
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Susana Braga | + 2930 word(s) | 2930 | 2020-12-28 09:16:25 | | | |
| 2 | Camila Xu | -1 word(s) | 2929 | 2021-02-07 11:49:30 | | |
Video Upload Options
Curcumin is a pigment with a strong yellow colour found and the main active component of Curcuma longa, a perennial Zingiberaceae plant native to southwest India, but now grown across the South and Southeast Asia, especially in China and India. It is used for centuries as a spice and currently it is viewed as a nutraceutical due to the increasing number of scientific studies demonstrating its anti-oxidant, anti-inflammatory, anti-tumoral and cancer preventive properties. Its chemical structure comprises two aromatic ring systems with o-methoxy phenol groups connected by a seven-carbon linker consisting of an α,β-unsaturated β-diketone with tautomerism when in solution.
1. History and Sources of Curcumin
The history of curcumin dates back about five thousand years. Curcumin is the main active ingredient of turmetic, a spice obtained by grinding the dried rhizomes of the plant Curcuma longa. [1][2][3]. Turmeric is referenced in Ayurvedic medicine, the characteristic medicinal system of Ancient India, as a home remedy for various diseases [4]. The expansion of the therapeutic use of curcumin to the Western civilizations dates from the time of the Portuguese "State of India", in the XVI century as it was mentioned by Garcia de Orta, the physician of the Viceroy of India, as “a medicine for jaundice” [5].Turmeric dry rhizome is composed mainly of starch, having also carbohydrates, proteins, lipids, fiber, curcuminoid pigments, sesquiterpenes (turmerone, atlantone, zingiberone, turmeronol, germacrone, α-curcumene, β-sesquiphellanderene, bisacurone, curcumenone, dehydrocurdione, procurcumadiol, bis-acumol, curcumenols, zedoaronediol, bisabolene, and curlone), and caffeic acid [6][7]. The curcuminoid content typically varies between 2% and 9%. Curcumin is the most abundant curcuminoid in turmeric, but traces of its precursors, desmethoxycurcumin and bisdemethoxycurcumin (Figure 1), are also present [8].
Besides turmetic, curcumin and its analogues can be found in Curcuma mangga, Curcuma zedoaria, Costus speciosus, Curcuma xanthorrhiza, Curcuma aromatica, Curcuma phaeocaulis, Etlingera elatior, and Zingiber cassumunar [9]. C. mangga is commonly named mango ginger and considered as a good dietary source of curcumin, although it is still far from reaching the widespread reputation of turmeric as a dietary supplement and functional food.
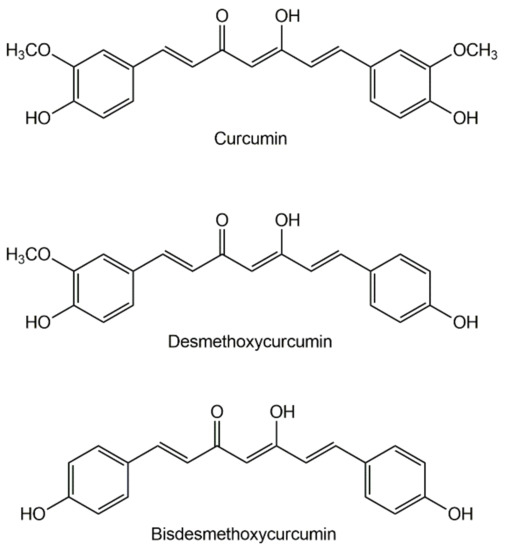
Figure 1. The three main curcuminoids found in Curcuma longa.
In western societies, turmeric consumption is a growing trend due to the recognition of its therapeutic properties against inflammation and cancer [10][11]. Turmeric has been granted the GRAS status (‘Generally Recognized as Safe’) by the FDA [12], with an allowed daily intake (ADI) limit of 2.5 mg/kg of body weight; for pure curcumin, the ADI is of 0.1 mg/kg weight [13]. The official acknowledgement of turmeric as a safe dietary supplement contributed strongly to its widespread use and expansion to other areas such as cosmetics. Since 2013, turmeric is the top-ranking herbal supplement in North America, with sales of that year having grown 26.2% [14]. India is the largest producer of curcumin and North America its largest market, with revenues over US$20 million in 2014 [15] and expected to grow up to a global value of $94.3 billion in 2022 [16].
2. Curcumin Chemistry
The isolation of the active phytochemicals in turmeric dates back to 1815, when the first crude extract was obtained and described as “a matter of yellow color” [17]. Many of the properties of curcumin could already be observed in this extract: it was insoluble in water, solubilizing upon the addition of alkali to form a reddish-brown solution, and able to react with salts of metals such as lead and tin [17]. The extract was later found to contain a mixture of curcuminoids along with some oils and resins. Curcumin was purified in 1870, having been isolated as orthorhombic crystals [18]. Its chemical structure was determined in 1910 [19].
Curcumin has the chemical formula C21H20O6 (Mw of 368.38): it is also referred to as diferuloylmethane, having a very long IUPAC denomination: (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione. Its chemical structure comprises two aromatic ring systems with o-methoxy phenol groups connected by a seven-carbon linker consisting of an α,β-unsaturated β-diketone moiety that exhibits keto-enol tautomerism in solution [20]. Due to extended conjugation, the π electron cloud is distributed all along the molecule making curcumin very hydrophobic, with a log p value of 3.38 and an extremely low solubility in water (1.34 ± 0.02 mg/L) [21]. According to the Biopharmaceutics Classification System (BCS) [22], curcumin is a class IV drug; that is, a compound having low solubility and low permeability. Class IV drugs usually are “not well absorbed over the intestinal mucosa and a high variability [in the absorption profile] is expected”.
Curcumin is reasonably stable in water at pH < 7.0 due to structural stabilization by the conjugated diene; in PBS and at pH > 8 it may degrade rapidly (10 min) [2]. In fact, curcumin possesses three ionizable protons with pKa values of approximately 8.5 (enolic proton) and 10–10.5 (two phenolic protons) [23][24].
Curcumin absorbs light from the near ultraviolet (around 340 nm) to the indigo-blue spectral region (450–460 nm), with absorption peaking at 410–430 nm (violet light) [25]. It presents a fluorescence band between 460 and 560 nm. Furthermore, curcumin is sensitive to ultraviolet radiation and its degradation is accelerated by exposure to sunlight [26][27]. When irradiated with light above 400 nm, curcumin undergoes a self-sensitized photo-decomposition where singlet oxygen is involved, but when reactive oxygen species are not available, other decomposition mechanisms are triggered. Photodegradation products include vanillin, vanillic acid, 4-vinyl-guaiacol, ferulic aldehyde, and ferulic acid [28].
3. Biological Actions of Curcumin
Curcumin is indicated in ayurvedic medicine for an enormous variety of pathologies and ailments [29]. Most of this knowledge is, however, empirical, or it has not been demonstrated by studies on human subjects. Most of studies available in the literature have been conducted either in vitro or in animal models (mostly rodents). They provide information on possible therapeutic indications of curcumin for conditions as varied as viral infections, scleroderma, atherosclerosis, myocardial infarction, brain ischemia, and Alzheimer’s [30], but such activities may not necessarily be manifested in human patients. Only in the latest decades has evidence from clinical trials been gathered on curcumin. This section presents the most relevant results of clinical trials, with highlight on cancer therapy.
3.1. Medicinal Activity in Humans
Turmeric has well-documented anti-inflammatory [31][32], antioxidant [33][34][35][36] and antitumor activities [37][38][39][40] that are mainly attributed to curcumin. Curcumin is a strongly pleiotropic molecule, able to modulate the activity of numerous signalling biomolecules, to interfere with different cellular and molecular cascades [41][42] and to interact with transcription factors, growth factors or their receptors, nuclear factors, cytokines, and hormone receptors. Curcumin is even able to regulate the expression of genes associated with the processes of cell proliferation and apoptosis [43]. Details on the different biochemical targets of curcumin are given in the Section 3.2.
3.1.1. Curcumin against Inflammation and Oxidative Stress
Curcumin interferes with various steps of the arachidonic acid inflammatory cascade, inhibiting the enzymes phospholipase, cyclooxygenase II, and lipo-oxygenase, and having also effects on cytokines [45][44]. Curcumin was shown to reduce post-operative inflammation in patients having had surgical repair of inguinal hernia and/or hydrocele [46], to ameliorate symptoms of chronic inflammation pathologies such as arthritis [47][48], psoriasis [49], and bowel conditions (IBS, Crown’s disease, and ulcerative colitis) [31][50][51][52][53] and to treat eye inflammations such as the “idiopathic orbital inflammatory syndrome”[54] and uveitis [55].
The antioxidant activity of curcumin is also the result of a multiplicity of actions. Not only does curcumin stabilize superoxide and hydroxyl free radicals due to the electron-donating properties of its phenolic groups [20][56][57][58], but it also induces the expression of antioxidant enzymes. In vitro tests with beta cells of human pancreas islets incubated with curcuminoids have shown increased levels of heme oxygenase 1, gamma-glutamyl-cysteine ligase, and NAD(P)H:quinone oxidoreductase and a consequent increase in glutathione levels [59]. Curcumin protects against oxidative stress caused by advanced glycation end products in patients with diabetes, being under evaluation as a new anti-diabetes drug candidate in a series of clinical and pre-clinical studies [60]. It should also be highlighted that the antioxidant benefits of curcumin do not cease with its metabolization, as many of the metabolites present significant antioxidant properties [61].
3.1.2. Antitumoral Action
The first report on the anticancer activity of curcumin, in 1987 [62], rekindled the interest in this compound and brought it to the spotlight of the western society. Curcumin has been the subject of over 30 clinical trials in the context of cancer, some of them still ongoing.
-
In colorectal cancer, curcumin was studied for both tumor prevention and chemotherapy. In cancer prevention, it was demonstrated to reduce by 40% the formation of aberrant crypt foci in smoking patients (intake of 4 g/day for one month) [63]. In a combination study, curcumin, and quercetin (1440 + 60 mg/day for six months) were shown to reduce the number and size of polyps in patients with familial adenomatous polyposis, a hereditary disorder characterized by the development of hundreds of colorectal adenomas which turn malign when left untreated [64]. In chemotherapy, 1 g/day curcumin for up to one month (prior to surgical removal of the tumor) was shown to improve the patient’s body weight and to increase the apoptosis rates of the patient’s tumor cells [65].
-
In prostate cancer, a trial has demonstrated that curcumin/flavone association reduces the chances of developing cancer by lowering the levels of prostate-specific antigen (PSA). PSA levels are increased due to the presence of chronic inflammation in the prostate, which is one of the most significant causes of tumorigenesis [66]. Association of curcumin (5.4 g/day for seven days around chemotherapy) with docetaxel/prednisone (75 mg/m2 + 24 mg, once every three weeks, for six cycles) demonstrated encouraging results, with a tumor objective response in 40% and a PSA response in 59% of the patients in a group having castration-resistant prostate cancer [67]. There is also preliminary evidence on the ability to reduce the formation of metastases. An association of polyphenols (pomegranate seed, green tea, broccoli, and turmeric), taken over six months, has lowered PSA by 63.8% (compared to placebo) in prostatectomized patients [68]. Note that, since these men have no prostate, PSA is produced only by neoplastic cells, thus being a good indicator of metastasis growth. Curcumin can confer radioprotective effect in patients with prostate cancer who undergo radiation therapy, reducing the severity of radiotherapy related urinary symptoms. Patients were given 3 g of curcuminoids per day (corresponding to ca. 2 g/day of curcumin) for one week before the onset of radiotherapy and until completion of radiotherapy [69][70].
-
In breast cancer, curcumin was used in co-therapy with both chemotherapeutic agents and radiation. A combination therapy with docetaxel and curcumin (in escalating doses of up to 6 g/day) was found to afford better therapeutic results than docetaxel used alone: histological improvements were observed in the fourteen patients under study, all having reduction or elimination of disseminated foci [71]. Curcumin was evaluated in two clinical trials regarding protective action against radiation-induced dermatitis during radiotherapy of breast cancer patients. Despite promising results on a pilot study, with slightly less severe dermatitis in the curcumin group, a second trial on 686 patients showed no significant changes in pain, symptoms, and quality of life of the patients taking curcumin (1.5 g daily) in regard to those taking placebo [72].
-
Pancreatic cancer: a phase II study with twenty-one patients taking curcumin (8 g/day for up to 18 months) showed partial regression during the treatment period; patients had different responses after treatment, one of them having become stable and another one having shown a strong tumor relapse [73]. Another trial evaluated the association of curcuminoids (8 g/day, corresponding to 6.14 g/day of curcumin) with a gemcitabine-based chemotherapeutic treatment. A total of 21 patients was divided into two groups: one, with 2 patients, received gemcitabine monotherapy; the other, with 19 patients, received a combination therapy of gemcitabine and S-1. S-1 is a novel oral antitumor formula based on fluorouracil, comprising three pharmacological agents: (i) tegafur, a prodrug of 5-fluorouracil, (ii) 5-chloro-2,4-dihydroxypyridine, which inhibits dihydropyrimidine dehydrogenase activity; and (iii) potassium oxonate, which reduces gastrointestinal toxicity was also evaluated. Eighty-one percent of the patients died during the study period. In the surviving patients, the treatment was able to stabilize the disease [74].
3.2. Molecular Targets of Curcumin
The various targets and effects of curcumin are summarized in the Table 1 and described with more detail in the following sub-sections.
3.2.1. Curcumin Modulates the Activity of Transcription Factors
Three main families of transcription factors are involved in cell proliferation, cell invasion, metastasis, angiogenesis, and resistance to chemotherapy and radiotherapy. They are:
-
the families of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and of the activated protein-1 (AP-1),
-
signal transducers and activators of transcription (STAT), and
-
steroid receptors [77].
NF-kB is involved in cell response to several external agents, including physical strain, oxidative stress (free radicals), and cytokines. NF-kB is usually inactive in the cytoplasm, but once activated by the adequate stimuli it can translocate to the nucleus, inducing expression of apoptosis-suppressing genes to promote cell proliferation and even metastasis. Curcumin was shown to inhibit the activation of the NF-kB pathway by studies in vitro [78] and in vivo [79] and in a phase II clinical trial [80].
AP-1 is involved in the differentiation, proliferation, apoptosis, and oncogenic transformations of the cells [81]. AP-1 can be activated by stimuli of growth factors, cytokines, or bacterial/viral infections. Activated AP-1 induces the expression of several genes that code proteins involved in the angiogenesis and growth of cancer cells, such as cyclin-D1, MMP, and VEGF [82]. Curcumin inhibits this pathway by direct interaction with the AP-1 DNA-binding site, namely in human leukemia cells, transformed keratinocytes and prostate cancer cells [83][84][85][86][87]. The effect of curcumin on the expression of NF-kB and AP-1 members was evaluated in an oral cancer cell line [88]. Nuclear extracts obtained from curcumin-treated cancer cell were evaluated regarding binding of the transcription factors AP-1 and NF-kB, to reveal that binding is reduced in a dose dependent manner and that in cells treated with 100 μM of curcumin, the DNA-binding activities of AP-1 and NF-kB were completely lost. These results confirmed the downregulation of several transcription factors and inhibition of NF-kB and AP-1.
The Janus kinase (JAK) signal transducer of activators of transcription (STAT) pathway signalling pathway is a signaling pathway employed by diverse cytokines, interferons, growth factors, and related molecules, allowing these extracellular factors control gene expression and regulate cell growth and differentiation [89]. In cancer cells, this pathway is consistently active, being involved in metastasis. Inhibition of the JAK/STAT pathway by curcumin was observed in prostate, lung, and glioblastoma cancer cell lines [90][91][92]. Curcumin was shown to inhibit STAT3 phosphorylation and to lower levels of interleukin-6 (IL-6), a pro-inflammatory cytokine involved in cell proliferation and survival. Curcumin also exhibited antineoplastic effects in K652 chronic leukemia, ovarian, and endometrial cancer cells by suppression of JAK/STAT signalling [93][94].
Table 1. Molecular targets and cell processes modulated by curcumin.
| Family | Molecular Target | Effect |
|---|---|---|
| Transcription factors | NF-kB | ↓ |
| Nrf2 | ↑ | |
| AP-1 | ↓ | |
| STAT-3 | ↓ | |
| STAT-5 | ↓ | |
| β-catenin | ↓ | |
| EGR-1 | ↓ | |
| HIF-1 | ↓ | |
| Notch-1 | ↓ | |
| Growth factors | EGF | ↓ |
| FGF | ↓ | |
| PDGF | ↓ | |
| TGF-β | ↓ | |
| VEGF | ↓ | |
| Cytokines, pro-inflammatory | TNF-α | ↓ |
| IL-1 | ↓ | |
| IL-2 | ↓ | |
| IL-5 | ↓ | |
| IL-6 | ↓ | |
| IL-8 | ↓ | |
| IL-12 | ↓ | |
| IL-18 | ↓ | |
| Enzymes | COX-2 | ↓ |
| iNOS | ↓ | |
| Lipoxygenase | ↓ | |
| MMP-9 | ↓ | |
| Kinases | JNK | ↑ |
| MAPK | ↓ | |
| PKC | ↓ | |
| Akt | ↓ | |
| CDKs | ↓ | |
| Receptors | AR | ↓ |
| EGFR | ↓ | |
| Adhesion molecules | ICAM-1 | ↓ |
| VCAM-1 | ↓ | |
| ELAM-1 | ↓ | |
| Antiapoptotic proteins | Bcl-2 | ↓ |
| Bcl-xL | ↓ | |
| Proapoptotic proteins | Bax | ↑ |
| Bak | ↑ | |
| Others | Cyclin D1 | ↓ |
| p53 | ↑ |
3.2.2. Curcumin Decreases Tumor Angiogenesis
Curcumin has anti-angiogenic properties by inhibition of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) [79]. Several in vitro and in vivo studies have demonstrated the association between the suppression of VEGF expression by curcumin with its inhibitory action on tumor growth [115][116][117]. In vivo studies in mice showed that VEGF expression and angiogenesis suppression is mediated through suppression of the osteopontin gene expression and the NF-κB/ATF-4 pathway [115].
3.2.3. Curcumin Inhibits Inflammatory Cytokines
Tumor necrosis factor (TNF) and interleukins (IL) are two kinds of inflammatory cytokines with an important mediating role in tumorigenesis. Curcumin was shown to have profound effects on TNF inhibition in dendritic cells, macrophages, monocytes, alveolar macrophages, and endothelial and bone marrow cells [118]. The suppression of the TNF-signalling pathway by curcumin is related to the inhibition of NF-kB phosphorylation, as detailed in 2.2.1 [119][120].
Interleukins (ILs) contribute to tumor invasiveness and angiogenesis by induction of the expression of metalloproteinases, adhesion molecules and STATs [144]. Curcumin inhibits the expression of IL-1 [121], IL-2 [122], IL-5 [123], IL-6 [118], IL-8 [122], IL-12 [124], and IL-18 [125], being thus a potent inhibitor of these classes of cytokines.
3.2.4. Curcumin Regulates the Activity of Enzymes with Roles in Inflammation and Cancer
Pro-inflammatory enzymes are linked with various types of cancer. COX-2 is known to participate in uncontrolled cell proliferation and suppression of apoptosis, while inducible nitric oxide synthase (iNOS) and matrix metalloproteinase-9 (MMP-9) are involved in the formation of metastases [145][146]. Several studies, both in vitro and in vivo, demonstrated that the inhibitory action of curcumin on COX-2 expression contributes significantly to its antitumor action [72][80][126]. Curcumin was also shown to inhibit the expression of MMP-9 in orthotopically implanted pancreatic [129] and ovarian [130] tumors in mice and the production of iNOS in chronic colitis [127].
3.2.5. Curcumin and Cell Cycle Regulation
Programmed cell death, or apoptosis, is a mechanism of vital importance in maintaining normal cell growth. Apoptosis is initiated by regulation of protein 53 (p53) and by proteins of the B-cell lymphoma 2 family (Bcl-2). Activated p53 induces activation of two pro-apoptotic proteins, Bcl-2 homologous antagonist killer (Bak), and Bcl-2 associated x protein (Bax), which in turn release cytochrome c into the cytoplasm to activate the caspase cascade. Curcumin is able to induce apoptosis in prostate cancer PC-3, DU-145, and LNCaP cells via p53-dependent mitochondrial pathways [148]. Activation of p53 by curcumin leads to over-expression of Bak, Bax, and several caspase proteins [136][137][138][139][140]. In addition, curcumin inhibits the activity of a few anti-apoptotic proteins, such as Bcl-2 and B-cell lymphoma extra-large protein (Bcl-XL) [136][137][138][139][140][141].
Cyclin-dependent kinases (CDKs) are also involved in the life cycle of cells, being in charge of its progression through the different stages [147]. Malignant cells have thus frequent alterations in CDK expression, with overexpression of cyclins and suppression of CDK inhibitors. Curcumin induces cell cycle arrest in colon cancer cells (HTC116 line) by CDK2-dependent effects [135]. The mechanism underlying cell cycle arrest by curcumin may involve overexpression of CDK inhibitors and blockage of the expression of cyclin E and cyclin D1 [142][143].
References
- Akram, M.; Uddin, S.; Ahmed, A.; Khan, U.; Hannan, A.; Mohihuddin, E.; Asif, M. Curcuma Longa and Curcumin. Rom. J. Biol. Plant. Biol. 2010, 55, 65–70.
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876, doi:10.1016/s0731-7085(96)02024-9.
- Nabavi, S.M.; Daglia, M.; Moghaddam, A.H.; Habtemariam, S. Curcumin and Liver Disease: From Chemistry to Medicine. Compr. Rev. Food Sci. Food Saf. 2013, 13, 62–77, doi:10.1111/1541-4337.12047.
- Mukherjee, P.K.; Wahile, A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J. Ethnopharmacol. 2006, 103, 25–35, doi:10.1016/j.jep.2005.09.024.
- de Orta, G. Colóquios Dos Simples, E Drogas E Coisas Medicinais Da India E Assim de Algumas Frutas Achadas Nela Onde Se Tratam Algumas Coisas Tocantes a Medicina Prática, E Outras Coisas Boas Para Saber; Ioannes de Endem: Goa, India, 1563.
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011.
- Govindarajan, V.; Stahl, W.H. Turmeric—chemistry, technology, and quality. CRC Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301, doi:10.1080/10408398009527278.
- Kita, T.; Imai, S.; Sawada, H.; Kumagai, H.; Seto, H. The Biosynthetic Pathway of Curcuminoid in Turmeric (Curcuma longa) as Revealed by13C-Labeled Precursors. Biosci. Biotechnol. Biochem. 2008, 72, 1789–1798, doi:10.1271/bbb.80075.
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian Solid Gold. In Results and Problems in Cell Differentiation; Springer Science and Business Media LLC: Berlin, Germany, 2007; Volume 595, pp. 1–75.
- Toda, S.; Miyase, T.; Arichi, H.; Tanizawa, H.; Takino, Y. Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chem. Pharm. Bull. 1985, 33, 1725–1728, doi:10.1248/cpb.33.1725.
- Osawa, T.; Sugiyama, Y.; Inayoshi, M.; Kawakishi, S. Antioxidative Activity of Tetrahydrocurcuminoids. Biosci. Biotechnol. Biochem. 1995, 59, 1609–1612, doi:10.1271/bbb.59.1609.
- FDA. Inventory of GRAS Notices, Number 460. Curcuminoids Purified from Turmeric (Curcuma longa L.); Food and Drugs Administration: Silver Spring (MD), USA, 2013. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=460&sort=GRN_No&order=DESC&startrow=1&type=basic&search=460 (accessed on 15 December 2020)
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Turmeric and curcumin (WHO Food Additives Series 6). Available online: http://www.inchem.org/documents/jecfa/jecmono/v06je29.htm (accessed on 16 November 2020).
- Anna’s Tuin & Ruigte. Available online: http://annastuinenruigte.nl/ (accessed on 24 March 2020).
- The State of the Curcumin Market. Available online: http://www.naturalproductsinsider.com/articles/2015/12/the-state-of-the-curcumin-market.aspx (accessed on 16 November 2020).
- Curcumin Market. Size, Share & Trends Analysis Report By Application (Pharmaceutical, Food, Cosmetics), By Region (North America, Europe, Asia Pacific, Central & South America, Middle East & Africa), And Segment Forecasts, 2020–2027. Available online: http://www.grandviewresearch.com/industry-analysis/turmeric-extract-curcumin-market (accessed on 16 November 2020).
- Pelletier, J.; Vogel, A. Examen Chimique de La Racine de Curcuma. J. Pharm. 1815, 1, 289–300.
- Daube, F.W. Ueber Curcumin, den Farbstoff der Curcumawurzel. Eur. J. Inorg. Chem. 1870, 3, 609–613, doi:10.1002/cber.187000301196.
- Lampe, V.; Milobedzka, J. Studien Über Curcumin. Berichte der Dtsch. Chem. Gesellschaft 1913, 46, 2235–2240.
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112, doi:10.3390/molecules191220091.
- Carvalho, D.D.M.; Takeuchi, K.P.; Geraldine, R.M.; De Moura, C.J.; Torres, M.C.L. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. 2015, 35, 115–119, doi:10.1590/1678-457x.6515.
- Chavda, H.; Patel, C.; Anand, I. Biopharmaceutics classification system. Syst. Rev. Pharm. 2010, 1, 62, doi:10.4103/0975-8453.59514.
- Hatcher, H.; Planalp, R.; Cho, J.; Tortia, F.M.; Tortic, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652, doi:10.1007/s00018-008-7452-4.
- Bernabé-Pineda, M.; Ramı́rez-Silva, M.T.; Romero-Romo, M.; González-Vergara, E.; Rojas-Hernández, A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2004, 60, 1091–1097, doi:10.1016/s1386-1425(03)00342-1.
- Chignell, C.F.; Bilskj, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. spectral and photochemical properties of curcumin. Photochem. Photobiol. 1994, 59, 295–302, doi:10.1111/j.1751-1097.1994.tb05037.x.
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95, doi:10.1016/j.jphotochemrev.2009.05.001.
- Khurana, A.; Ho, C.-T. High Performance Liquid Chromatographic Analysis of Curcuminoids and Their Photo-oxidative Decomposition Compounds in Curcuma Longa L. J. Liq. Chromatogr. 1988, 11, 2295–2304, doi:10.1080/01483918808067200.
- Tønnesen, H.H.; Karlsen, J.; van Henegouwen, G.B. Studies on Curcumin and Curcuminoids VIII. Photochemical Stability of Curcumin. Z. Lebensm. Unters. Forsch. 1986, 183, 116–122.
- De Jager, P. Turmeric: The Ayurvedic Spice of Life, 2nd ed.; Pioneer imprints: Maui, Hawaii, 2010.
- De Oliveira, M.R.; Jardim, F.R.; Setzer, W.N.; Nabavi, S.M. Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol. Adv. 2016, 34, 813–826, doi:10.1016/j.biotechadv.2016.04.004.
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin Therapy in Inflammatory Bowel Disease: A Pilot Study. Dig. Dis. Sci. 2005, 50, 2191–2193, doi:10.1007/s10620-005-3032-8.
- Shishodia, S.; Sethi, G.; Aggarwal, B.B. Curcumin: Getting Back to the Roots. Ann. New York Acad. Sci. 2005, 1056, 206–217, doi:10.1196/annals.1352.010.
- Subramanian, M.; Sreejayan; Devasagayam, T.P.; Singh, B. Diminution of singlet oxygen-induced DNA damage by curcmin and related antioxidants. Mutat. Res. Mol. Mech. Mutagen. 1994, 311, 249–255, doi:10.1016/0027-5107(94)90183-x.
- Iqbal, M.; Sharma, S.D.; Okazaki, Y.; Fujisawa, M.; Okada, S. Dietary Supplementation of Curcumin Enhances Antioxidant and Phase II Metabolizing Enzymes in ddY Male Mice: Possible Role in Protection against Chemical Carcinogenesis and Toxicity. Pharmacol. Toxicol. 2003, 92, 33–38, doi:10.1034/j.1600-0773.2003.920106.x.
- Kuo, M.-L.; Huang, T.-S.; Lin, J.-K. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 1996, 1317, 95–100, doi:10.1016/s0925-4439(96)00032-4.
- Sreejayan; Rao, M.N.A. Curcuminoids as Potent Inhibitors of Lipid Peroxidation. J. Pharm. Pharmacol. 1994, 46, 1013–1016, doi:10.1111/j.2042-7158.1994.tb03258.x.
- Busquets, S.; Carbó, N.; Almendro, V.; Quiles, M.T.; López-Soriano, F.J.; Argilés, J.M. Curcumin, a natural product present in turmeric, decreases tumor growth but does not behave as an anticachectic compound in a rat model. Cancer Lett. 2001, 167, 33–38, doi:10.1016/s0304-3835(01)00456-6.
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190, doi:10.1016/j.canlet.2004.09.041.
- Garcea, G.; Berry, D.P.; Jones, D.J.L.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidem. Biomarkers Prev. 2005, 14, 120–5.
- Bentham Science Publisher Tzeng-Horng Leu; Bentham Science Publisher Ming-Chei Maa The Molecular Mechanisms for the Antitumorigenic Effect of Curcumin. Curr. Med. Chem. Agents 2002, 2, 357–370, doi:10.2174/1568011024606370.
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by Curcumin as Revealed by Molecular Interaction Studies. Nat. Prod. Rep. 2011, 28, 1937.
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and Cancer Cells: How Many Ways Can Curry Kill Tumor Cells Selectively? AAPS J. 2009, 11, 495–510, doi:10.1208/s12248-009-9128-x.
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and Therapeutic Properties in Laboratory Studies and Clinical Trials. Antioxidants Redox Signal. 2008, 10, 511–546, doi:10.1089/ars.2007.1769.
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168, doi:10.1089/107555303321223035.
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Russo, L.L.; De Lillo, A.; Laino, L.; Muzio, L.L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp. Ther. Med. 2015, 10, 1615–1623, doi:10.3892/etm.2015.2749.
- Satoskar, R.R.; Shah, S.J.; Shenoy, S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654.
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70, doi:10.1016/j.hermed.2015.03.001.
- Chandran, B.; Goel, A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients with Active Rheumatoid Arthritis. Phytotherapy Res. 2012, 26, 1719–1725, doi:10.1002/ptr.4639.
- Heng, M.; Song, M.; Harker, J. Drug‐induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br. J. Dermatol. 2000, 143, 937–949, doi:10.1046/j.1365-2133.2000.03767.x.
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506, doi:10.1016/j.cgh.2006.08.008.
- Lahiff, C.; Moss, A.C. Curcumin for clinical and endoscopic remission in ulcerative colitis. Inflamm. Bowel Dis. 2011, 17, E66, doi:10.1002/ibd.21710.
- Epstein, J.; Docena, G.; MacDonald, T.T.; Sanderson, I.R. Curcumin Suppresses p38 Mitogen-Activated Protein Kinase Activation, Reduces IL-1beta and Matrix Metalloproteinase-3 and Enhances IL-10 in the Mucosa of Children and Adults with Inflammatory Bowel Disease. Br. J. Nutr. 2010, 103, 824–832.
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Booth, J. Turmeric Extract May Improve Irritable Bowel Syndrome Symptomology in Otherwise Healthy Adults: A Pilot Study. J. Altern. Complement. Med. 2004, 10, 1015–1018, doi:10.1089/acm.2004.10.1015.
- Lal, B.; Kapoor, A.K.; Agrawal, P.K.; Asthana, O.P.; Srimal, R.C. Role of curcumin in idiopathic inflammatory orbital pseudotumours. Phytotherapy Res. 2000, 14, 443–447, doi:10.1002/1099-1573(200009)14:63.0.co;2-v.
- Lal, B.; Kapoor, A.K.; Asthana, O.P.; Agrawal, P.K.; Prasad, R.; Kumar, P.; Srimal, R.C. Efficacy of Curcumin in the Management of Chronic Anterior Uveitis. Phytother. Res. 1999, 13, 318–322.
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.-H. Recent advances in the investigation of curcuminoids. Chin. Med. 2008, 3, doi:10.1186/1749-8546-3-11.
- Reddy, A.P.; Lokesh, B. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 1992, 111, 117–124, doi:10.1007/bf00229582.
- Noorafshan, A.; Ashkani-Esfahani, S. A Review of Therapeutic Effects of Curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046.
- Balamurugan, A.; Akhov, L.; Selvaraj, G.; Pugazhenthi, S. Induction of Antioxidant Enzymes by Curcumin and Its Analogues in Human Islets. Pancreas 2009, 38, 454–460, doi:10.1097/mpa.0b013e318196c3e7.
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, doi:10.3390/nu11081837.
- Hatcher, H.C.; Torti, F.M.; Torti, S.V. Curcumin, Oxidative Stress, and Cancer Therapy. In Oxidative Stress in Cancer Biology and Therapy; Springer Science and Business Media LLC: Berlin, Germany, 2012; pp. 233–256.
- Kuttan, R.; Sudheeran, P.; Josph, C. Turmeric and Curcumin as Topical Agents in Cancer Therapy. Tumori J. 1987, 73, 29–31, doi:10.1177/030089168707300105.
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L.; et al. Phase IIa Clinical Trial of Curcumin for the Prevention of Colorectal Neoplasia. Cancer Prev. Res. 2011, 4, 354–364, doi:10.1158/1940-6207.capr-10-0098.
- Cruz–Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination Treatment With Curcumin and Quercetin of Adenomas in Familial Adenomatous Polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038, doi:10.1016/j.cgh.2006.03.020.
- He, Z.; Shi, C.-B.; Wen, H.; Li, F.-L.; Wang, B.-L.; Wang, J. Upregulation of p53 Expression in Patients with Colorectal Cancer by Administration of Curcumin. Cancer Investig. 2011, 29, 208–213, doi:10.3109/07357907.2010.550592.
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 2010, 70, 1127–1133, doi:10.1002/pros.21147.
- Mahammedi, H.; Planchat, E.; Pouget, M.; Durando, X.; Curé, H.; Guy, L.; Van-Praagh, I.; Savareux, L.; Atger, M.; Bayet-Robert, M.; et al. The New Combination Docetaxel, Prednisone and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study. Oncology 2016, 90, 69–78, doi:10.1159/000441148.
- Thomas, R.J.; Williams, M.M.A.; Sharma, H.; Chaudry, A.; Bellamy, P. A double-blind, placebo RCT evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer: The U.K. National Cancer Research Network (NCRN) Pomi-T study. J. Clin. Oncol. 2013, 31, doi:10.1200/jco.2013.31.15_suppl.5008.
- Taleban, R.R.F.-A.; Hejazi, J. A Pilot Clinical Trial of Radioprotective Effects of Curcumin Supplementation in Patients with Prostate Cancer. J. Cancer Sci. Ther. 2013, 5, 320–324, doi:10.4172/1948-5956.1000222.
- Hejazi, J.; Rastmanesh, R.; Taleban, F.-A.; Molana, S.-H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85, doi:10.1080/01635581.2016.1115527.
- Bayet-Robert, M.; Kwiatkowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14, doi:10.4161/cbt.9.1.10392.
- Ryan, J.L.; Heckler, C.E.; Guido, J.J.; Peoples, A.R.; Gewandter, J.S.; Ling, M.; Vinciguerra, V.P.; Anderson, T.; Evans, L.; Wade, J.; et al. Oral curcumin for radiation dermatitis: A URCC NCORP study of 686 breast cancer patients. Support. Care Cancer 2017, 26, 1543–1552, doi:10.1007/s00520-017-3957-4.
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499, doi:10.1158/1078-0432.ccr-08-0024.
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2010, 68, 157–164, doi:10.1007/s00280-010-1470-2.
- Devi, K.P.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.M.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028, doi:10.1007/s13277-016-5183-y.
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299, doi:10.1111/j.1440-1681.2011.05648.x.
- Libermann, T.A.; Zerbini, L.F. Targeting Transcription Factors for Cancer Gene Therapy. Curr. Gene Ther. 2006, 6, 17–33, doi:10.2174/156652306775515501.
- Xiang, L.; He, B.; Liu, Q.; Hu, D.; Liao, W.; Li, R.; Peng, X.; Wang, Q.; Zhao, G. Antitumor effects of curcumin on the proliferation, migration and apoptosis of human colorectal carcinoma HCT 116 cells. Oncol. Rep. 2020, 44, 1997–2008, doi:10.3892/or.2020.7765.
- Juturu, V.; Sahin, K.; Pala, R.; Tuzcu, M.; Ozdemir, O.; Orhan, C.; Sahin, N. Curcumin prevents muscle damage by regulating NF-kB and Nrf2 pathways and improves performance: An in vivo model. J. Inflamm. Res. 2016, 9, 147–154, doi:10.2147/jir.s110873.
- Vadhan-Raj, S.; Weber, D.M.; Wang, M.; Giralt, S.A.; Thomas, S.K.; Alexanian, R.; Zhou, X.; Patel, P.; Bueso-Ramos, C.E.; Newman, R.A.; et al. Curcumin Downregulates NF-kB and Related Genes in Patients with Multiple Myeloma: Results of a Phase I/II Study. Blood 2015, 110, 1177.
- Wagner, E.F. Functions of AP1 (Fos/Jun) in bone development. Ann. Rheum. Dis. 2002, 61, 40–42, doi:10.1136/ard.61.suppl_2.ii40.
- Dorai, T.; Aggarwal, B.B. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004, 215, 129–140, doi:10.1016/j.canlet.2004.07.013.
- Bierhaus, A.; Zhang, Y.; Quehenberger, P.; Luther, T.; Haase, M.; Müller, M.; Mackman, N.; Ziegler, R.; Nawroth, P.P. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb. Haemost. 1997, 77, 772–782.
- Han, S.-S.; Keum, Y.-S.; Seo, H.-J.; Surh, Y.-J. Curcumin Suppresses Activation of NF-kappaB and AP-1 Induced by Phorbol Ester in Cultured Human Promyelocytic Leukemia Cells. J. Biochem. Mol. Biol. 2002, 35, 337–342.
- Balasubramanian, S.; Eckert, R.L. Curcumin Suppresses AP1 Transcription Factor-dependent Differentiation and Activates Apoptosis in Human Epidermal Keratinocytes. J. Biol. Chem. 2007, 282, 6707–6715, doi:10.1074/jbc.m606003200.
- Nakamura, K.; Yasunaga, Y.; Segawa, T.; Ko, D.; Moul, J.W.; Srivastava, S.; Rhim, J.S. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int. J. Oncol. 2002, 21, 825–830, doi:10.3892/ijo.21.4.825.
- Teiten, M.-H.; Gaascht, F.; Eifes, S.; Dicato, M.; Han, B.W. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010, 5, 61–74, doi:10.1007/s12263-009-0152-3.
- Mishra, A.; Kumar, R.; Tyagi, A.; Kohaar, I.; Hedau, S.; Bharti, A.C.; Sarker, S.; Dey, D.; Saluja, D.; Das, B. Curcumin modulates cellular AP-1, NF-kB, and HPV16 E6 proteins in oral cancer. Ecancermedicalscience 2015, 9, doi:10.3332/ecancer.2015.525.
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention. Annu. Rev. Med. 2015, 66, 311–328, doi:10.1146/annurev-med-051113-024537.
- Yang, C.-L.; Liu, Y.; Ma, Y.-G.; Xue, Y.-X.; Liu, D.-G.; Ren, Y.; Liu, X.-B.; Li, Y.; Li, Z. Curcumin Blocks Small Cell Lung Cancer Cells Migration, Invasion, Angiogenesis, Cell Cycle and Neoplasia through Janus Kinase-STAT3 Signalling Pathway. PLoS ONE 2012, 7, e37960, doi:10.1371/journal.pone.0037960.
- Kroon, P.; Berry, P.A.; Stower, M.J.; Rodrigues, G.; Mann, V.M.; Simms, M.; Bhasin, D.; Chettiar, S.; Li, C.; Li, P.-K.; et al. JAK-STAT Blockade Inhibits Tumor Initiation and Clonogenic Recovery of Prostate Cancer Stem-like Cells. Cancer Res. 2013, 73, 5288–5298, doi:10.1158/0008-5472.can-13-0874.
- Weissenberger, J.; Priester, M.; Bernreuther, C.; Rakel, S.; Glatzel, M.; Seifert, V.; Kögel, D. Dietary Curcumin Attenuates Glioma Growth in a Syngeneic Mouse Model by Inhibition of the JAK1,2/STAT3 Signaling Pathway. Clin. Cancer Res. 2010, 16, 5781–5795, doi:10.1158/1078-0432.ccr-10-0446.
- Blasius, R.; Reuter, S.; Henry, E.; Dicato, M.; Diederich, M. Curcumin regulates signal transducer and activator of transcription (STAT) expression in K562 cells. Biochem. Pharmacol. 2006, 72, 1547–1554, doi:10.1016/j.bcp.2006.07.029.
- Saydmohammed, M.; Joseph, D.; Syed, V. Curcumin suppresses constitutive activation of STAT-3 by up-regulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J. Cell. Biochem. 2010, 110, 447–456, doi:10.1002/jcb.22558.
- Kumar, A.; Dhawan, S.; Hardegen, N.J.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Inhibition of Tumor Necrosis Factor (TNF)-Mediated Adhesion of Monocytes to Endothelial Cells by Suppression of Cell Surface Expression of Adhesion Molecules and of Nuclear Factor-kappaB Activation. Biochem. Pharmacol. 1998, 55, 775–783.
- Kang, E.S.; Woo, I.S.; Kim, H.J.; Eun, S.Y.; Paek, K.S.; Chang, K.C.; Lee, J.H.; Lee, H.T.; Kim, J.-H.; Kim, H.J.; et al. Up-regulation of aldose reductase expression mediated by phosphatidylinositol 3-kinase/Akt and Nrf2 is involved in the protective effect of curcumin against oxidative damage. Free. Radic. Biol. Med. 2007, 43, 535–545, doi:10.1016/j.freeradbiomed.2007.05.006.
- Kahl, G. Nuclear Factor. In The Dictionary of Genomics, Transcriptomics and Proteomics; Wiley: Hoboken, NJ, USA, 2015; Volume 103, p. 1.
- Bharti, A.C.; Donato, N.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Inhibits Constitutive and IL-6-Inducible STAT3 Phosphorylation in Human Multiple Myeloma Cells. J. Immunol. 2003, 171, 3863–3871, doi:10.4049/jimmunol.171.7.3863.
- Chen, W.; Chen, Y.; Cui, G.-H.; Gu, J.; Hu, N.; Li, X.-G. Effect of curcumin on STAT5 signaling pathway in primary CML cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2004, 12, 572–576.
- Chen, W.; Chen, Y.; Gu, J.; He, J. Effect of curcumin on STAT5 signaling molecule in K562 cells. Zhonghua Xue Ye Xue Za Zhi 2004, 25, 151–153.
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. Beta-Catenin-Mediated Transactivation and Cell-Cell Adhesion Pathways Are Important in Curcumin (Diferuylmethane)-Induced Growth Arrest and Apoptosis in Colon Cancer Cells. Oncogene 2002, 21, 8414–8427.
- Park, C.H.; Hahm, E.R.; Park, S.; Kim, H.-K.; Yang, C.H. The inhibitory mechanism of curcumin and its derivative against β-catenin/Tcf signaling. FEBS Lett. 2005, 579, 2965–2971, doi:10.1016/j.febslet.2005.04.013.
- Pendurthi, U.R.; Rao, L.M. Suppression of Transcription Factor Egr-1 by Curcumin. Thromb. Res. 2000, 97, 179–189, doi:10.1016/s0049-3848(99)00148-6.
- Chen, A.; Xu, J.; Johnson, A.C. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 2006, 25, 278–287, doi:10.1038/sj.onc.1209019.
- Bae, M.-K.; Kim, S.-H.; Jeong, J.-W.; Lee, Y.M.; Kim, H.-S.; Kim, S.-R.; Yun, I.; Bae, S.-K.; Kim, K.-W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol. Rep. 2006, 15, 1557–1562, doi:10.3892/or.15.6.1557.
- Wang, Z.; Zhang, Y.; Banerjee, S.; Li, Y.; Sarkar, F.H. Retracted: Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer 2006, 106, 2503–2513, doi:10.1002/cncr.21904.
- Zhou, Y.; Zheng, S.; Lin, J.; Zhang, Q.-J.; Chen, A. The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARγ in rat activated hepatic stellate cell in vitro. Lab. Investig. 2007, 87, 488–498, doi:10.1038/labinvest.3700532.
- Mohan, R.; Sivak, J.; Ashton, P.; Russo, L.A.; Pham, B.Q.; Kasahara, N.; Raizman, M.B.; Fini, M.E. Curcuminoids Inhibit the Angiogenic Response Stimulated by Fibroblast Growth Factor-2, Including Expression of Matrix Metalloproteinase Gelatinase B. J. Biol. Chem. 2000, 275, 10405–10412, doi:10.1074/jbc.275.14.10405.
- Yang, X.; Thomas, D.P.; Zhang, X.; Culver, B.W.; Alexander, B.M.; Murdoch, W.J.; Rao, M.N.; Tulis, D.A.; Ren, J.; Sreejayan, N. Curcumin Inhibits Platelet-Derived Growth Factor–Stimulated Vascular Smooth Muscle Cell Function and Injury-Induced Neointima Formation. Arter. Thromb. Vasc. Biol. 2006, 26, 85–90, doi:10.1161/01.atv.0000191635.00744.b6.
- Santibanez, J.F.; Quintanilla, M.; Martínez, J. Genistein and Curcumin Block TGF-β1-Induced u-PA Expression and Migratory and Invasive Phenotype in Mouse Epidermal Keratinocytes. Nutr. Cancer 2000, 37, 49–54, doi:10.1207/s15327914nc3701_6.
- Gaedeke, J.; Noble, N.A.; Border, W.A. Curcumin blocks multiple sites of the TGF-β signaling cascade in renal cells. Kidney Int. 2004, 66, 112–120, doi:10.1111/j.1523-1755.2004.00713.x.
- Hu, Y.; Liang, H.; Du, Y.; Zhu, Y.; Wang, X. Curcumin Inhibits Transforming Growth Factor-β Activity via Inhibition of Smad Signaling in HK-2 Cells. Am. J. Nephrol. 2010, 31, 332–341, doi:10.1159/000287230.
- Song, K.; Peng, S.; Sun, Z.; Li, H.; Yang, R. Curcumin suppresses TGF-β signaling by inhibition of TGIF degradation in scleroderma fibroblasts. Biochem. Biophys. Res. Commun. 2011, 411, 821–825, doi:10.1016/j.bbrc.2011.07.044.
- Zhang, L.; Cheng, X.; Gao, Y.; Zhang, C.; Bao, J.; Guan, H.; Yu, H.; Lu, R.; Xu, Q.; Sun, Y. Curcumin Inhibits Metastasis in Human Papillary Thyroid Carcinoma BCPAP Cells via down-Regulation of the TGF-β/Smad2/3 Signaling Pathway. Exp. Cell Res. 2016, 341, 157–165.
- Chakraborty, G.; Jain, S.; Kale, S.; Raja, R.; Kumar, S.; Mishra, R.; Kundu, G.C. Curcumin suppresses breast tumor angiogenesis by abrogating osteopontin-induced VEGF expression. Mol. Med. Rep. 2008, 1, 641–646, doi:10.3892/mmr_00000005.
- Yoysungnoen, P.; Wirachwong, P.; Changtam, C.; Suksamrarn, A.; Patumraj, S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J. Gastroenterol. 2008, 14, 2003–2009, doi:10.3748/wjg.14.2003.
- Ferreira, L.C.; Arbab, A.S.; Jardim-Perassi, B.V.; Borin, T.F.; Gonçalves, N.N.; Nadimpalli, R.S.V.; Zuccari, D.A.P.D.C. Abstract A02: Effect of curcumin on the tumor growth and angiogenesis of breast cancer. Tumor-Assoc. Blood Vessels Lymph. 2015, 75, A02, doi:10.1158/1538-7445.chtme14-a02.
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225, doi:10.1016/j.canlet.2008.03.009.
- Aggarwal, B.B.; Bhatt, I.D.; Ichikawa, H. Curcumin-Biological and Medicinal Properties. In Tumeric: The Genus Curcuma; CRC Press: Boca Raton, FL, USA, 2006; pp 297–368.
- Aggarwal, B.B. Activation of Transcription Factor NF-kappaB Is Suppressed by Curcumin (Diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000.
- Cho, J.-W.; Lee, K.-S.; Kim, C.-W. Curcumin Attenuates the Expression of IL-1beta, IL-6, and TNF-Alpha as Well as Cyclin E in TNF-Alpha-Treated HaCaT Cells; NF-kappaB and MAPKs as Potential Upstream Targets. Int. J. Mol. Med. 2007, 19, 469–474.
- Ranjan, D.; Chen, C.; Johnston, T.D.; Jeon, H.; Nagabhushan, M. Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFκB activation, and IL-2 signaling. J. Surg. Res. 2004, 121, 171–177, doi:10.1016/j.jss.2004.04.004.
- Kobayashi, T.; Hashimoto, S.; Horie, T. Curcumin inhibition of Dermatophagoides farinea-induced interleukin-5 (IL-5) and granulocyte macrophage-colony stimulating factor (GM-CSF) production by lymphocytes from bronchial asthmatics. Biochem. Pharmacol. 1997, 54, 819–824, doi:10.1016/s0006-2952(97)00220-7.
- Fahey, A.J.; Robins, R.A.; Constantinescu, C.S. Curcumin modulation of IFN-β and IL-12 signalling and cytokine induction in human T cells. J. Cell. Mol. Med. 2007, 11, 1129–1137, doi:10.1111/j.1582-4934.2007.00089.x.
- Grandjean-Laquerriere, A.; Antonicelli, F.; Gangloff, S.C.; Guenounou, M.; Le Naour, R. UVB-Induced IL-18 Production in Human Keratinocyte Cell Line NCTC 2544 through NF-κB Activation. Cytokine 2007, 37, 76–83.
- Chun, K.-S.; Keum, Y.-S.; Han, S.S.; Song, Y.-S.; Kim, S.-H.; Surh, Y.-J. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF- B activation. Carcinogenesis 2003, 24, 1515–1524, doi:10.1093/carcin/bgg107.
- Camacho-Barquero, L.; Villegas, I.; Sánchez-Calvo, J.M.; Talero, E.; Sánchez-Fidalgo, S.; Motilva, V.; De La Lastra, C.A. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int. Immunopharmacol. 2007, 7, 333–342, doi:10.1016/j.intimp.2006.11.006.
- Huang, M.T.; Lysz, T.; Ferraro, T.; Abidi, T.F.; Laskin, J.D.; Conney, A.H. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991, 51, 813–9.
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin Potentiates Antitumor Activity of Gemcitabine in an Orthotopic Model of Pancreatic Cancer through Suppression of Proliferation, Angiogenesis, and Inhibition of Nuclear Factor-κB–Regulated Gene Products. Cancer Res. 2007, 67, 3853–3861, doi:10.1158/0008-5472.can-06-4257.
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.S.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin Inhibits Tumor Growth and Angiogenesis in Ovarian Carcinoma by Targeting the Nuclear Factor- B Pathway. Clin. Cancer Res. 2007, 13, 3423–3430, doi:10.1158/1078-0432.ccr-06-3072.
- Woo, M.-S.; Jung, S.-H.; Kim, S.-Y.; Hyun, J.-W.; Ko, K.-H.; Kim, W.-K.; Kim, H.-S. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem. Biophys. Res. Commun. 2005, 335, 1017–1025, doi:10.1016/j.bbrc.2005.07.174.
- Chen, Y.-R.; Tan, T.-H. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 1998, 17, 173–178, doi:10.1038/sj.onc.1201941.
- Salh, B.; Assi, K.; Templeman, V.; Parhar, K.K.S.; Owen, D.; Gomez-Muñoz, A.; Jacobson, K. Curcumin attenuates DNB-induced murine colitis. Am. J. Physiol. Liver Physiol. 2003, 285, G235–G243, doi:10.1152/ajpgi.00449.2002.
- Hussain, A.R.; Al-Rasheed, M.; Manogaran, P.S.; Al-Hussein, K.A.; Platanias, L.C.; Al Kuraya, K.; Uddin, S. Curcumin induces apoptosis via inhibition of PI3′-kinase/AKT pathway in Acute T cell Leukemias. Apoptosis 2006, 11, 245–254, doi:10.1007/s10495-006-3392-3.
- Surh, Y.-J.; Lee, S.-Y.; Huang, Z.; Lim, D.Y.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin Suppresses Proliferation of Colon Cancer Cells by Targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474, doi:10.1158/1940-6207.capr-13-0387.
- Bush, J.A.; Cheung, K.-J.J.; Li, G. Curcumin Induces Apoptosis in Human Melanoma Cells through a Fas Receptor/Caspase-8 Pathway Independent of p53. Exp. Cell Res. 2001, 271, 305–314, doi:10.1006/excr.2001.5381.
- Anto, R.J.; Mukhopadhyay, A.; Denning, K.; Aggarwal, B.B. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: Its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis 2002, 23, 143–150, doi:10.1093/carcin/23.1.143.
- Lin, S.-S.; Huang, H.-P.; Yang, J.-S.; Wu, J.-Y.; Hsai, T.-C.; Lin, C.-C.; Lin, C.-W.; Kuo, C.-L.; Wood, W.G.; Chung, J. DNA damage and endoplasmic reticulum stress mediated curcumin-induced cell cycle arrest and apoptosis in human lung carcinoma A-549 cells through the activation caspases cascade- and mitochondrial-dependent pathway. Cancer Lett. 2008, 272, 77–90, doi:10.1016/j.canlet.2008.06.031.
- Jiang, A.-J.; Jiang, G.; Li, L.-T.; Zheng, J. Curcumin induces apoptosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol. Biol. Rep. 2014, 42, 267–275, doi:10.1007/s11033-014-3769-2.
- Li, F.; Chen, X.; Xu, B.; Zhou, H. Curcumin induces p53-independent necrosis in H1299 cells via a mitochondria-associated pathway. Mol. Med. Rep. 2015, 12, 7806–7814, doi:10.3892/mmr.2015.4395.
- Shishodia, S.; Amin, H.M.; Lai, R.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Inhibits Constitutive NF-κB Activation, Induces G1/S Arrest, Suppresses Proliferation, and Induces Apoptosis in Mantle Cell Lymphoma. Biochem. Pharmacol. 2005, 70, 700–713.
- Choudhuri, T.; Pal, S.; Das, T.; Sa, G. Curcumin Selectively Induces Apoptosis in Deregulated Cyclin D1-expressed Cells at G2Phase of Cell Cycle in a p53-dependent Manner. J. Biol. Chem. 2005, 280, 20059–20068, doi:10.1074/jbc.m410670200.
- Srivastava, R.K.; Chen, Q.; Siddiqui, I.; Sarva, K.; Shankar, S. Linkage of Curcumin-Induced Cell Cycle Arrest and Apoptosis by Cyclin-Dependent Kinase Inhibitor p21/WAF1/CIP1. Cell Cycle 2007, 6, 2953–2961.
- Dinarello, C.A. The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev. 2006, 25, 307–313, doi:10.1007/s10555-006-9000-8.
- Williams, C.S.; Mann, M.; Dubois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916, doi:10.1038/sj.onc.1203286.
- Kumar, A.; Dhawan, S.; Mukhopadhyay, A.; Aggarwal, B.B. Human Immunodeficiency Virus-1-Tat Induces Matrix Metalloproteinase-9 in Monocytes through Protein Tyrosine Phosphatase-Mediated Activation of Nuclear Transcription Factor NF-kappaB. FEBS Lett. 1999, 462, 140–144.
- John, P.C.L.; Mews, M.; Moore, R. Cyclin/cdk complexes: Their involvement in cell cycle progression and mitotic division. Protoplasma 2001, 216, 119–142, doi:10.1007/bf02673865.
- Shankar, S.; Srivastava, R.K. Involvement of Bcl-2 family members, phosphatidylinositol 3’-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 2007, 30, 905–918, doi:10.3892/ijo.30.4.905.




