
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Patrick Van Dijck | + 3005 word(s) | 3005 | 2021-01-12 06:58:42 | | | |
| 2 | Camila Xu | Meta information modification | 3005 | 2021-01-12 10:48:18 | | |
Video Upload Options
Polyenes were, after griseofulvin, the first fungal-specific antibiotics on the market and ever since, more than 200 polyene antifungals have been discovered, of which amphotericin B, nystatin and natamycin are most commonly used in antifungal therapy .
1. History of Polyenes as Antifungal Drugs
In 1949, microbiologist Elizabeth L. Hazen and chemist Rachel F. Brown isolated the first antifungal polyene—fungicidin—later called nystatin after New York State, where the meeting of the National Academy of Sciences in which fungicidin was presented, took place that year [1][2][3]. Nystatin was purified as a fermentation product of Streptomyces noursei, cultured from a soil sample of the farm of W. Nourses, after which the antibiotic producing actinomycete species was named [3]. Nystatin was patented by the E.R. Squibb and Sons Institute and became one of the first antimycotic drugs on the market. Early on, it became apparent, however, that nystatin had poor gastrointestinal absorption and could thus only be used to treat topical mycoses [4]. Therefore, the same research group continued their broad screening of soil-cultivated fermentation broths and in January 1953, a fermentation broth from the Streptomyces nodosus culture M4575, cultured from a soil sample of the Orinoco basin in Venezuela, showed remarkable antifungal activity [2][4]. Two active compounds were isolated: amphotericin A and amphotericin B (AmB), named after their amphoteric properties. These polyenes were chemically similar to nystatin, but the ultraviolet absorption spectrum showed additional maxima at longer wavelengths [4]. After successful purification, the tetraene amphotericin A showed an antifungal spectrum similar to nystatin, while the heptaene AmB had a significantly greater antifungal activity compared to nystatin and amphotericin A [4]. It took over one and a half decades to completely unravel the chemical structure of AmB [5]. AmB is, like other polyenes, a complex macrolide antibiotic, characterized by an almost flat macrolactone ring (hence the name macrolide) with a series of conjugated double bonds (Figure 1) [6]. The latter discriminates polyenes from antibacterial macrolides such as erythromycin [6]. Depending on the number of conjugated double bonds, polyenes can be classified into trienes, tetraenes, pentaenes, hexaenes, heptaenes, etc. [6][7]. In general, polyenes consist of a hydrophobic polyene “tail” and a hydrophilic ”head” with a mycosamine group and a polyol chain that holds a number of hydroxyl groups [8].
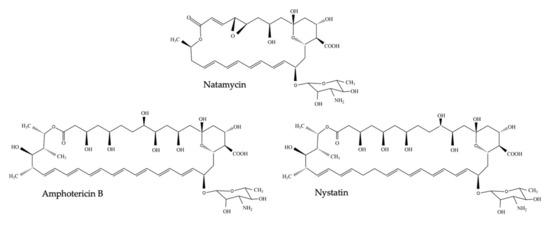
Figure 1. Chemical structure of nystatin, amphotericin B and natamycin (pimaricin).
Due to its amphipathic character, AmB is poorly water soluble and although initially oral treatment of infected mice seemed successful [4], no such effect was obtained in humans [3][4]. Eventually, researchers at the E.R. Squibb and Sons institute used a formulation in which AmB and sodium deoxycholate formed a micellar suspension when reconstituted in a glucose solution [3][4]. This preparation, named Fungizone® and commonly referred to as AmB deoxycholate, could yield high blood concentrations of AmB upon intravenous administration and was very effective against systemic cryptococcosis, histoplasmosis and other deep mucosal infections [4]. Ever since the discovery of AmB, multiple different derivates and formulations have been developed. The latter to counteract the dose-limiting toxicities of AmB, which comprise nephrotoxicity and infusion-related complications [9].
2. Strengths and Drawbacks of Polyene Use in the Clinic
Clinical use of AmB and other polyenes has extensively been reviewed elsewhere [8][9][10][11][12]; here, we only provide a short summary on the most common applications and drawbacks of the use of polyenes as antifungal drugs. About six polyene antifungals have been used for antifungal therapy: AmB, nystatin, natamycin (also called pimaricin), candicidin, trichomycin and methyl partricin [8]. However, only three polyenes remain in widespread therapeutic use today: AmB for systemic mycoses, nystatin for mucosal infections such as oral or vulvovaginal candidiasis and natamycin for ophthalmic infection [8][13][10]. Polyenes are still in use because of their broad spectrum of activity (cf. echinocandins, 1st and 2nd generation azoles) against pathogenic yeasts and molds, including Candida spp., Aspergillus spp., Cryptococcus spp., Fusarium spp., Mucorales (e.g., Rhizopus spp.) and endemic mycosis (e.g., Histoplasma spp.) [10]. Nevertheless, several studies show that AmB treatment of systemic mycoses caused by species such as Aspergillus terreus [14], Scedosporium spp. [15] and Candida auris [16] might not always be successful, often due to intrinsic or acquired resistance [11]. Still, polyene resistance is rarer [10][17][18], and the relative decrease in susceptibility is smaller, compared to resistance to other drug antifungal classes such as azoles or echinocandins [19][20]. Nevertheless, due to its toxicity and the availability of the (tri)azole and echinocandin antifungals, the use of AmB to treat the most common systemic mycoses such as candidiasis and aspergillosis has decreased [10]. According to the Infectious Diseases Society of America (IDSA) [21] and European Confederation of Medical Mycology [22] (ECMM) guidelines, AmB is still recommended as first line treatment for severe cryptococcosis (often in combination with flucytosine), disseminated histoplasmosis and mucormycosis, while it remains an alternative for other infections upon intolerance, limited availability or failure of other treatments [10][21]. Furthermore, AmB has been recommended as a prophylaxis for invasive Candida [21] and Aspergillus [23] infections in solid-organ transplant recipients and patients receiving immunosuppressive treatment, respectively.
The biggest constraint concerning the use of AmB in the clinic is its intrinsic host toxicity. This dose-related toxicity limits the maximum tolerated dose to for example 0.7–1.0 mg/kg/day for AmB deoxycholate, which may be suboptimal to acquire clinical success [9]. Although the affinity of AmB to fungal ergosterol is over ten-fold higher compared to mammalian cholesterol, non-selective disruption of mammalian cell membranes does occur [24]. Renal toxicity and acute infusion-related adverse effects such as fever and nausea are most commonly associated with intravenous AmB administrations, while liver damage occurs but is less common [10]. Acute infusion-related toxicity is due to the fact that AmB, a molecule of microbial origin, is recognized by TLR2 (Toll-like receptor 2) and CD14 on mononuclear immune cells, leading to the initiation of an inflammatory response [9]. Nephrotoxicity is thought to be caused by increased exposure of AmB to renal cells via low-density lipoprotein (LDL) receptor mediated endocytosis. Moreover, AmB causes vasoconstriction in afferent renal arterioles, which decreases renal blood flow and glomerular filtration [9]. Lipid-associated AmB formulations, such as AmB lipid complex (ABLC) and liposomal AmB (L-AmB), have been developed with the main goal of improving the therapeutic index and reducing toxic complications compared to conventional AmB deoxycholate [9]. The pharmacokinetic parameters for these formulations differ substantially. For example, ABLC is large and taken up rapidly by macrophages in tissues such as the liver, spleen and lungs, while L-AmB is small and negatively charged, resulting in higher peak plasma levels compared to other formulations [9]. The effects of these lipid formulations on the clinical success and mortality of the patient are a subject of debate, and largely depend on the study, varying for type of infection (e.g., cryptococcosis and histoplasmosis) and background of the patients (e.g., AIDS and neutropenic) [9]. Overall, lipid AmB formulations show less nephrotoxicity compared to AmB deoxycholate, while L-AmB also exhibits less infusion-related reactions [9]. Still, new structures and formulations are developed to optimize the use of polyenes in the clinic. A recent example is the discovery of amphamide, an amide of AmB and termed a “second generation polyene antifungal” [25]. Amphamide was developed to increase the water solubility of AmB and shows an over 20-fold higher therapeutic index compared to AmB. Additionally, it has a superior antifungal activity and lower acute host toxicity in vivo [25]. Nanotechnology-based formulations of polyenes have also been investigated, aimed at decreasing their toxicity and/or increase their solubility, therapeutic index and/or oral availability. Nanoformulations include nanocrystals, nanotubes, polymeric nanocarriers, cubosomal and cochleate nanoparticles [26]. Although the low solubility and gastrointestinal absorption initially redeemed polyenes as oral drugs, recent research such as nanobody delivery shows great potential for the future [26][27].
3. Mode of Action of Polyene Antifungal Drugs
3.1. Polyene—Sterol Interactions
Overall, polyenes have an unusual mode of action compared to other antifungal drug classes, as they do not target a specific enzyme but rather interact with a vital molecule—ergosterol [28]. The first indications of the mode of action of polyenes were published in 1958, when Gottlieb et al. [29] discovered that the addition of sterols such as cholesterol, lanosterol and ergosterol could inhibit the fungicidal effect of nine polyenes including filipin, AmB and nystatin on three fungal species. They suggested that polyenes could prevent the synthesis of sterols (as the, then not yet discovered, azole, allylamine and phenylmorpholine antifungals do [28]) or competitively replace the sterols as a cofactor of an essential metabolic reaction [29]. Later, however, it became apparent that polyenes can alter the permeability of the membrane by reacting with sterols [7]. How this process of sterol sequestration works, remains subject to debate. The most studied mechanism of action is the pore forming model in which polyenes interact with ergosterol to form ion-leaking pores in the membrane [17]. Nevertheless, it has been shown that pore formation can also be established in the absence of sterols [30]. Early on, Cotero et al. [30] proposed that sterols have an essential role in the structure of the membrane itself during amphotericin activity, but might not be directly involved in the pore formation [30]. Later, other studies supported this idea [31][32][33][34][35][36]. For example, the polyene natamycin was shown to bind ergosterol without altering the cell membrane permeability [36]. Further research pointed out that natamycin inhibits various ergosterol-dependent membrane proteins and so disturbs essential cellular processes such as glucose transport, amino acid transport [37] and vacuolar fusion [35]. Currently, four models of the polyene mode of antifungal action have been proposed: the pore forming model, the surface adsorption model, the sterol sponge model and the oxidative damage model [38].
In every proposed model, the binding of the polyene with ergosterol is key to its antifungal effect [38]. Ergosterol plays an essential role in many cellular processes of fungi, including regulation of membrane proteins, endocytosis, cell division, membrane fluidity and cell signaling [39][40][41]. The specificity of therapeutic polyenes to ergosterol comes from the fact that ergosterol has a significantly different three-dimensional structure compared to mammalian cholesterol, enabling better binding into the hydrophobic ”pocket” of polyenes such as AmB, as depicted in Figure 2 [28]. Three interactive forces play a role in the binding of AmB and ergosterol: Van der Waals powers which are highest when both molecules are orientated co-planar and parallel, a hydrogen bond network between the 3β-OH group of the sterol and the polar mycosamine group of AmB and π–π electronic interactions between the ergosterol side chain and the polyene “tail” of AmB [42]. The latter essential “attach point” does not occur when AmB binds to cholesterol [38][42]. Moreover, Van Der Waals interactions are weaker between cholesterol and AmB due to the sigmoidal conformation of the sterol side-chain [38]. The specific binding, along with the higher ergosterol:phospholipid ratio in fungal cell membranes, compared to the cholesterol:phospholipid ratio in mammalian cells, explains the selectivity of most polyenes to fungal cells [28].
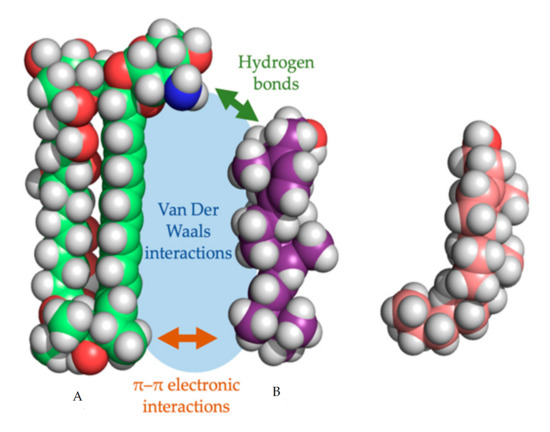
Figure 2. Three-dimensional model of amphotericin B (a) with the cylindrical ergosterol (b) and sigmoidal cholesterol (c). The three types of non-covalent interactions between amphotericin B and ergosterol are shown.
3.2. Pore Forming Models
The most studied model for polyene action is the pore formation model, in which polyenes and ergosterol interact to form an ion channel-like complex that leaks ions and small organic molecules from the cell, eventually leading to cell death [17]. Based on the amphipathic properties of polyenes, they would orientate in the plasma membrane with their hydrophobic polyene “tail” interacting with ergosterol, directed to the inner lipid environment of the membrane, while the hydrophilic polyol portion would form an aqueous channel, as illustrated in Figure 3A,B. Intermolecular hydrogen bonds between amino and carboxyl groups of the hydrophilic “heads” of neighboring polyene molecules further stabilize this channel [17]. Neutron diffraction studies have confirmed that such an architecture can exist when AmB interacts with ergosterol [43]. Typically, 4 to 12 polyene monomers would form a pore [38]. As the length of AmB is almost equal to the length of a membrane phospholipid on average, two types of channels can be made: a full pore consisting of two ring complexes of polyenes (see Figure 3A) and a ”half-pore”, containing only one polyene ring (see Figure 3B). Both types essentially have the same structure but the latter would induce a conformational thinning of the lipid bilayer [17].
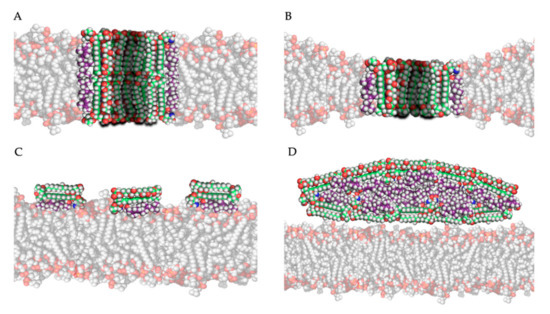
Figure 3. Four mechanistic models of the interaction of amphotericin B with ergosterol in/near the plasma membrane: (A) the pore forming model, (B) the half-pore forming model, (C) the surface adsorption model and (D) the sterol sponge model (Legend: see Figure 2).
Which pore is formed would primarily depend on the polyene, and the composition and thus thickness of the membrane [19]. For example, AmB would primarily form half-pores in a membrane mainly composed of dimyristoylphosphatidylcholine (DMPC) [44]. Pores are only formed after a certain threshold of polyene molecules in the membrane is reached. Below this threshold, aggregate complexes termed “non-aqueous pores” or “cation-selective pores” can increase the membrane permeability to monovalent cations, while true pores and half-pores can also transport larger nonelectrolyte molecules [45]. This threshold is significantly lower (by factor 5–10) in ergosterol containing membranes, compared to cholesterol containing membranes [45]. Moreover, patch clamp experiments with artificial AmB channels have shown that the ion transport occurs faster (a shorter channel ”dwell time”) in ergosterol containing membranes compared to cholesterol containing membranes [46], showcasing their antifungal specificity. The diameter of the pore determines the selectivity of transport or “leakage” out of the cell and depends on the type and concentration of polyene, while sterol type and sterol concentration have minor influences [19][39][47]. This is shown in the study by Yang et al., in which the channel diameter increases 100-fold when the concentration of AmB multiplies with factor 40 in an ergosterol-rich membrane [47]. In general, AmB forms relatively wide pores (approximately 0.46 nm) and can transport molecules as big as sucrose, while nystatin forms smaller pores (approximately 0.36 nm) [19].
3.3. Surface Adsorption and Sterol Sponge Models
The second and third models for polyene mode of action both hypothesize that, by adsorption or extraction of ergosterol from the membrane, the phospholipid membrane is destabilized, and essential cellular processes such as endocytosis and regulation of membrane protein function are disturbed [19]. Polyenes could adsorb ergosterol molecules to the “surface” of the phospholipid bilayer as illustrated in Figure 3C, termed the ”surface adsorption model” [33][48]. In light of this theory, Anderson and colleagues [32] conducted a series of NMR studies to determine the localization and structure of AmB interacting with ergosterol, and they observed that these complexes are not (always) inserted in the membrane and can form extra-membranous aggregates. They suggested that in such a mechanism, large aggregates of parallelly positioned AmB molecules can form at the membrane, functioning as a “sterol sponge” [32] as illustrated in Figure 3D. Extracting ergosterol from the membrane in such a “sponge” would perturbate a vast array of ergosterol-dependent cellular processes, many of which governed by membrane proteins that directly bind to ergosterol. This might also explain why resistance to polyenes is rarely observed, as in resistant cells an alternative membrane sterol such as lanosterol (a precursor of ergosterol) will probably malfunction in these processes and reduce the fitness and pathogenicity of the cell [32]. Anderson et al. [32] also suggest that the extraction of cholesterol by large extra membranous aggregates of AmB is the primary cause of toxicity of this drug towards mammalian cells and thus, optimizing the binding affinity of AmB derivatives to ergosterol could significantly improve its therapeutic efficacy. Some remarks have to be made regarding the sterol sponge model proposed by Anderson et al. [32]. First, the ergosterol-to-lipid ratio used in their experimental set-up is different from the one observed in natural systems [39]. As the ergosterol-to-lipid ratio is essential regarding the polyene susceptibility of fungi, this might play a vital role. Secondly, it has been proven that, in a cholesterol-saturated environment such as the mammalian cell membrane, the thermodynamical balance between ergosterol-AmB vs. cholesterol-AmB would shift towards the latter, meaning that the “sterol sponge” would be saturated with cholesterol rather than ergosterol. A third argument against this model is that fungi have a rigid hydrophilic cell wall composed of polymers of chitin that might prevent the passage of hydrophobic ergosterol and so the formation of super-aggregates or “sponges” outside the cell wall [39].
Ion channel formation, small membrane-spanning aggregates and large extra-membranous aggregates may exist at the same time [32], although the chemical structure of the polyene probably influences the primary mode of action. Several studies have shown that the elimination of the C35 hydroxyl group of AmB does not alter the sterol-adsorption capacity and cytotoxic effect of the molecule but eliminates its pore-forming capacity [33][49]. It was suggested that the ability to form pores depends on the dimensions of the polyene macrolactone ring and, therefore, polyenes can be divided into non-pore forming polyenes (e.g., natamycin) and the pore-forming polyenes (e.g., AmB and nystatin) [19].
3.4. Other Proposed Modes of Action
Several observations point towards oxidative damage as an additional mode of action of AmB [51][52]. One example is the rescue effect of hypoxia, exogenous catalase and super oxide dismutase (SOD) during AmB treatment of C. albicans without hindering AmB induced K+-leakage [52]. Another example is the enhanced resistance to oxidative damage by H2O2 of AmB resistant C. albicans strains [51]. Currently, several studies have provided evidence that polyenes can induce oxidative stress and cause DNA damage, protein carbonylation and lipid peroxidation, eventually leading to or contributing to cell death in fungi [53]. Moreover, metabolomic analysis of C. albicans exposed to AmB pointed out that AmB induced cell death was attenuated through increased production of polyamines such as putrescine, spermidine and spermine which have a role in scavenging reactive oxygen species (ROS) [54]. This is supported by gene expression analysis of C. albicans exposed to AmB, showing an increased expression of stress-related genes besides genes involved in membrane sterol homeostasis [55]. In Cryptococcus neoformans, it was shown that, after addition of AmB, cells become metabolically inactive and encounter a strong oxidative burst suggested to contribute to AmB induced cell death apart from membrane interactions and pore formation [35]. How this oxidative stress is exactly caused is still not clear, although it was suggested that polyene binding to the membrane triggers this response that leads to an apoptotic such as phenotype that includes ROS production or that, since AmB auto-oxidizes and forms free radicals [55], the antifungal itself causes oxidative stress [35]. In the latter model, the oxidative stress effect of polyenes would be distinct from its membrane permeabilization properties, although the free radicals produced would affect the membrane itself through lipid peroxidation [35].
References
- Hazen, E.L.; Brown, R. Fungicidin, an antibiotic produced by a soil actinomycete. Proc. Soc. Exp. Biol. Med. 1951, 76, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Lattif, A.A.; Swindell, K. History of Antifungals. In Antifungal Therapy; Ghannoum, A.M., Perfect, R.J., Eds.; CRC Press: Boca Raton, FL, USA, 2009; Volume 1. [Google Scholar]
- Espinell-Ingroff, A. Medical Mycology and Training in the United States: A Historical Analysis (1894–1996); Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Dutcher, J.D. The discovery and development of amphotericin B. Dis. Chest 1968, 54, 296–298. [Google Scholar]
- Borowski, E.; Zieliński, J.; Ziminski, T.; Falkowski, L.; Kołodziejczyk, P.; Golik, J.; Jereczek, E.; Adlercreutz, H. Chemical studies with amphotericin B III. The complete structure of the antibiotic. Tetrahedron Lett. 1970, 11, 3909–3914. [Google Scholar]
- Hamilton-Miller, J. Chemistry and biology of the polyene macrolide antibiotics. Bacteriol. Rev. 1973, 37, 166. [Google Scholar]
- Kinsky, S.C. Polyene Antibiotics. In Antibiotics; Springer: Berlin/Heidelberg, Germany, 1967; pp. 122–141. [Google Scholar]
- Zotchev, S.B. Polyene macrolide antibiotics and their applications in human therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [PubMed]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar]
- Chandrasekar, P. Management of invasive fungal infections: A role for polyenes. J. Antimicrob. Chemother. 2011, 66, 457–465. [Google Scholar]
- Cornely, O.A.; Vehreschild, J.J.; Ullmann, A.J. Is there a role for polyenes in treating invasive mycoses? Curr. Opin. Infect. Dis. 2006, 19, 565–570. [Google Scholar]
- Stone, N.R.; Bicanic, T.; Salim, R.; Hope, W. Liposomal amphotericin B (AmBisome®): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016, 76, 485–500. [Google Scholar] [PubMed]
- Dixon, D.M.; Walsh, T.J. Antifungal Agents. In Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Steinbach, W.J.; Benjamin, D.K., Jr.; Kontoyiannis, D.P.; Perfect, J.R.; Lutsar, I.; Marr, K.A.; Lionakis, M.S.; Torres, H.A.; Jafri, H.; Walsh, T.J. Infections due to Aspergillus terreus: A multicenter retrospective analysis of 83 cases. Clin. Infect. Dis. 2004, 39, 192–198. [Google Scholar] [PubMed]
- Steinbach, W.J.; Schell, W.A.; Miller, J.L.; Perfect, J.R. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J. Clin. Microbiol. 2003, 41, 3981–3985. [Google Scholar] [PubMed]
- Escandon, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin. Infect. Dis. 2019, 68, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kristanc, L.; Božič, B.; Jokhadar, Š.Z.; Dolenc, M.S.; Gomišček, G. The pore-forming action of polyenes: From model membranes to living organisms. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 418–430. [Google Scholar]
- Vincent, B.M.; Lancaster, A.K.; Scherz-Shouval, R.; Whitesell, L.; Lindquist, S. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol. 2013, 11, e1001692. [Google Scholar]
- Rambach, G.; Oberhauser, H.; Speth, C.; Lass-Flörl, C. Susceptibility of Candida species and various moulds to antimycotic drugs: Use of epidemiological cutoff values according to EUCAST and CLSI in an 8-year survey. Med. Mycol. 2011, 49, 856–863. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A. Treatment of aspergillosis: Clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef]
- Readio, J.D.; Bittman, R. Equilibrium binding of amphotericin B and its methyl ester and borate complex to sterols. Biochim. Biophys. Acta (BBA)-Biomembr. 1982, 685, 219–224. [Google Scholar] [CrossRef]
- Tevyashova, A.N.; Bychkova, E.N.; Solovieva, S.E.; Zatonsky, G.V.; Grammatikova, N.E.; Isakova, E.B.; Mirchink, E.P.; Treshchalin, I.D.; Pereverzeva, E.R.; Bykov, E.E. Discovery of Amphamide, a Drug Candidate for the Second Generation of Polyene Antibiotics. ACS Infect. Dis. 2020, 6, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, M.; Yang, Z. Design of amphotericin B oral formulation for antifungal therapy. Drug Deliv. 2017, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cuddihy, G.; Wasan, E.K.; Di, Y.; Wasan, K.M. The development of oral amphotericin b to treat systemic fungal and parasitic infections: Has the myth been finally realized? Pharmaceutics 2019, 11, 99. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar]
- Gottlieb, D.; Carter, H.E.; Sloneker, J.H.; Ammann, A. Protection of fungi against polyene antibiotics by sterols. Science 1958, 128, 361. [Google Scholar] [CrossRef]
- Cotero, B.V.; Rebolledo-Antúnez, S.; Ortega-Blake, I. On the role of sterol in the formation of the amphotericin B channel. Biochim. Biophys. Acta (BBA)-Biomembr. 1998, 1375, 43–51. [Google Scholar] [CrossRef]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400. [Google Scholar] [CrossRef]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef]
- Palacios, D.S.; Anderson, T.M.; Burke, M.D. A Post-PKS Oxidation of the Amphotericin B Skeleton Predicted to be Critical for Channel Formation Is Not Required for Potent Antifungal Activity. J. Am. Chem. Soc. 2007, 129, 13804–13805. [Google Scholar] [CrossRef]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Gianinni, M.J.S.M.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Te Welscher, Y.M.; Jones, L.; Van Leeuwen, M.R.; Dijksterhuis, J.; De Kruijff, B.; Eitzen, G.; Breukink, E. Natamycin inhibits vacuole fusion at the priming phase via a specific interaction with ergosterol. Antimicrob. Agents Chemother. 2010, 54, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Te Welscher, Y.M.; ten Napel, H.H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; De Kruijff, B.; Breukink, E. Natamycin blocks fungal growth by binding specifically to ergosterol without permeabilizing the membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Te Welscher, Y.M.; van Leeuwen, M.R.; de Kruijff, B.; Dijksterhuis, J.; Breukink, E. Polyene antibiotic that inhibits membrane transport proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 11156–11159. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, D.M. Recent progress in the study of the interactions of amphotericin B with cholesterol and ergosterol in lipid environments. Eur. Biophys. J. 2014, 43, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L. The multifunctional fungal ergosterol. mBio 2018, 9, e01755-18. [Google Scholar] [CrossRef] [PubMed]
- Heese-Peck, A.; Pichler, H.; Zanolari, B.; Watanabe, R.; Daum, G.; Riezman, H. Multiple functions of sterols in yeast endocytosis. Mol. Biol. Cell 2002, 13, 2664–2680. [Google Scholar] [CrossRef]
- Jin, H.; McCaffery, J.M.; Grote, E. Ergosterol promotes pheromone signaling and plasma membrane fusion in mating yeast. J. Cell Biol. 2008, 180, 813–826. [Google Scholar] [CrossRef]
- Baran, M.; Borowski, E.; Mazerski, J. Molecular modeling of amphotericin B–ergosterol primary complex in water II. Biophys. Chem. 2009, 141, 162–168. [Google Scholar] [CrossRef]
- Foglia, F.; Lawrence, M.J.; Demeė, B.; Fragneto, G.; Barlow, D. Neutron diffraction studies of the interaction between amphotericin B and lipid-sterol model membranes. Sci. Rep. 2012, 2, 778. [Google Scholar] [CrossRef]
- Cohen, B.E. Amphotericin B membrane action: Role for two types of ion channels in eliciting cell survival and lethal effects. J. Membr. Biol. 2010, 238, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ostroumova, O.S.; Efimova, S.S.; Schagina, L.V. Probing amphotericin B single channel activity by membrane dipole modifiers. PLoS ONE 2012, 7, e30261. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-S.; Ou, K.-L.; Peng, P.-W.; Liou, B.-C.; Wang, W.-T.; Huang, Y.-C.; Tsai, C.-M.; Su, C.-H. Quantifying membrane permeability of amphotericin B ion channels in single living cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Mouri, R.; Konoki, K.; Matsumori, N.; Oishi, T.; Murata, M. Complex formation of amphotericin B in sterol-containing membranes as evidenced by surface plasmon resonance. Biochemistry 2008, 47, 7807–7815. [Google Scholar] [CrossRef] [PubMed]
- Szpilman, A.M.; Cereghetti, D.M.; Manthorpe, J.M.; Wurtz, N.R.; Carreira, E.M. Synthesis and Biophysical Studies on 35-Deoxy Amphotericin B Methyl Ester. Chem. Eur. J. 2009, 15, 7117–7128. [Google Scholar] [CrossRef] [PubMed]
- Szpilman, A.M.; Cereghetti, D.M.; Manthorpe, J.M.; Wurtz, N.R.; Carreira, E.M. Synthesis and Biophysical Studies on 35-Deoxy Amphotericin B Methyl Ester. Chem. Eur. J. 2009, 15, 7117–7128. [Google Scholar] [CrossRef] [PubMed]
- Sokol-Anderson, M.; Sligh, J.E., Jr.; Elberg, S.; Brajtburg, J.; Kobayashi, G.S.; Medoff, G. Role of cell defense against oxidative damage in the resistance of Candida albicans to the killing effect of amphotericin B. Antimicrob. Agents Chemother. 1988, 32, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Sokol-Anderson, M.L.; Brajtburg, J.; Medoff, G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 1986, 154, 76–83. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, Z.; Chen, X.; Yao, X.; Zhao, L.; Wang, H.; Yan, L.; Wu, H.; Chai, Y.; Jiang, Y. Effect of amphotericin B on the metabolic profiles of Candida albicans. J. Proteome Res. 2013, 12, 2921–2932. [Google Scholar] [CrossRef]
- Liu, T.T.; Lee, R.E.; Barker, K.S.; Lee, R.E.; Wei, L.; Homayouni, R.; Rogers, P.D. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother. 2005, 49, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Lamp-Freund, M.T.; Ferreira, V.F.; Schreier, S. Mechanism of inactivation of the polyene antibiotic amphotericin B. J. Antibiot. 1985, 38, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Lamp-Freund, M.T.; Ferreira, V.F.; Schreier, S. Mechanism of inactivation of the polyene antibiotic amphotericin B. J. Antibiot. 1985, 38, 753–757. [Google Scholar] [CrossRef] [PubMed]




