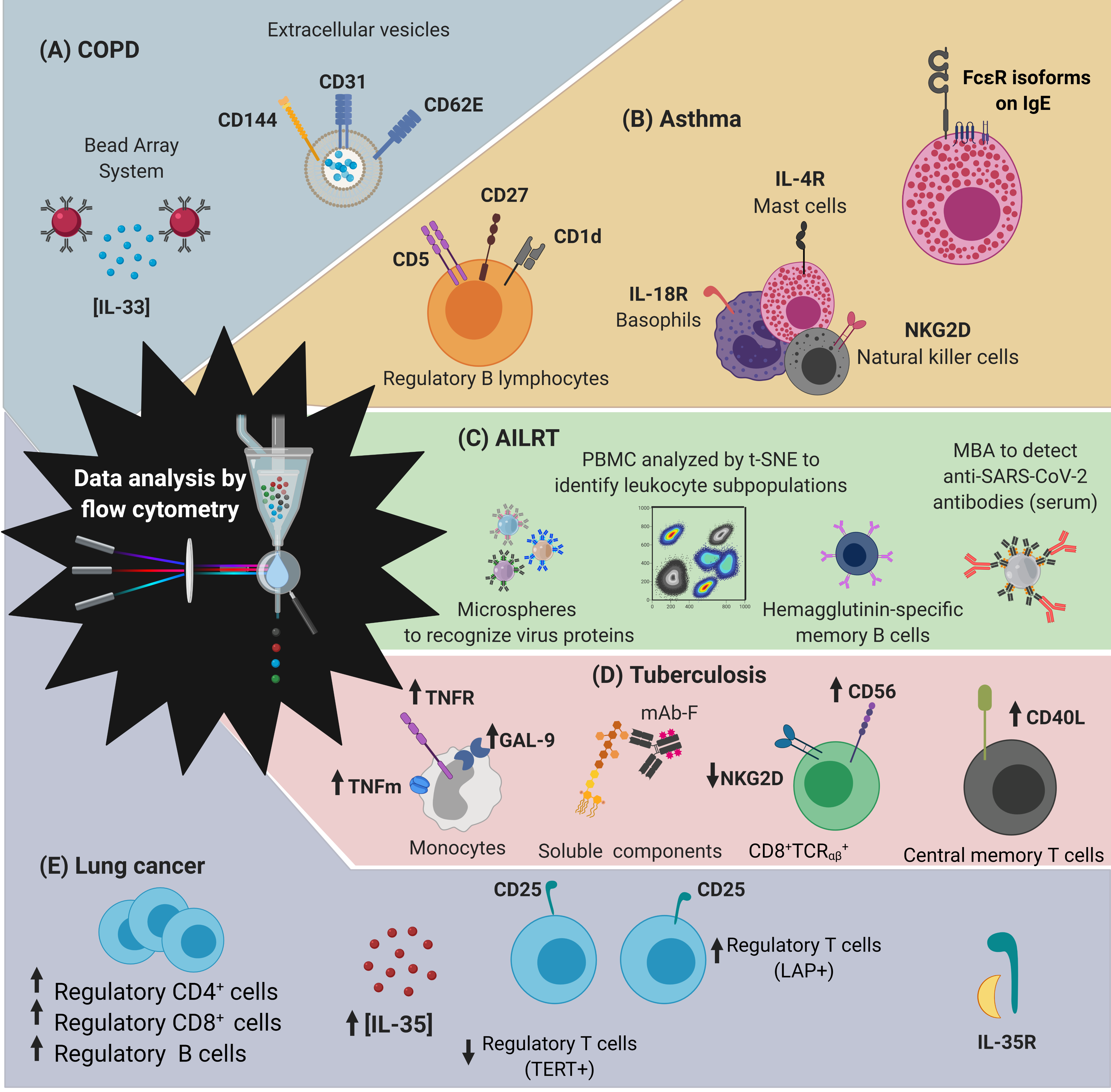
The respiratory diseases are the most common causes of death worldwide [10]. The Forum of International Respiratory Societies (FIRS) defines respiratory disease as those that “affect the airway and lungs, impacting lung capacity or gas distribution in our body”, and the five main ones are chronic obstructive pulmonary disease (COPD), asthma, acute lower respiratory tract infections, tuberculosis (TB), and lung cancer (Figure 3).
Techniques such as single-cell RNA sequencing, mass cytometry and FCM have been essential to describe pathophysiological mechanisms and to search for diagnostic markers in respiratory diseases [11]. In the next sections, we have demonstrated the experimental evidence that exists on the importance of using FCM as a tool for monitoring and diagnosing respiratory diseases.
Figure 3. Developments in the diagnosis of respiratory diseases obtained by flow cytometry (FCM). FCM has made it possible to establish parameters for the diagnosis and study of the five main respiratory diseases: (A) chronic obstructive pulmonary disease (COPD), (B) asthma, (C) acute lower respiratory tract infections (AILRT), (D) tuberculosis and (E) lung cancer. Abbreviations: t-SNE, high-dimensional unbiased stoichiometric analysis; PBMC, peripheral mononuclear cells; MBA, microsphere-based antibody assay; mAB-F, Monoclonal antibodies coupled to fluorochromes; GAL-9, Galectine 9; LAP, Latency Associated Peptide; TERT, telomerase reactivity.
6. Advantages and limitations of conventional methods, compared to FCM, in the diagnosis of respiratory diseases
Currently, specialized agencies in public health such as the WHO and the centers for disease control and prevention, establish and approve guidelines for the diagnostic and follow-up of different pathologies, including those of respiratory origin.
As we discussed above, FCM is a technique that has been showing is useful to improve the diagnosis and monitoring of respiratory diseases, majorly in those pathologies where the conventional technique for diagnostics (provided in the guidelines) has inconvenient like low sensitivity and specificity, highly invasive technique to obtain the tissue sample, expensive, long time to deliver results, among other. The measuring of molecules by FCM usually is implemented in peripheral mononuclear cells or serum, this means; a blood sample is sufficient to develop the quantification of molecules by FCM. Moreover, although this technique requests sophisticated equipment and special capacitation to use it, FCM results are delivered in a short time, and they are relatively easy to interpretive. Finally, flow cytometer is a stable machine that has the potential to implement a point-of-care test.
Guidelines provided by European Respiratory Society (ERS), American Thoracic Society (ATS) and Global Initiative for Chronic Obstructive Lung Disease (GOLD), recommend the use of lung function tests and imaging tests in the diagnosis of respiratory diseases [12]. For instance, spirometry test is relevant for COPD diagnosis, and though it is an excellent tool to support a clinical decision, to have a quality of spirometric measurements is requested a consensus among manufacturers, clinicians, operators, and researchers in order to improve the accuracy and precision of the test [13][14][15].
Methods used to TB diagnosis also show limitations, for instance, culture-based methods need of a long time to deliver the result, and it delays the starting of the treatment, and another test as the tuberculin skin test has a low specificity [16]. Currently, serological and nucleic acid amplification tests are useful to identify influenza or SARS-CoV-2 infection. However, RT-PCR test is expensive, and although it is highly specific and sensitive, there are reports of negative- and positive-false that are majorly associated to the high homology between strains or because the test is detecting non-viable virus or cell debris; whereas, some serological test requests improve its sensibility and specificity [17].
Finally, the biopsy is a method used for lung cancer diagnosis is a highly invasive method to get the tissue sample. Moreover, it should be done by competent personal that request training for a long time.
7. Conclusions
Diseases that affect the respiratory system influence humans to high mortality and morbidity, regardless of age, gender or social status. These conditions are subject to our lifestyle, geographic environment, and climate change.
In the history of humanity, diseases that arise with clinical characteristics of their pathophysiology have been described. Therefore, tools are needed to address the following criteria:
- Quick and specific diagnosis.
- Identification of clinical phases.
- Follow-up biomarkers to evaluate adequate response to treatments.
- Cost-benefit ratio.
Within the current clinical evaluation tools, special attention has been paid to the specificity and sensitivity for the diagnosis and follow-up of diseases offered by FCM. Since its inception, it has emerged as a tool for rapid and individualized interrogation of suspended events. Through continuous improvement in the basic principles of fluorochrome excitation and emission, monoclonal antibody production, and data analysis systems, today's cytometrists have been equipped with powerful tools to extend our knowledge of biological systems.
References
- Louis A. Kamentsky; Myron R. Melamed; Herbert Derman; Spectrophotometer: New Instrument for Ultrarapid Cell Analysis. Science 2006, 150, 630-631, 10.1126/science.150.3696.630.
- V T Oi; A N Glazer; L Stryer; Fluorescent phycobiliprotein conjugates for analyses of cells and molecules.. Journal of Cell Biology 1982, 93, 981-986, 10.1083/jcb.93.3.981.
- G. Köhler; C. Milstein; Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495-497, 10.1038/256495a0.
- Leonard A Herzenberg; David Parks; Bita Sahaf; Omar Perez; Mario Roederer; Leonore A Herzenberg; The history and future of the fluorescence activated cell sorter and flow cytometry: a view from Stanford.. Clinical Chemistry 2002, 48, 1819, 10.1093/clinchem/48.10.1819.
- Bajgelman, M.C.. Principles and applications of flow cytometry. In Data Processing Handbook for Complex Biological Data Sources; Misra G, Eds.; Elsevier: USA, 2019; pp. 119–124.
- Laura Ferrer-Font; Christophe Pellefigues; Johannes U. Mayer; Sam J. Small; Maria C. Jaimes; Kylie M. Price; Panel Design and Optimization for High‐Dimensional Immunophenotyping Assays Using Spectral Flow Cytometry. Current Protocols in Cytometry 2020, 92, e70, 10.1002/cpcy.70.
- Maryam Khordad; Robert E. Mercer; Identifying genotype-phenotype relationships in biomedical text. Journal of Biomedical Semantics 2017, 8, 57-57, 10.1186/s13326-017-0163-8.
- Stephania Vázquez Rodríguez; Lourdes Arriaga-Pizano; Estibalitz Laresgoiti-Servitje; Javier Mancilla Ramirez; Omar L. Peralta Méndez; Gisela Villalobos Alcazar; Martha L. Granados Cepeda; Maria Graciela Hernandez Pelaez; Guadalupe Cordero Gonzalez; Roberto Arizmendi Villanueva; et al.José Luis Cruz RamírezArmando IsibasiConstantino López-MacíasLeopoldo Flores-RomoLuis A. Jimenez ZamudioArturo Cérbulo-Vázquez Multiparameter flow cytometry analysis of leukocyte markers for diagnosis in preterm neonatal sepsis. The Journal of Maternal-Fetal & Neonatal Medicine 2019, 2019, 1-11, 10.1080/14767058.2019.1666100.
- Julia I. Ellyard; Robert Tunningley; Ayla May Lorenzo; Simon H. Jiang; Amelia Cook; Rochna Chand; Dipti Talaulikar; Ann-Maree Hatch; Anastasia Wilson; Carola G. Vinuesa; et al.Matthew C. CookDavid A. Fulcher Non-parametric Heat Map Representation of Flow Cytometry Data: Identifying Cellular Changes Associated With Genetic Immunodeficiency Disorders. Frontiers in Immunology 2019, 10, 2134, 10.3389/fimmu.2019.02134.
- Haidong Wang; Mohsen Naghavi; Christine Allen; Ryan M Barber; Zulfiqar A Bhutta; Austin Carter; Daniel C Casey; Fiona J Charlson; Alan Zian Chen; Matthew M Coates; et al.Megan CoggeshallLalit DandonaDaniel J DickerHolly E ErskineAlize J FerrariChristina FitzmauriceKyle ForemanMohammad H ForouzanfarMaya S FraserNancy FullmanPeter W GethingEllen M GoldbergNicholas GraetzJuanita A HaagsmaSimon I HayChantal HuynhCatherine O JohnsonNicholas J KassebaumYohannes KinfuXie Rachel KulikoffMichael KutzHmwe H KyuHeidi J LarsonJanni LeungXiaofeng LiangStephen S LimMargaret LindRafael LozanoNeal MarquezGeorge A MensahJoe MikesellAli H MokdadMeghan D MooneyGrant NguyenElaine NsoesieDavid M PigottChristine PinhoGregory A RothJoshua A SalomonLogan SandarNaris SilpakitAmber SligarReed J D SorensenJeffrey StanawayCaitlyn SteinerStephanie TeepleBernadette A ThomasChristopher TroegerAmelia VanderzandenStein Emil VollsetValentine WangaHarvey A WhitefordTimothy WolockLeo ZoecklerKalkidan Hassen AbateCristiana AbbafatiKaja M AbbasFoad Abd-AllahSemaw Ferede AberaDaisy M X AbreuLaith J Abu-RaddadGebre Yitayih AbyuTom AchokiAdemola Lukman AdelekanZanfina AdemiArsène Kouablan AdouJosé C AdsuarKossivi Agbelenko AfanviAshkan AfshinEmilie Elisabet AgardhArnav AgarwalAnurag AgrawalAliasghar Ahmad KiadaliriOluremi N AjalaAli Shafqat AkandaRufus Olusola AkinyemiTomi F AkinyemijuNadia AkseerFaris Hasan Al LamiSamer AlabedZiyad Al-AlyKhurshid AlamNoore K M AlamDeena AlasfoorSaleh Fahed AldhahriRobert William AldridgeMiguel Angel AlegrettiAlicia V AlemanZewdie Aderaw AlemuQuistberg D. AlexSamia AlhabibAmmar WalidAla'a AlkerwiFrançois AllaPeter AllebeckRajaa Al-RaddadiUbai AlsharifKhalid A AltirkawiElena Alvarez MartinNelson Alvis-GuzmanTedla Bemnet AmareAdeladza Kofi AmegahEmmanuel A AmehHeresh AminiWalid AmmarStephen Marc AmrockHjalte H AndersenBenjamin O AndersonGregory M AndersonCarl Abelardo T AntonioAtsede Fantahun AregayJohan ÄrnlövValentina S Arsic ArsenijevicAl ArtamanHamid AsayeshRana Jawad AsgharSuleman AtiqueEuripide Frinel G Arthur AvokpahoAshish AwasthiPeter AzzopardiUmar BachaAlaa BadawiMaria C BahitKalpana BalakrishnanAmitava BanerjeeAleksandra BaracSuzanne L Barker-ColloTill BärnighausenLars BarregardLope H BarreroArindam BasuSanjay BasuYibeltal Tebekaw BayouShahrzad Bazargan-HejaziJustin BeardsleyNeeraj BediEttore BeghiHaileeyesus Adamu BelayBrent BellMichelle L BellAminu K BelloDerrick A BennettIsabela M BensenorAdugnaw BerhaneEduardo BernabéBalem Demtsu BetsuAddisu Shunu BeyeneNeeraj BhalaAshish BhallaSibhatu BiadgilignBoris BikbovAref A Bin AbdulhakBrian J BiroscakStan BiryukovEspen BjertnessJed D BloreChristopher D BlosserMegan A BohenskyRohan BorschmannDipan BoseRupert R A BourneMichael BraininCarol E G BrayneAlexandra BrazinovaNicholas J K BreitbordeHermann BrennerJerry D BrewerAlexandria BrownJonathan BrownTraolach S BrughaGeoffrey Colin BuckleZahid A ButtBianca CalabriaIsmael Ricardo Campos-NonatoJulio Cesar CampuzanoJonathan R CarapetisRosario CárdenasDavid O CarpenterJuan Jesus CarreroCarlos A Castañeda-OrjuelaJacqueline Castillo RivasFerrán Catalá-LópezFiorella CavalleriKelly CercyJorge CerdaWanqing ChenAdrienne ChewChiang Peggy Pei -ChiaMirriam ChibalabalaChioma Ezinne ChibuezeOdgerel Chimed-OchirVesper Hichilombwe ChisumpaJee-Young Jasmine ChoiRajiv ChowdhuryHanne ChristensenDevasahayam Jesudas ChristopherLiliana G CiobanuMassimo CirilloAaron J CohenValentina ColistroMercedes ColomarSamantha M ColquhounCyrus CooperLeslie Trumbull CooperMonica CortinovisBenjamin C CowieJohn A CrumpJames Damsere-DerryHadi DanawiRakhi DandonaFarah DaoudSarah C DarbyPaul I DarganJosé Das NevesGail DaveyAdrian C DavisDragos V DavitoiuE Filipa De CastroPieter De JagerDiego De LeoLouisa DegenhardtRobert P DellavalleKebede DeribeAmare DeribewSamath D DharmaratnePreet K DhillonCesar Diaz-TornéEric L DingKadine Priscila Bender Dos SantosEdem DossouTim R DriscollLeilei DuanManisha DubeyBruce Bartholow DuncanRichard G EllenbogenChristian Lycke EllingsenIqbal ElyazarAman Yesuf EndriesSergey Petrovich ErmakovBabak EshratiAlireza EsteghamatiKara EstepImad D A FaghmousSaman FahimiEmerito Jose Aquino FaraonTalha A FaridCarla Sofia E Sa FarinhaAndré FaroMaryam S FarvidFarshad FarzadfarValery L FeiginSeyed-Mohammad FereshtehnejadJefferson G FernandesJoao C FernandesFlorian FischerJoseph R A FitchettAbraham FlaxmanNataliya FoigtF Gerry R FowkesElisabeth Barboza FrancaRichard C FranklinJoseph FriedmanJoseph FrostadThomas FürstNeal D FutranSeana L GallKetevan GambashidzeAmiran GamkrelidzeParthasarathi GangulyFortuné Gbètoho GankpéTeshome GebreTsegaye Tsewelde GebrehiwotAmanuel Tesfay GebremedhinAlemseged Aregay GebruJohanna M GeleijnseBradford D GessnerAloke Gopal GhoshalKather Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet 2016, 388, 1459-1544, 10.1016/s0140-6736(16)31012-1.
- Karolyn A. Oetjen; Katherine E. Lindblad; Meghali Goswami; Gege Gui; Pradeep K. Dagur; Catherine Lai; Laura W. Dillon; J. Philip McCoy; Christopher S. Hourigan; Human Bone Marrow Assessment by Single Cell RNA Sequencing, Mass Cytometry and Flow Cytometry. null 2018, 3, 416750, 10.1101/416750.
- Brian L. Graham; Irene Steenbruggen; Martin R. Miller; Igor Barjaktarevic; Brendan G. Cooper; Graham L. Hall; Teal S. Hallstrand; David A. Kaminsky; Kevin McCarthy; Meredith C. McCormack; et al.Cristine E. OropezMargaret RosenfeldSanja StanojevicMaureen P. SwanneyBruce R. Thompson Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. American Journal of Respiratory and Critical Care Medicine 2019, 200, e70-e88, 10.1164/rccm.201908-1590st.
- Agnaldo José Lopes; Advances in spirometry testing for lung function analysis. Expert Review of Respiratory Medicine 2019, 13, 559-569, 10.1080/17476348.2019.1607301.
- Marcin Bednarek; Marcin Grabicki; Tomasz Piorunek; Halina Batura-Gabryel; “Current place of impulse oscillometry in the assessment of pulmonary diseases.”. Respiratory Medicine 2020, 170, 105952, 10.1016/j.rmed.2020.105952.
- David M. G. Halpin; Gerard J. Criner; Alberto Papi; Dave Singh; Antonio Anzueto; Fernando J. Martinez; Alvar A. Agusti; Claus F. Vogelmeier; Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine 2020, 203, 24-36, 10.1164/rccm.202009-3533so.
- Evan Bursle; Jennifer Robson; Non-culture methods for detecting infection. Australian Prescriber 2016, 39, 171-175, 10.18773/austprescr.2016.059.
- Laura García-Arroyo; Núria Prim; Neus Martí; Maria Carme Roig; Ferran Navarro; Nuria Rabella; Benefits and drawbacks of molecular techniques for diagnosis of viral respiratory infections. Experience with two multiplex PCR assays. Journal of Medical Virology 2015, 88, 45-50, 10.1002/jmv.24298.