
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianping Xu | + 2861 word(s) | 2861 | 2021-01-11 07:43:44 | | | |
| 2 | Camila Xu | Meta information modification | 2861 | 2021-01-14 10:21:14 | | | | |
| 3 | Camila Xu | Meta information modification | 2861 | 2021-01-14 10:22:02 | | |
Video Upload Options
Sphinganine-analog mycotoxins (SAMs) including fumonisins and Alternaria alternata f. sp. Lycopersici (AAL) toxins are a group of related mycotoxins produced by plant pathogenic fungi in the Fusarium genus and in A. alternata f. sp. Lycopersici, respectively. SAMs have shown diverse cytotoxicity and phytotoxicity, causing adverse impacts on plants, animals, and humans, and are a destructive force to crop production worldwide.
1. Introduction
Mycotoxins are secondary metabolites produced by various fungi. These metabolites have important ecological functions on living systems in their natural habitats. As secondary metabolites, mycotoxins are regarded as not essential for fungal growth or reproduction. However, their toxic effects to plants, animals, as well as humans are attracting increasing attention from chemists, biologists, food scientists, and healthcare professionals. Many fungi are capable of synthesizing mycotoxins, including certain saprophytic molds, poisonous mushrooms, human fungal pathogens, and plant fungal pathogens. Mycotoxins produced by plant pathogenic fungi can be divided into two groups: (i) host-selective (or host-specific) toxins (HSTs) and (ii) non-host-specific toxins (nHSTs), depending on whether they are specifically toxic to host plant (HSTs) or to a wide range of species (nHSTs). The known mycotoxins are typically low molecular-weight chemicals but with diverse structures and modes of actions. One group of mycotoxins are structurally analogous to sphingosine, the backbone precursor of sphingolipids that play essential structural and cellular roles in eukaryotic cells. These toxins are called sphinganine-analog mycotoxins (SAMs), with fumonisins and the Alternaria alternata f. sp. Lycopersici (AAL) toxins as the two most widely studied groups of SAMs. SAMs are toxic to plants and animals. They act by inhibiting the ceramide synthase (CerS), thereby influencing the sphingolipid metabolism and initiating apoptosis in animals and programmed cell death (PCD) in plants [1][2][3]. The paper provides an overview on the structural diversity, syntheses, modes of action, and health impacts of SAMs.
The discovery of fumonisin was first reported in 1988 and the organism producing it was Fusarium verticillioides (syn. Gibberella fujikuroi mating population A, syn. G. moniliformis Wineland, syn. F. moniliforme Sheldon) [4]. Fumonisins have since been found to be produced by at least 18 species of the Fusarium genus, with F. verticillioides and F. proliferatum being the most prominent, and by three unrelated fungal genera, Aspergillus section Nigri (such as Asp. niger, Asp. Welwitschiae (syn. Asp. awamori) and so on, known as black aspergilli), Tolypocladium (T. inflatum, T. cylindrosporum, and T. geodes), and Alternaria (the tomato pathotype of A. alternata, formerly known as A. arborescens) [5][6][7][8][9][10]. Species of the Fusarium genus can be found as saprophytes in soil and as endophytes and pathogens of many plants worldwide. A common group of diseases caused by Fusarium pathogens is rotting that can happen to all tissues during all stages of plant development [11][12]. In addition, the Fusarium species can infect crops at the post-harvest period during storage [13]. The fungal propagules surviving in the soil can also infect new crop plants and can be carried to new fields by wind or by anthropogenic activities, such as when seedlings are transplanted [14]. Fusarium strains can synthesize fumonisins during all stages of their growth, including the saprophytic stage in the soil, during their pathogenesis, and as endophytes in different parts of plants, as well as during crop storage after harvest [15].
Fumonisins, as a nHST, are major contaminants of cereals and grains, including corn, rice, wheat, barley, rye, oat, millet, and products made based on these crops [16]. The consumption of food contaminated by fumonisins significantly increases health problems for humans, leading to a variety of cancers such as esophageal cancer and neurological defects [17][18]. For example, the International Agency for Research on Cancer (IARC) characterized fumonisin FB1 as a group 2B carcinogens for humans [16]. Fumonisins can also cause diseases and adverse effects in other species, especially in livestock when the feeds are contaminated [19]. Well-known diseases in livestock caused by fumonisins include leukoencephalomalacia in horses and pulmonary edema syndrome in pigs [20][21].
Similar to fuminisins, the AAL-toxins include a family of structurally analogous metabolites. AAL-toxins are a group of HST produced by the ascomycete fungal pathogen A. alternata f. sp. Lycopersici, the causal agent of tomato stem canker disease [22]. It should be noted that several other pathotypes of A. alternata could also produce other HSTs responsible for fungal pathogenesis on their specific host plants, respectively [23]. Unlike other HSTs produced by A. alternata, besides the susceptible tomato host, AAL-toxins can also affect many other weeds and crops of dicotyledonous species and at least 25 species of Solanaceae [24][25]. Furthermore, the tomato pathotype of A. alternata was also reported to produce fumonisins B (FBs) [8][26]. AAL-toxin and FBs were not only detected in the necrosis plant tissues and culture media inoculated by A. alternata but also in spores and mycelia of this pathogen [27]. However, AAL-toxin remains the only toxin as a pathogenicity factor for stem canker disease of sensitive tomato varieties, while fumonisins are toxigenic virulence factors [28].
2. Chemical and Structural Properties
2.1. Chemical and Structural Properties of Sphingolipids
SAMs have a distinct structural similarity to sphinganine (Figure 1). Sphinganine (dihydrosphingosine, DHS) is the simplest class of sphingolipids and has a backbone that consists of a linear aliphatic group with 18-carbon, an amino at C-2, and two hydroxyls (-OH) at C-1 and C-3, respectively. Phytosphingosine is obtained if a hydroxyl is introduced at C-4. Sphingosine consists of the sphinganine backbone but with a double bond at C-4. Ceramides are synthesized by linking an amide fatty acid at C-2 of sphingosine. Ceramides is a waxy lipid molecule, which is found in high concentrations in the membrane of eukaryotic cells. More complex sphingolipids can be formed by linking different chemical groups to hydroxyl (C1) of ceramides. Sphingolipids are one type of lipids widely found in their membranes in eukaryotes and a few prokaryotes, and they form complex and diverse interactions with other molecules [29]. Sphingolipids play important structural and functional roles, they are involved in a variety of signal transductions and crucial cellular processes [30][31]. For example, in humans, ceramides help form the skin’s barrier and regulate immune response, protecting the skin against environmental irritants, pollutants, and water loss. Without the proper ratio of ceramides on our epidermal cells, the barrier of the skin will be damaged, resulting in dryness, itching, and irritation [32].
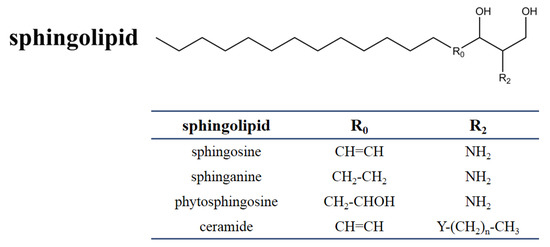
Figure 1. Chemical structure of sphingolipids. The table shows the different substituents in the chemical scaffold of the most essential sphingolipid.
2.2. Chemical and Structural Properties of Fumonisins
SAMs consist of two main types of toxins, fuminisins and AAL-toxins. Fumonisins can be divided into seven groups (FA, FB, FC, FD, FP, FPy, and FLa). These groups differ in the nitrogen functional group and the length of the carbon backbone [5]. Most fumonisins contain a 19–20 (FD contain 17 or 18 carbon) linear backbone similar to sphinganine with one nitrogen functional group (except for FPys and FLas), two to four hydroxyl, two methyl, and two propane-1,2,3-tricarboxylic acid (PTCA) side chains esterified to the backbones [26][33]. The structural features of the seven groups of fumonisins are shown in Figure 2. Among them, the B group is the dominant one. For example, FB1 accounts for 70–80% of the total fumonisins produced by F. verticillioides and is the predominant toxic form [5]. FB2 and FB3 are isomers of each other but with one less hydroxyl group than FB1. The B series of fumonisins (FBs) are also the main food contaminants. Group A fumonisins (FA) are acetylated derivates of group B toxins, with lower toxicity and bioactivity than their FB counterparts [34]. Group C fumonisins (FC) have the same nitrogen functional group as FB1 but lack the terminal methyl group at C-1 [35]. Three forms of acetylated FC1 have been discovered in F. oxysporum [36]. Group P fumonisins (FP) have a nitrogen functional group of 3-hydroxypyridinium instead of the amino group in FB at the R2 position [37]. The FC and FP groups have similar phytotoxic and cytotoxic effects to those caused by FB1 or AAL-toxin [38]. Aside from these four main groups, there are several other lesser-known fumonisin analogs, with one or two PTCA replaced by a hydroxyl or carbonyl or other carboxylic acids group at C-13 and/or C-14 of the backbone (for example, HFB1, as show in Figure 2). Rheeder et al. summarized the 28 fumonisin analogs that have been characterized between 1988 and 2002 [5]. By reversed-phase high-performance liquid chromatography/electrospray ionization ion trap multistage mass spectrometry (RP-HPLC/ESI-IT-MSn), Bartok et al. detected 58 fumonisins (including FD) or fumonisin-like compounds from F. verticillioides in rice cultures, and 28 isomers of FB1 [33][39]. Indeed, the recent application of a semi-targeted method revealed over 100 structurally related compounds from SAMs-producing fungi, including a hydroxyl-FB1, and two new classes of non-aminated fumonisins (FPys and FLas) [26].
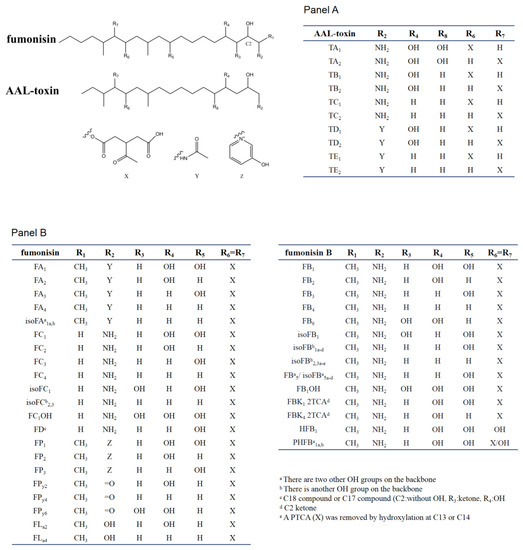
Figure 2. Chemical structure of sphinganine-analog mycotoxins (SAMs). Panel A shows the AAL-toxins, Panel B shows fumonisin. In the table of each panel, the different substituents present in the chemical scaffolds of individual compounds are shown.
2.3. Chemical and Structural Properties of AAL-Toxin
The AAL-toxins have a structural similarity to fumonisins (Figure 2). The main difference between fumonisins and AAL-toxins is that AAL-toxins have one fewer PTCA side chain than fumonisins. The AAL-toxins have been divided into five pairs based on their side chain structures: A, B, C, D, and E pairs (TA, TB, TC, TD and TE). These pairs differ in their nitrogen functional group and hydroxylation at C-4 or C-5 positions of the backbone [40][41][42]. Each pair of AAL-toxins is composed of two regioisomers with PTCA esterified to C-13 or C-14 of the backbone, respectively. The TA pair is the major pair of toxins, with the TB and TC pairs formed by removing hydroxyl groups one by one from C-5 and C-4 of the TA pair. The TD and TE pairs were acetylated derivatives of TB and TC respectively, while the acetylated form of TA and keto derivatives of AAL-toxins (2-keto or 14-keto analogues predicted) were also found in 2015 [26]. These four regioisomeric pairs (TB, TC, TD, and TE) of AAL-toxins can all induce genotype-specific necrosis characteristics in tomato leaflets in the same pattern as that of the TA pair, but they differ as much as 1000-fold in their relative toxicity [42].
2.4. Chemical and Structural Properties of Analogs of SAMs
In addition to fumonisins and AAL-toxins, several fungal secondary metabolites have also been identified as structural analogs of sphinganine and CerS inhibitors (summarized in Figure 3 and Table 1). These metabolites include myriocins, sphingofungins, viridiofungins, 2-amino-14,16-dimethyl-octadecan-3-ol (2-AOD-3-ol), and a new C17-SAM identified from mussels contaminated by marine fungi including Aspergillus, Fusarium, and Trichoderma. Australifungin, a structurally unrelated mycotoxin produced by Sporormiella australis, was also shown to inhibit sphingolipid synthesis in plants, similar to those of SAMs.
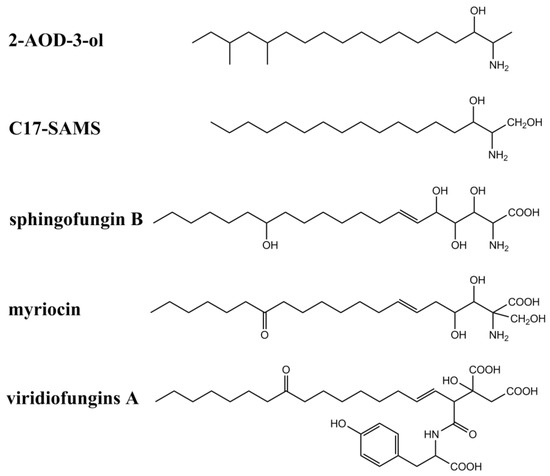
Figure 3. Chemical structures of other sphinganine-analog metabolites.
Table 1. Analogs of sphinganine-analog mycotoxins (SAMs), their fungal producer(s), and their activities.
| Analog of SAMs | Fungi/Origin | Activities | Scopus Citation (Review) | Reference | |
|---|---|---|---|---|---|
| Myriocins (thermozymocidin, ISP-I) |
Myriococcum albomyces Melanconis flavovirens Isaria sinclairii |
Antifungal activity Inhibitor of serine palmitoyltransferase (SPT) Immunosuppressive activity Protective effect on hepatotoxicity Relieve fumonisin B1 (FB1)-induced toxicity and cell death Multi-pharmacological function on human |
421(34) | [46][47][48][49][50][51][52][53] | |
| Sphingofungins | E/F | Paecilomyces variotii | Inhibitor of SPT Antifungal activity |
65(15) | [54][55][56][57][58] |
| A/B/C/D/I | Asp. fumigatus | ||||
| G/H | Asp. penicilliodes | ||||
| Viridiofungins | A/B/C | Trichoderma viride Pers | Inhibitors of SPT and squalene synthase Antifungal but lack antibacterial activity |
21(5) | [59][60][61] |
| Tri. harzianum | |||||
| Australifungin | Sporormiella australis | Inhibitors of sphinganine-N acyl transferase Antifungal activity, phytotoxicity |
26(7) | [62][63] | |
| 2-AOD-3-ol | F. avenaceum | Animal cell toxicity as fumonisin B | 5 | [64] | |
| C17-sphinganine analog mycotoxin | Contaminated mussels | Blocking skeletal muscle contraction | 1 | [65] | |
Myriocins, sphingofungins, and viridiofungins inhibit serine palmitoyltransferase (SPT), while fumonisins, AAL-toxin, and australifungin inhibit sphinganine-N acyltransferase. Serine palmitoyl transferase, one of the key enzymes in the synthesis of sphingolipids, was also reported to play a positive role in PCD regulation. The increase of SPT activity promoted PCD in plants. In contrast, by inhibiting SPT activity, the excessive accumulation of sphingosine can be alleviated, leading to reduced PCD [43]. Therefore, myriocin are usually used as a SPT inhibitor to pretreat Arabidopsis thaliana and tomato plants to induce their resistance to FB1 and AAL-toxin, respectively [44][45].
3. Relationships between SAMs’ Structure and Biological Activities
The biological effects of SAMs, such as their toxicity, are similar among different SMAs. Many SAMs have a similar spectrum of susceptible plant species [34]. Tomato tissues and cells are similarly sensitive to AAL-toxins and to FB1 and FB2 toxins. In some other plants, AAL-toxins can cause necrotic cell death, similar to that of fumonisins [66]. For animal tissue cultures, the TA toxins can induce cytotoxicity in both rat liver and dog kidney cells as FB1 toxin [67][68]. Besides, AAL-toxin and F. verticillioides could also inhibit larval growth and reduced pupal weights of tobacco budworrn Heliothis virescens [69]. Such similarities have been attributed to the structural similarities between the SAMs and sphinganine. However, there are differences among SAMs in their biological effects and those differences are related to their structural differences. Below, we summarize the main findings in this area.
The amino functional group of SAMs is essential for their toxic activity. The peracetylated derivatives of AAL-toxins and FB1 are biologically inactive or have significantly reduced toxicity in both the plant bioassay and the animal tissue culture systems [66][70][71]. These results were consistent with initial reports on these toxins showing that blocking the free primary amines of AAL-toxins by specific reagents could abolish the biological activities of these toxins in plants [72]. In an in vitro test of rat primary hepatocytes, it was noted that the N-acetyl analogue of FB1, FA1, also showed CerS inhibition [68]. Later, FA was found to spontaneously undergo isomerization, rearranging its O-acetylation group to form different analogs. The impact of these rearrangement products on inhibition of CerS in rat liver slices also supported the important role of a primary amino for both CerS inhibition and toxicity [73]. Derivatization of the amino group with fluorogenic reagents also makes the FBs’ detection possible by the high-performance liquid chromatography (HPLC) assay [74]. FBs can bind covalently to proteins by reacting with amino groups in abiogenic conversions, which may increase the toxicity of those conversion products [75]. Similarly, the terminal amino group of FB1 can conjugate to bovine serum albumin (BSA) and work as an immunogen to produce monoclonal antibodies for enzyme-linked immunosorbent assay (ELISA) detection [76]. Amino group of fumonisins can also work as an electron donor and react with the electrophilic carbon within the isothiocyanate (ITC) group. Consequently, FBs can be degraded by fumigation treatment with ITC-containing compounds [77].
The hydrolysis product of FB1 (HFB1) was shown as less toxic than both FB1 and TA to plants [78]. Neither HFB1 nor the yeast sphingolipids (completely acetylated) contain PTCA. While both had adverse effects on duckweed growth, they showed lower phytotoxicity than TA and FB1 that contained one and two PTCA, respectively [79]. In contrast, the hydrolysis products of AAL-toxins largely maintain the toxicities of their parental compounds to the susceptible tomato lines [66]. These results indicate that PTCA is important to phytotoxicity of FBs and there is specificity of interaction between AAL-toxins and tomatoes.
Different from those in plants, an in vitro test using primary hepatocytes of rat showed that the HFBs had greater cytotoxicity than FBs. However, the HFBs could not initiate cancer development due to the lack of PTCA moiety, which was proposed to play an active role in the fumonisins absorption from the gut [70]. In the pregnant LM/Bc mouse model, HFB1 did not cause neural tube defects. In contrast, 10 mg of FB1/kg body weight of mice disrupted maternal sphingolipid metabolism, caused hepatic apoptosis in the female mice, increased fetus mortality, and reduced fetus weight [80]. In the SAMs-sensitive pig model, HFB1 was shown to have limited intestinal or hepatic toxicity but only slightly disrupted sphingolipids metabolism [81]. The toxic effects of FB1 and HFB1 exposure on intestinal barrier function and immunity in a pig intestinal porcine epithelial cells and porcine peripheral blood mononuclear cells co-culture model was also investigated. FB1 aggravated lipopolysaccharide (LPS)/deoxynivalenol (DON)-induced intestinal inflammation, while HFB1 showed less toxicity to the immune system [82]. In addition, when HFB1 and HFB2 were acylated by CerS, the N-acyl-metabolites were toxic in vitro to the human colonic cell line and in vivo to the intraperitoneal rat tissues [83].
Fumonisins are capable of binding to polysaccharides and proteins via their two PTCA side chains in thermal-treated food and form fumonisin artifacts [84]. The activities of SAMs vary depending on where hydroxylation occurs along the carbon backbone. For example, FB2 had a greater cytotoxic effect than FB3 and FB1 in primary rat hepatocytes [70]. However, different from most other side groups, the C-1 terminal methyl group, which differed between FC and AAL-toxin from other fumonisins, seemed not required for the biological activity in SAMs.
Similar symptoms but less phytotoxicities of SAMs were observed when long-chain sphingoid bases or simple sphingolipids were applied to duckweed, which indicated that the phytotoxicity of SAMs might be resulted from the accumulation of phytotoxic sphingolipid intermediates [71][85]. This result was consistent with the induction of PCD through ceramide-based signaling pathways (described below).
Although AAL-toxins and fumonisins are structurally related chemicals with similar phytotoxicity, the latter are 10 times less efficient. AAL-toxins have been considered to serve as a herbicide at a very low dosage against a wide variety of broadleaf weeds (e.g., jimsonweed, prickly sida, and black nightshade). However, monocotyledonous crops (e.g., maize and wheat) are tolerant to AAL-toxins [24][86][87]. Until 2013, the mode of action through CerS inhibition was not among the 21 molecular target sites of the commonly used herbicides. Using AAL-toxin as a lead compound has the potential to develop novel and safe bio-herbicide, which has phytotoxicity but reduced or no mammalian toxicity [88][89].
References
- Gilchrist, D.G. Programmed cell death in plant disease: The purpose and promise of cellular suicide. Annu. Rev. Phytopathol. 1998, 36, 393–414. [Google Scholar] [CrossRef]
- Riley, R.T.; Wang, E.; Schroeder, J.J.; Smith, E.R.; Plattner, R.D.; Abbas, N.; Yoo, H.S.; Merrill, A.H., Jr. Evidence for disruption of sphingolipid metabolism as a contributing factor in the toxicity and carcinogenicity of fumonisins. Nat. Toxins 1996, 4, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Merrill, A.H., Jr.; Sullards, M.C.; Wang, E.; Voss, K.A.; Riley, R.T. Sphingolipid metabolism: Roles in signal transduction and disruption by fumonisins. Environ. Health Persp. 2001, 109, 283–289. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Jaskiewicz, K.; Marasas, W.F.O.; Thiel, P.G.; Horak, R.M.; Vleggaar, R.; Kriek, N.P.J. Fumonisins-novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microb. 1988, 54, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microb. 2002, 68, 2101–2105. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Smedsgaard, J.; Samson, R.A.; Larsen, T.O.; Thrane, U. Fumonisin B2 production by Aspergillus niger. J. Agric. Food Chem. 2007, 55, 9727–9732. [Google Scholar] [CrossRef]
- Susca, A.; Proctor, R.H.; Butchko, R.A.E.; Haidukowski, M.; Stea, G.; Logrieco, A.; Moretti, A. Variation in the fumonisin biosynthetic gene cluster in fumonisin-producing and nonproducing black aspergilli. Fungal Genet. Biol. 2014, 73, 39–52. [Google Scholar] [CrossRef]
- Chen, J.; Mirocha, C.J.; Xie, W.; Hogge, L.; Olson, D. Production of the mycotoxin fumonisin B1 by Alternaria alternata f. sp. lycopersici. Appl. Environ. Microb. 1992, 58, 3928–3931. [Google Scholar] [CrossRef]
- Proctor, R.H.; Van Hove, F.; Susca, A.; Stea, G.; Busman, M.; van der Lee, T.; Waalwijk, C.; Moretti, A.; Ward, T.J. Birth, death and horizontal transfer of the fumonisin biosynthetic gene cluster during the evolutionary diversification of Fusarium. Mol. Microbiol. 2013, 90, 290–306. [Google Scholar] [CrossRef]
- Mogensen, J.M.; Møller, K.A.; Von Freiesleben, P.; Labuda, R.; Varga, E.; Sulyok, M.; Kubátová, A.; Thrane, U.; Andersen, B.; Nielsen, K.F. Production of fumonisins B2 and B4 in Tolypocladium species. J. Ind. Microbiol. Biot. 2011, 38, 1329–1335. [Google Scholar] [CrossRef]
- Alabouvette, C.; Lemanceau, P.; Steinberg, C. Recent advances in the biological control of fusarium wilts. Pestic. Sci. 1993, 37, 365–373. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Plattner, R.D.; Nelsen, T.C.; Leslie, J.F. Genetic analysis of fumonisin production and virulence of Gibberella fujikuroi mating population A (Fusarium moniliforme) on maize (Zea mays) seedlings. Appl. Environ. Microb. 1995, 61, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. A 2010, 27, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; McGee, D.C.; Carlton, W.M. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology 1997, 87, 209–217. [Google Scholar] [CrossRef]
- Williams, L.D.; Glenn, A.E.; Bacon, C.W.; Smith, M.A.; Riley, R.T. Fumonisin production and bioavailability to maize seedlings grown from seeds inoculated with Fusarium verticillioides and grown in natural soils. J. Agric. Food Chem. 2006, 54, 5694–5700. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Chu, F.S.; Li, G.Y. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Appl. Environ. Microb. 1994, 60, 847–852. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-Van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.A.; et al. Fumonisins Disrupt Sphingolipid Metabolism, Folate Transport, and Neural Tube Development in Embryo Culture and In Vivo: A Potential Risk Factor for Human Neural Tube Defects among Populations Consuming Fumonisin-Contaminated Maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef]
- Ross, P.F.; Rice, L.G.; Osweiler, G.D.; Nelson, P.E.; Richard, J.L.; Wilson, T.M. A review and update of animal toxicoses associated with fumonisin-contaminated feeds and production of fumonisins by Fusarium isolates. Mycopathologia 1992, 117, 109–114. [Google Scholar] [CrossRef]
- Harrison, L.R.; Colvin, B.M.; Greene, J.T.; Newman, L.E.; Cole, J.R., Jr. Pulmonary Edema and Hydrothorax in Swine Produced by Fumonisin B1, a Toxic Metabolite of Fusarium Moniliforme. J. Vet. Diagn. Investig. 1990, 2, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, D.G.; Grogan, R.G. Production and nature of a host-specific toxin from Alternaria alternata f. sp. lycopersici. Phytopathology 1976, 66, 165–171. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Duke, S.O.; Paul, R.N.; Riley, R.T.; Tanaka, T. AAL-toxin, a potent natural herbicide which disrupts sphingolipid metabolism of plants. Pestic. Sci. 1995, 43, 181–187. [Google Scholar] [CrossRef]
- Mesbah, L.A.; Van Der Weerden, G.M.; Nijkamp, H.J.J.; Hille, J. Sensitivity among species of Solanaceae to AAL toxins produced by Alternaria alternata f.sp. lycopersici. Plant Pathol. 2000, 49, 734–741. [Google Scholar] [CrossRef]
- Renaud, J.B.; Kelman, M.J.; Qi, T.F.; Seifert, K.A.; Sumarah, M.W. Product ion filtering with rapid polarity switching for the detection of all fumonisins and AAL-toxins. Rapid Commun. Mass Sp. 2015, 29, 2131–2139. [Google Scholar] [CrossRef]
- Abbas, H.K.; Riley, R.T. The presence and phytotoxicity of fumonisins and AAL-toxin in Alternaria alternata. Toxicon 1996, 34, 133–136. [Google Scholar] [CrossRef]
- Yamagishi, D.; Akamatsu, H.; Otani, H.; Kodama, M. Pathological evaluation of host-specific AAL-toxins and fumonisin mycotoxins produced by Alternaria and Fusarium species. J. Gen. Plant Pathol. 2006, 72, 323–327. [Google Scholar] [CrossRef]
- Merrill, A.H. Sphingolipid Biosynthesis. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 281–286. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Huby, E.; Napier, J.A.; Baillieul, F.; Michaelson, L.V.; Dhondt-Cordelier, S. Sphingolipids: Towards an integrated view of metabolism during the plant stress response. New Phytol. 2020, 225, 659–670. [Google Scholar] [CrossRef]
- Li, Q.; Fang, H.; Dang, E.; Wang, G. The role of ceramides in skin homeostasis and inflammatory skin diseases. J. Dermatol. Sci. 2020, 97, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Bartók, T.; Szécsi, Á.; Szekeres, A.; Mesterházy, Á.; Bartók, M. Detection of new fumonisin mycotoxins and fumonisin-like compounds by reversed-phase high-performance liquid chromatography/electrospray ionization ion trap mass spectrometry. Rapid Commun. Mass Sp. 2006, 20, 2447–2462. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Duke, S.O.; Tanaka, T. Phytotoxicity of fumonisins and related compounds. Toxin Rev. 1993, 12, 225–251. [Google Scholar] [CrossRef]
- Branham, B.E.; Plattner, R.D. Isolation and characterization of a new fumonisin from liquid cultures of Fusarium moniliforme. J. Nat. Prod. 1993, 56, 1630–1633. [Google Scholar] [CrossRef]
- Seo, J.A.; Kim, J.C.; Lee, Y.W. N-acetyl derivatives of type C fumonisins produced by Fusarium oxysporum. J. Nat. Prod. 1999, 62, 355–357. [Google Scholar] [CrossRef]
- Musser, S.M.; Gay, M.L.; Mazzola, E.P.; Plattner, R.D. Identification of a new series of fumonisins containing 3-hydroxypyridine. J. Nat. Prod. 1996, 59, 970–972. [Google Scholar] [CrossRef]
- Abbas, H.K.; Shier, W.T.; Seo, J.A.; Lee, Y.W.; Musser, S.M. Phytotoxicity and cytotoxicity of the fumonisin C and P series of mycotoxins from Fusarium spp. fungi. Toxicon 1998, 36, 2033–2037. [Google Scholar] [CrossRef]
- Bartók, T.; Tölgyesi, L.; Szekeres, A.; Varga, M.; Bartha, R.; Szécsi, A.; Bartók, M.; Mesterházy, A. Detection and characterization of twenty-eight isomers of fumonisin B1 (FB1) mycotoxin in a solid rice culture infected with Fusarium verticillioides by reversed-phase high-performance liquid chromatography/electrospray ionization time-of-flight and ion trap mass spectrometry. Rapid Commun. Mass Sp. 2010, 24, 35–42. [Google Scholar] [CrossRef]
- Bottini, A.T.; Bowen, J.R.; Gilchrist, D.G. Phytotoxins. II. Characterization of a phytotoxic fraction from Alternaria alternata f. sp. lycopersici. Tetrahedron Lett. 1981, 22, 2723–2726. [Google Scholar] [CrossRef]
- Bottini, A.T.; Gilchrist, D.G. Phytotoxins. I. A 1-amino dimethyl heptadecapentol from alternaria alternata f. sp. lycopersici. Tetrahedron Lett. 1981, 22, 2719–2722. [Google Scholar] [CrossRef]
- Caldas, E.D.; Jones, A.D.; Ward, B.; Winter, C.K.; Gilchrist, D.G. Structural Characterization of Three New AAL Toxins Produced by Alternaria alternata f. sp. lycopersici. J. Agric. Food Chem. 1994, 42, 327–333. [Google Scholar] [CrossRef]
- Saucedo-García, M.; Guevara-García, A.; González-Solís, A.; Cruz-García, F.; Vázquez-Santana, S.; Markham, J.E.; Lozano-Rosas, M.G.; Dietrich, C.R.; Ramos-Vega, M.; Cahoon, E.B.; et al. MPK6, sphinganine and the LCB2a gene from serine palmitoyltransferase are required in the signaling pathway that mediates cell death induced by long chain bases in Arabidopsis. New Phytol. 2011, 191, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Bielawski, J.; Mu, J.; Dong, H.; Teng, C.; Zhang, J.; Yang, X.; Tomishige, N.; Hanada, K.; Hannun, Y.A.; et al. Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Res. 2007, 17, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Spassieva, S.D.; Markham, J.E.; Hille, J. The plant disease resistance gene Asc-1 prevents disruption of sphingolipid metabolism during AAL-toxin-induced programmed cell death. Plant J. 2002, 32, 561–572. [Google Scholar] [CrossRef]
- Kluepfel, D.; Bagli, J.; Baker, H.; Charest, M.P.; Kudelski, A.; Sehgal, S.N.; Vézina, C. Myriocin, a new antifungal antibiotic from Myriococcum albomyces. J. Antibiot. 1972, 25, 109–115. [Google Scholar] [CrossRef]
- Šašek, V.; Sailer, M.; Vokoun, J.; Musílek, V. Production of thermozymocidin (myriocin) by the pyrenomycete Melanconis flavovirens. J. Basic Microb. 1989, 29, 383–390. [Google Scholar] [CrossRef]
- Fujita, T.; Inoue, K.; Yamamoto, S.; Ikumoto, T.; Sasaki, S.; Toyama, R.; Chiba, K.; Hoshino, Y.; Okumoto, T. Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J. Antibiot. 1994, 47, 208–215. [Google Scholar] [CrossRef]
- Miyake, Y.; Kozutsumi, Y.; Nakamura, S.; Fujita, T.; Kawasaki, T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Bioph. Res. Co. 1995, 211, 396–403. [Google Scholar] [CrossRef]
- Tatematsu, K.; Tanaka, Y.; Sugiyama, M.; Sudoh, M.; Mizokami, M. Host sphingolipid biosynthesis is a promising therapeutic target for the inhibition of hepatitis B virus replication. J. Med. Virol. 2011, 83, 587–593. [Google Scholar] [CrossRef]
- Yu, S.; Jia, B.; Yang, Y.; Liu, N.; Wu, A. Involvement of PERK-CHOP pathway in fumonisin B1- induced cytotoxicity in human gastric epithelial cells. Food Chem. Toxicol. 2020, 136. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Withers, B.R.; Blalock, E.; Liu, K.; Dickson, R.C. Reducing sphingolipid synthesis orchestrates global changes to extend yeast lifespan. Aging Cell 2013, 12, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Reforgiato, M.R.; Milano, G.; Fabriàs, G.; Casas, J.; Gasco, P.; Paroni, R.; Samaja, M.; Ghidoni, R.; Caretti, A.; Signorelli, P. Inhibition of ceramide de novo synthesis as a postischemic strategy to reduce myocardial reperfusion injury. Basic Res. Cardiol. 2016, 111. [Google Scholar] [CrossRef] [PubMed]
- Horn, W.S.; Smith, J.L.; Bills, G.F.; Raghoobar, S.L.; Helms, G.L.; Kurtz, M.B.; Marrinan, J.A.; Frommer, B.R.; Thornton, R.A.; Mandala, S.M. Sphingofungins E and F: Novel serinepalmitoyl trans-ferase inhibitors from Paecilomyces variotii. J. Antibiot. 1992, 45, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- VanMiddlesworth, F.; Giacobbe, R.A.; Lopez, M.; Garrity, G.; Bland, J.A.; Bartizal, K.; Fromtling, R.A.; Polishook, J.; Zweerink, M.; Edison, A.M.; et al. Sphingofungins a, b, c, and d; a new family of antifungal agents: I. Fermentation, isolation, and biological activity. J. Antibiot. 1992, 45, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, Y.; Gao, J.; Hu, J.; He, H.; Dai, S.; Wang, L.; Dai, H.; Zhang, L.; Song, F. Antitubercular metabolites from the marine-derived fungus strain Aspergillus fumigatus MF029. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Sphingofungins G and H: New five-membered lactones from Aspergillus penicilliodes Speg. Nat. Prod. Res. 2019, 33, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Nishijima, M.; Fujita, T.; Kobayashi, S. Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells. A novel evaluation system using an SPT-defective mammalian cell mutant. Biochem. Pharmacol. 2000, 59, 1211–1216. [Google Scholar] [CrossRef]
- Harris, G.H.; Turner Jones, E.T.; Meinz, M.S.; Nallin-Omstead, M.; Helms, G.L.; Bills, G.F.; Zink, D.; Wilson, K.E. Isolation and structure elucidation of viridiofungins A, B and C. Tetrahedron Lett. 1993, 34, 5235–5238. [Google Scholar] [CrossRef]
- Mandala, S.M.; Thornton, R.A.; Frommer, B.R.; Dreikorn, S.; Kurtz, M.B. Viridiofungins, novel inhibitors of sphingolipid synthesis. J. Antibiot 1997, 50, 339–343. [Google Scholar] [CrossRef]
- El-Hasan, A.; Walker, F.; Schöne, J.; Buchenauer, H. Detection of viridiofungin A and other antifungal metabolites excreted by Trichoderma harzianum active against different plant pathogens. Eur. J. Plant Pathol. 2009, 124, 457–470. [Google Scholar] [CrossRef]
- Abbas, H.K.; Duke, S.O.; Merrill, A.H., Jr.; Wang, E.; Shier, W.T. Phytotoxicity of Australifungin, AAL-toxins and fumonisin B1 to Lemna pausicostata. Phytochemistry 1998, 47, 1509–1514. [Google Scholar] [CrossRef]
- Mandala, S.M.; Thornton, R.A.; Frommer, B.R.; Curotto, J.E.; Rozdilsky, W.; Kurtz, M.B.; Giacobbe, R.A.; Bills, G.F.; Cabello, M.A. The Discovery of Australifungin, a Novel Inhibitor of Sphinganine N-Acyltransferase from Sporormiella australis Producing Organism, Fermentation, Isolation, and Biological Activity. J. Antibiot. 1995, 48, 349–356. [Google Scholar] [CrossRef]
- Uhlig, S.; Petersen, D.; Flåøyen, A.; Wilkins, A. 2-Amino-14,16-dimethyloctadecan-3-ol, a new sphingosine analogue toxin in the fungal genus Fusarium. Toxicon 2005, 46, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Marrouchi, R.; Benoit, E.; Le Caer, J.P.; Belayouni, N.; Belghith, H.; Molgó, J.; Kharrat, R. Toxic C17-sphinganine analogue mycotoxin, contaminating Tunisian mussels, causes flaccid paralysis in rodents. Mar. Drugs 2013, 11, 4724–4740. [Google Scholar] [CrossRef] [PubMed]
- Mirocha, C.J.; Gilchrist, D.G.; Shier, W.T.; Abbas, H.K.; Wen, Y.; Vesonder, R.F. AAL Toxins, funionisms (biology and chemistry) and host-specificity concepts. Mycopathologia 1992, 117, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Shier, W.T.; Abbas, H.K.; Mirocha, C.J. Toxicity of the mycotoxins fumonisins B1 and B2 and Alternaria alternata f. sp. lycopersici toxin (AAL) in cultured mammalian cells. Mycopathologia 1991, 116, 97–104. [Google Scholar] [CrossRef]
- Van Der Westhuizen, L.; Shephard, G.S.; Snyman, S.D.; Abel, S.; Swanevelder, S.; Gelderblom, W.C.A. Inhibition of sphingolipid biosynthesis in rat primary hepatocyte cultures by fumonisin B1 and other structurally related compounds. Food Chem. Toxicol. 1998, 36, 497–503. [Google Scholar] [CrossRef]
- Abbas, H.K.; Mulrooney, J.E. Effect of Some Phytopathogenic Fungi and Their Metabolites on Growth of Heliothis virescens (F.) and Its Host Plants. Biocontrol Sci. Techn. 1994, 4, 77–87. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Cawood, M.E.; Snyman, S.D.; Vleggaar, R.; Marasas, W.F.O. Structure-activity relationships of fumonisins in short-term carcinogenesis and cytotoxicity assays. Food Chem. Toxicol. 1993, 31, 407–414. [Google Scholar] [CrossRef]
- Tanaka, T.; Abbas, H.K.; Duke, S.O. Structure-dependent phytotoxicity of fumonisins and related compounds in a duckweed bioassay. Phytochemistry 1993, 33, 779–785. [Google Scholar] [CrossRef]
- Siler, D.J.; Gilchrist, D.G. Properties of host specific toxins produced by Alternaria alternata f. sp. lycopersici in culture and in tomato plants. Physiol. Plant Pathol. 1983, 23, 265–274. [Google Scholar] [CrossRef]
- Norred, W.P.; Riley, R.T.; Meredith, F.I.; Poling, S.M.; Plattner, R.D. Instability of N-acetylated fumonisin B1 (FA1) and the impact on inhibition of ceramide synthase in rat liver slices. Food Chem. Toxicol. 2001, 39, 1071–1078. [Google Scholar] [CrossRef]
- Shier, W.T.; Abbas, H.K. Current issues in research on fumonisins, mycotoxins which may cause nephropathy. J. Toxicol. Toxin Rev. 1999, 18, 323–335. [Google Scholar] [CrossRef]
- Shier, W.T.; Abbas, H.K.; Abou-Karam, M.; Badria, F.A.; Resch, P.A. Fumonisins: Abiogenic Conversions of an Environmental Tumor Promoter and Common Food Contaminant. J. Toxicol. Toxin Rev. 2003, 22, 591–616. [Google Scholar] [CrossRef]
- Savard, M.E.; Sinha, R.C.; Lau, R.; Séguin, C.; Buffam, S. Monoclonal antibodies for fumonisins B1, B2 and B3. Food Agric. Immunol. 2003, 15, 127–134. [Google Scholar] [CrossRef]
- Azaiez, I.; Meca, G.; Manyes, L.; Luciano, F.B.; Fernández-Franzón, M. Study of the chemical reduction of the fumonisins toxicity using allyl, benzyl and phenyl isothiocyanate in model solution and in food products. Toxicon 2013, 63, 137–146. [Google Scholar] [CrossRef]
- Lamprecht, S.C.; Marasas, W.F.O.; Alberts, J.F.; Cawood, M.E.; Gelderblom, W.C.A.; Shephard, G.S.; Thiel, P.G.; Calitz, F.J. Phytotoxicity of fumonisins and TA-toxin to corn and tomato. Phytopathology 1994, 84, 383–391. [Google Scholar] [CrossRef]
- Vesonder, R.F.; Peterson, R.E.; Labeda, D.; Abbas, H.K. Comparative phytotoxicity of the fumonisins, AAL-toxin and yeast sphingolipids in Lemna minor L. (duckweed). Arch. Environ. Con. Tox. 1992, 23, 464–467. [Google Scholar] [CrossRef]
- Voss, K.A.; Riley, R.T.; Snook, M.E.; Gelineau-van Waes, J. Reproductive and sphingolipid metabolic effects of fumonisin B1 and its alkaline hydrolysis product in LM/Bc mice: Hydrolyzed fumonisin B1 did not cause neural tube defects. Toxicol. Sci. 2009, 112, 459–467. [Google Scholar] [CrossRef]
- Grenier, B.; Bracarense, A.P.F.L.; Schwartz, H.E.; Trumel, C.; Cossalter, A.M.; Schatzmayr, G.; Kolf-Clauw, M.; Moll, W.D.; Oswald, I.P. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochem. Pharmacol. 2012, 83, 1465–1473. [Google Scholar] [CrossRef]
- Gu, M.J.; Han, S.E.; Hwang, K.; Mayer, E.; Reisinger, N.; Schatzmayr, D.; Park, B.C.; Han, S.H.; Yun, C.H. Hydrolyzed fumonisin B1 induces less inflammatory responses than fumonisin B1 in the co-culture model of porcine intestinal epithelial and immune cells. Toxicol. Lett. 2019, 305, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Seiferlein, M.; Humpf, H.U.; Voss, K.A.; Sullards, M.C.; Allegood, J.C.; Wang, E.; Merrill, A.H., Jr. Hydrolyzed fumonisins HFB1 and HFB2 are acylated in vitro and in vivo by ceramide synthase to form cytotoxic N-acyl-metabolites. Mol. Nutr. Food Res. 2007, 51, 1120–1130. [Google Scholar] [CrossRef]
- Seefelder, W.; Knecht, A.; Humpf, H.U. Bound fumonisin B1: Analysis of fumonisin-B1 glyco and amino acid conjugates by liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Agric. Food Chem. 2003, 51, 5567–5573. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Tanaka, T.; Duke, S.O.; Porter, J.K.; Wray, E.M.; Hodges, L.; Sessions, A.E.; Wang, E.; Merrill Jnr, A.H.; Riley, R.T. Fumonisin- and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiol. 1994, 106, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.; Lima, M.; Rosa, S.; Dias, A.S.; Dias, L.S. Allelopathic plants. XVIII. Solanum nigrum L. Allelopathy J. 2006, 17, 1–15. [Google Scholar]
- Duke, S.O.; Dayan, F.E. Modes of action of microbially-produced phytotoxins. Toxins 2011, 3, 1038–1064. [Google Scholar] [CrossRef]
- Abbas, H.K.; Tanaka, T.; Shier, W.T. Biological activities of synthetic analogues of Alternaria alternata toxin (AAL-toxin) and fumonisin in plant and mammalian cell cultures. Phytochemistry 1995, 40, 1681–1689. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E. Clues to new herbicide mechanisms of action from natural sources. ACS Symposium Series 2013, 1141, 203–215.




