| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Intan Rosalina Suhito | + 990 word(s) | 990 | 2021-01-07 08:41:10 |
Video Upload Options
Hormones are secreted primarily by glands or specific cells, circulate in the bloodstream, and specialize in targeting cells. The electrochemical biosensing of hormones has emerged for treating human diseases and performing clinical diagnosis.
1. Introduction
The development of biosensors enables detecting biomolecules and other phenomena, including hormones [1][2]. The quantity of hormones that regulate and control the metabolism of the human body is very low, leading to efforts to develop a highly sensitive tool to detect them. The electrochemical approach has typically been used for hormone sensing because it can overcome the limitations of other well-established methods (e.g., ELISA) in terms of sensitivity, selectivity, and time performance [3][4]. One example is the modified screen-printed carbon electrode (SPCE) with cobalt nanoparticles (CoNPs) with chitosan and multi-walled carbon nanotubes (MWCNTs) (CoNPs/chitosan-MWCNTs/SPCE) that can successfully detect insulin with concentrations down to 25 nM. This finding could confirm the advantages of an electrochemical detection system [5]. This entry describes several current sensors for hormone detection that may contribute to the development of electrochemical-based hormone sensors.
2. Electrochemical Detection of Estrogen Hormone
Estrogen is a naturally occurring steroid hormone in mammals with unusual behavior when it reacts with its receptor [6]. It is an essential hormone in the female reproductive cycle, menstrual cycle, and growth, while it can also lead to obesity and infertility at abnormal levels. Eighty percent of breast cancers are affected by estrogen, indicating its association with cancer [7]. Therefore, estrogen-level detection is highly favorable due to its promotion effects and initial tumor formation. A detection platform that uses the estrogen receptor has been studied through an electrochemical detection platform that is non-destructive with high selectivity and sensitivity [8][9][10]. In 2018, Liu et al. developed an electrode surface transformation with a gold electrode on which 6-mercapto-1-hexanol (MCH) was used for a self-assembled monolayer (SAM) [11]. Graphene was treated with a bi-function to adsorb the E2-binding aptamers and the SAM on the MCH/Au modified electrode. Electrochemical detection was performed with a 20 mM PBS containing 5 mM FcCOOH and 0.1 M NaClO4. The DPV performance confirmed the enhanced detection of E2, respectively.
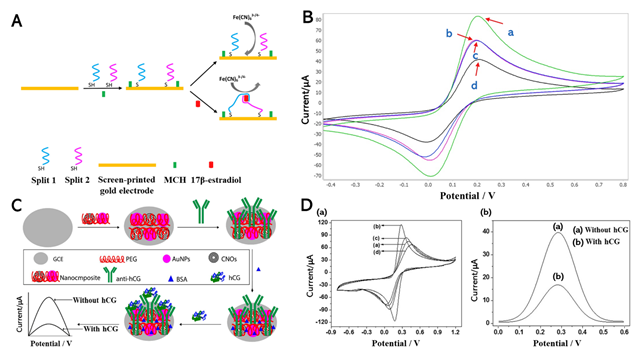
Nameghi et al. (2019) used the gold electrode and aptamers for the substrate to detect estrogen (Figure 1A). Previously, aptasensors have demonstrated satisfactory results at detecting their targets [9][10]. The immobilization was performed via thiol-modified split aptamers that can react with the gold surface [6]. CV was conducted through the proposed platform because the interfacial reaction could be determined via this method, thus enabling easy discrimination between estrogen and the control group through their signal (Figure 1B). The bare electrode presented the maximum CV current (curve A), which indicated excellent electron transfer between the bare gold electrode and [Fe(CN)6]3−/4−. When split aptamers were conjugated onto the gold electrode’s surface, the redox current decreased (curve B). From curve D, when adding the E2, the electrochemical signal was significantly reduced because the split1–E2–split2 complex was formed on the electrode. However, in the presence of bisphenol A (BPA) as non-target substances, the current signal of the split DNA aptamer modified electrode did not change (curve C). Furthermore, DPV analysis was performed, in which the concentrations of E2 were measured from 1.2 pM to 100 pM and 100 nM to 7 nM with a detection limit of 0.5 pM (S/N = 3). The outstanding performance of the proposed biosensors demonstrates their reproducibility, high sensitivity, and selectivity.
3. Electrochemical Detection of Human Chorionic Gonadotropin (hCG) Hormone
Human chorionic gonadotropin (hCG) is a glycoprotein hormone secreted by placenta trophoblast cells that functions as a diagnostic marker for pregnancy and a tumor marker [12]. The early quantitative detection of hCG is particularly challenging. Numerous hCG analysis methods have been reported, such as ELISA, fluorescence-labeled immunoassay, and radioimmunoassay [13][14]. Electrochemical detection could overcome the limitations of other methods (e.g., high cost, laborious, slow performance, and risk potential of radioactivity) [15]. A highly sensitive electrochemical immunosensor based on carbon nano-onions (CNOs), gold nanoparticles (GNPs), a polyethylene glycol (PEG) composite, and a glassy carbon electrode (GCE) was reported by Rizwan et al. [16]. This composite was drop-casted onto a pre-cleaned GCE as a self-assembled monolayer via chemisorption. The anti-hCG was then immobilized onto the modified CNOs/AuNPs/PEG/GCE biocompatible interface (Figure 1C). Before detecting the hCG, this fabricated sensor was incubated for 45 min at room temperature. This layer-by-layer fabrication was conducted through CV characterization. The detection of hCG was performed using square wave voltammetry (SWV), as illustrated in Figure 1D. This hCG immunosensor exhibited high sensitivity and productivity at a low detection concentration of 100 fg/mL.
Damiati et al. (2019) developed a screen-printed sensor based on the modified carbon macro- and micro-electrodes with a linker, 1-pyrenebutyric acid-N-hydroxy-succinimide ester (PANHS), and the immobilization of anti-hCG antibodies to detect hCG [17]. CV was conducted to characterize the modified electrode by increasing the scan rate from 10 to 100 mV/s. Furthermore, the SWV detection of the micro-electrode exhibited a higher sensitivity (1 pg/mL) than the macro-electrode sensor with a lower detection limit of 100 pg/mL. The working electrode’s physical size directly impacted the electrochemical sensitivity of biosensors that used macro- and micro-electrodes. The results of CV and SWV performance on the modified BSA/anti-hCG antibody/PANHS/SPCE demonstrated that the low-cost, label-free biosensor has high selectivity for hCG detection.
Figure 1. (A) Electrochemical aptasensor for sensing 17β-estradiol (E2) based on split DNA aptamers. (B) Electrochemical characterization of the electrode modification and the aptasensor function. CV profiles of: bare electrode (green curve, curve a), split DNA aptamers-modified electrode (pink curve, curve b), split DNA aptamers-modified electrode + bisphenol A (BPA) (lack of bridge) (blue curve, curve c), split DNA aptamers-modified electrode + E2 (bridge assembly) (black curve, curve d). (C) Fabrication of the hCG-immunosensor. (D) Assessment of the step-wise fabrication of hCG-immunosensors and electrochemical signal: CV curve of (a) bare-GCE, (b) CNOs/AuNPs/PEG/GCE, (c) anti-hCG/CNOs/AuNPs/PEG/GCE, and (d) BSA/anti-hCG/CNOs/AuNPs/PEG/GCE; SWV curves of immunosensor (a) without hCG and (b) with hCG. Reprinted with permission from [6]. Copyright 2019, Elsevier; Reprinted with permission from [16]. Copyright 2019, Elsevier.
References
- J.G. Manjunatha; Electroanalysis of estriol hormone using electrochemical sensor. Sensing and Bio-Sensing Research 2017, 16, 79-84, 10.1016/j.sbsr.2017.11.006.
- Mani Govindasamy; Bowya Subramanian; Sea-Fue Wang; Sathishkumar Chinnapaiyan; Jothi Ramalingam Rajabathar; Hamad A. Al-Lohedan; Ultrasound-assisted synthesis of tungsten trioxide entrapped with graphene nanosheets for developing nanomolar electrochemical (hormone) sensor and enhanced sensitivity of the catalytic performance. Ultrasonics Sonochemistry 2019, 56, 134-142, 10.1016/j.ultsonch.2019.03.021.
- Selma Cifrić; Jasna Nuhić; Dina Osmanović; Emina Kišija; Review of Electrochemical Biosensors for Hormone Detection. XXVI Brazilian Congress on Biomedical Engineering 2019, -, 173-177, 10.1007/978-3-030-17971-7_27.
- My Van Tieu; Anna Go; Young Ju Park; Huynh Vu Nguyen; Sei Young Hwang; Min-Ho Lee; Highly Sensitive ELISA Using Membrane-Based Microwave-Mediated Electrochemical Immunoassay for Thyroid-Stimulating Hormone Detection. IEEE Sensors Journal 2019, 19, 9826-9831, 10.1109/jsen.2019.2925020.
- Ivana Šišoláková; Jana Hovancová; Renáta Oriňaková; Andrej Oriňak; Libuše Trnková; Iveta Třísková; Zdeněk Farka; Matěj Pastucha; Jozef Radoňák; Electrochemical determination of insulin at CuNPs/chitosan-MWCNTs and CoNPs/chitosan-MWCNTs modified screen printed carbon electrodes. Journal of Electroanalytical Chemistry 2020, 860, 113881, 10.1016/j.jelechem.2020.113881.
- Morteza Alinezhad Nameghi; Noor Mohammad Danesh; Mohammad Ramezani; Mona Alibolandi; Khalil Abnous; Seyed Mohammad Taghdisi; An ultrasensitive electrochemical sensor for 17β-estradiol using split aptamers. Analytica Chimica Acta 2019, 1065, 107-112, 10.1016/j.aca.2019.02.062.
- Carolina V. Uliana; Camila R. Peverari; André S. Afonso; Marcia R. Cominetti; Ronaldo C. Faria; Fully disposable microfluidic electrochemical device for detection of estrogen receptor alpha breast cancer biomarker. Biosensors and Bioelectronics 2018, 99, 156-162, 10.1016/j.bios.2017.07.043.
- Rajesh Ahirwar; Anita Dalal; Jai Gopal Sharma; Birendra Kumar Yadav; Pradip Nahar; Ashok Kumar; Saroj Kumar; An aptasensor for rapid and sensitive detection of estrogen receptor alpha in human breast cancer. Biotechnology and Bioengineering 2018, 116, 227-233, 10.1002/bit.26819.
- Jinlong Li; Guangwu He; Bei Wang; Liu Shi; Tao Gao; Genxi Li; Fabrication of reusable electrochemical biosensor and its application for the assay of α-glucosidase activity. Analytica Chimica Acta 2018, 1026, 140-146, 10.1016/j.aca.2018.04.015.
- Chao Li; Xiaolu Hu; Jianyang Lu; Xiaoxia Mao; Yang Xiang; Yongqian Shu; Genxi Li; Design of DNA nanostructure-based interfacial probes for the electrochemical detection of nucleic acids directly in whole blood. Chemical Science 2018, 9, 979-984, 10.1039/c7sc04663d.
- Meichuan Liu; Hongyang Ke; Caiqin Sun; Guoqiang Wang; Yu Wang; Guohua Zhao; A simple and highly selective electrochemical label-free aptasensor of 17β-estradiol based on signal amplification of bi-functional graphene. Talanta 2019, 194, 266-272, 10.1016/j.talanta.2018.10.035.
- Ning Xia; Zhihua Chen; Yadong Liu; Huizhu Ren; Lin Liu; Peptide aptamer-based biosensor for the detection of human chorionic gonadotropin by converting silver nanoparticles-based colorimetric assay into sensitive electrochemical analysis. Sensors and Actuators B: Chemical 2017, 243, 784-791, 10.1016/j.snb.2016.12.066.
- Weiguo Wang; Junmin Li; Changzhi Dong; Yafang Li; Qiuye Kou; Jin-Wu Yan; Lei Zhang; Ultrasensitive ELISA for the detection of hCG based on assembled gold nanoparticles induced by functional polyamidoamine dendrimers. Analytica Chimica Acta 2018, 1042, 116-124, 10.1016/j.aca.2018.08.038.
- Zahra Pourmoghadam; Mohammad Sadegh Soltani-Zangbar; Golshan Sheikhansari; Ramyar Azizi; Shadi Eghbal-Fard; Hamed Mohammadi; Homayoon Siahmansouri; Leili Aghebati-Maleki; Shahla Danaii; Amir Mehdizadeh; et al.Mohammad Hojjat-FarsangiRoza MotavalliMehdi Yousefi Intrauterine administration of autologous hCG- activated peripheral blood mononuclear cells improves pregnancy outcomes in patients with recurrent implantation failure; A double-blind, randomized control trial study. Journal of Reproductive Immunology 2020, 142, 103182, 10.1016/j.jri.2020.103182.
- Nguyen Xuan Viet; Nguyen Xuan Hoan; Yuzuru Takamura; Development of highly sensitive electrochemical immunosensor based on single-walled carbon nanotube modified screen-printed carbon electrode. Materials Chemistry and Physics 2019, 227, 123-129, 10.1016/j.matchemphys.2019.01.068.
- Mohammad Rizwan; Muhammad Hazmi; Syazana Abdullah Lim; Minhaz Uddin Ahmed; A highly sensitive electrochemical detection of human chorionic gonadotropin on a carbon nano-onions/gold nanoparticles/polyethylene glycol nanocomposite modified glassy carbon electrode. Journal of Electroanalytical Chemistry 2019, 833, 462-470, 10.1016/j.jelechem.2018.12.031.
- Samar Damiati; Carrie Haslam; Sindre Sopstad; Martin Peacock; Toby Whitley; Paul Davey; Shakil A. Awan; Sensitivity Comparison of Macro- and Micro-Electrochemical Biosensors for Human Chorionic Gonadotropin (hCG) Biomarker Detection. IEEE Access 2019, 7, 94048-94058, 10.1109/access.2019.2928132.