
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lucio Diaz-Flores | + 4190 word(s) | 4190 | 2021-01-05 10:04:52 | | | |
| 2 | Rita Xu | -1255 word(s) | 2935 | 2021-01-12 04:57:45 | | |
Video Upload Options
The pathologic processes in which TCs/CD34+SCs are studied in adipose tissue include inflammation and repair through granulation tissue, iatrogenic insulin-amyloid type amyloidosis, non-adipose tissue components (nerve fascicles and fibres in neuromas and hyperplastic neurogenic processes) and tumours (signet ring carcinoma with Krukenberg tumour and colon carcinoma) growing in adipose tissue, adipose tissue tumours (spindle cell lipoma, dendritic fibromyxolipoma, pleomorphic lipoma, infiltrating angiolipoma of skeletal muscle and elastofibrolipoma), lipomatous hypertrophy of the interatrial septum, nevus lipomatosus cutaneous superficialis of Hoffman–Zurhelle and irradiated adipose tissue of the perirectal and thymic regions.
1. Introduction
White adipose tissue is formed by unilocular adipocytes and the extracellular vascular fraction, which includes endothelial cells of both blood and lymphatic vessels, pericytes, Schwann cells of peripheral nerves, fibroblasts, macrophages and telocytes/CD34+ stromal cells (TCs/CD34+SCs). Adipose tissue participates in (a) fat storage (triglycerides) (energy storage), (b) the production of hormones (e.g., lectin, adiponectin and resistin), growth factors and cytokines, (c) thermal regulation, mainly subcutaneous fact and (d) support and mechanical protection (lower effect of impacts on organs). Other “non-traditional” functions, widely reviewed in different locations by Zwick et al. (2017) [1], include (a) the modulation of tissue growth and regeneration by paracrine signals (e.g., progenitor signalling to hair follicles, mammary gland and bone marrow cell components), (b) participation in repair and cancer by crosstalk signalling and (c) the contribution to innate immunity in the skin and intestine by cytokines and antimicrobial peptides. In addition, human adipose tissue is an abundant and easily procured source of CD34+ adipose-derived stromal cells (ASCs) for studies in vitro and tissue engineering, which have received great attention [2][3][4][5].
Telocytes are a type of stromal cell. Under electron microscopy and in normal conditions, they show a small somatic body and two or several long, slender, moniliform cytoplasmic processes (telopodes) with podomeres (thin segments) and podoms (dilated portions) [6][7]. Since their discovery in 2010 [7], telocytes have been described in the interstitium of many tissues, including adipose tissue. However, no review has focused on studying the current state of telocyte behaviour in adipose tissue affected by pathologic processes. Telocytes express CD34, among other markers, and therefore correspond to the CD34+ stromal cells observed in light microscopy [8][9][10][11][12][13][14][15][16][17]. This immunohistochemical expression of telocytes, which allows us to consider them as telocytes/CD34+stromal cells (TCs/CD34+SCs), facilitates the correlation with previous descriptions or original observations of CD34+ resident/native stromal/interstitial cells in pathologic adipose tissue.
2. TCs/CD34+SCs in Normal Adipose Tissue
In adipose tissue, TCs/CD34+SCs are widely distributed in the septa and to a lesser extent in intralobular regions (Figure 1A). This distribution is important because different progenitor roles have been attributed to these cells, depending on their septal or intralobular location [18] (see below). In intralobular regions, TCs/CD34+SCs show a small somatic body and long, thin processes, which extend alongside adipocytes (Figure 1B–D) and the microvasculature (Figure 1E,F). Extracellular multivesicular bodies are present near telopodes (Figure 1F), a finding previously described in other tissues, with functional importance in intercellular communication [19] and in accordance with the hypothesis about the role of TCs/CD34+SCs in regulating the local microenvironment by cell-to-cell contacts and extracellular shedding vesicles [6][9][10][20][21][22][23]. In the septa, these cells form a network, which surrounds differently sized vessels (location in the vessel adventitia) and between collagen fibres. Thus, when stained with anti-CD34, the vessels show a double-ring appearance with two CD34-stained concentric circles “sandwiching” the unstained media layer (Figure 1G). Indeed, by double staining with anti-CD34 and anti-αSMA (α smooth muscle actin), the blood vessels in the adipose tissue present CD34+ αSMA- endothelial cells (ECs) in the intima layer, CD34- αSMA+ pericytes/vascular smooth muscle cells in the media layer and CD34+ αSMA- stromal cells in the external layer (insert in Figure 1G). The long, thin processes of the TCs/CD34+SCs in the external layer of these vessels also extend into the collagenous tissue of the septa. The small lymphatic vessels in adipose tissue present TCs/CD34+SCs around ECs. TCs/CD34+SCs are also observed surrounding small groups of smooth muscle cells in the pre-collector and collector lymphatic vessels (Figure 1H,I).
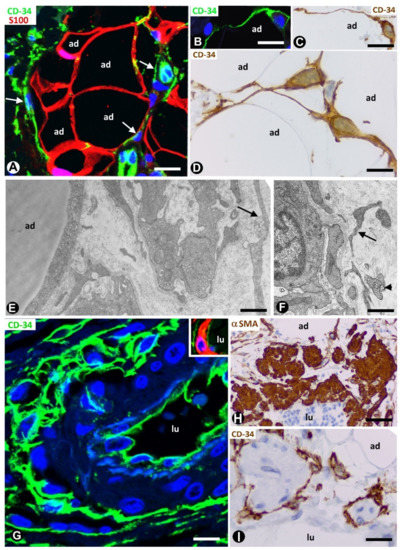
Figure 1. Telocytes/CD34+ stromal cells (TCs/CD34+SCs) in the normal adipose tissue. (A): TCs/CD34+SCs (green, arrows) in a septum and in a lobular region, in which adipocytes (ad) express S100 protein (red). (B–D): TCs/CD34+SCs (green in B, and brown in C and D) with small somatic bodies and long, thin processes between adipocytes (ad). (E,F): telopodes of telocytes (arrows) are ultrastructurally observed around small vessels. Note in F an extracellular multivesicular body (arrowhead) next to the telopodes. G and insert: a large septal vessel showing a double-ring appearance with anti-CD34 staining (green). Note that CD34 stained ECs in the intima and TCs/CD34+SCs in the adventitia form two stained circles “sandwiching” the unstained (G) or red αSMA stained (Insert) media layer. (H,I): lymphatic collectors in which parietal smooth muscle cells (expressing αSMA, H) are surrounded by TCs/CD34+SCs (I). Lumen = lu in blood (G) and lymphatic (H,I) vessels. A: double immunofluorescence labelling for CD34 (green) and protein S100 (red). B and G: immunofluorescence labelling for CD34 (green). C, D and I: immunochemistry for CD34. E and F: ultrathin sections. Uranyl acetate and lead citrate. Insert of G: double immunofluorescence staining for CD34 (green) and αSMA (red). H: immunochemistry for αSMA (brown). DAPI (4′,6-diamidino-2-phenylindole) (blue) counterstain for nuclei in A, B, G and insert of G, and haematoxylin counterstain in C, D, H and I. Bar: (A,H,I): 50 µm; (B,C,G): 30 µm; (D): 20 µm; (E,F): 4 µm.
3. TCs/CD34+SCs in Pathologically Affected Adipose Tissue
3.1. TCs/CD34+SCs of Adipose Tissue in Early and Most Advanced Stages of Inflammation and Repair through Granulation Tissue
Native (resident) TCs/CD34+SCs lose their CD34 expression during inflammatory/repair processes in adipose tissue, while numerous cells expressing αSMA are seen in the affected tissue region [24][25]. Successive culture passages of CD34+ cells that form part of the freshly isolated stromal fraction obtained from adipose tissue give a similar finding. Indeed, the CD34 expression of these cells only occurs during early passages [2][3][4][5]. In successive passages, the loss of CD34 expression is followed by the acquisition of other markers, depending on culture conditions [2][3][4][5]. Thus, CD34+ cells adhere to culture plastic, proliferate and differentiate in several tissue components, including adipocytes, chondrocytes, osteoblasts and myocytes. αSMA is among the new markers and when it is expressed, the cells acquire a myofibroblastic-like aspect. In human adipose tissue with inflammatory/repair processes (peri-appendicitis, peri-diverticulitis and actinomycosis with localised peritoneal abscesses), the number, morphology and expression of the markers in the stromal cells depend on the evolutionary stage and the distance from the lesion location. Thus, in early stages, TCs/CD34+SCs increase the somatic volume and size of the nucleus (Figure 2A,B), which present one or two prominent nucleoli. Frequent mitoses and a high proliferative index are seen in these cells, which show the co-expression of CD34 and Ki-67 (Figure 2C,D). Subsequently, the co-expression of CD34 and αSMA can be observed in some stromal cells (Figure 2E–G). In the most advanced stages, the stromal cells express αSMA (Figure 2H). Therefore, resident TCs/CD34+SCs in adipose tissue may be a source of stromal αSMA+ cells with a myofibroblastic aspect. As for the influence of the distance from the lesion location, it has been demonstrated that the increased number and size of activated stromal cells close to actinomycotic abscesses show αSMA expression, while those further away retain their CD34 expression [24][26].
Repair includes two types of processes: regeneration and repair through granulation tissue. The participation of TCs/CD34+SCs in regeneration has been well studied in several tissues and organs [25][27][28][29][30][31][32][33][34][35][36][37][38][39][40], while the role of these cells in repair through granulation tissue has received less attention [24][25]. In this provisional tissue, myofibroblasts are a main component, with associated macrophages and numerous small blood vessels. Granulation tissue may remain as such (mainly in the stroma of certain tumours, see below) or may be followed by (a) fibrous tissue, with scarring or organisation (formation of new masses of fibrous tissue in blood clots or inflammatory exudates with fibrin deposits) and (b) other tissues, such as cartilage and bone. Adipose tissue, plastic, abundant and rich in CD34+ resident stromal cells, is therefore highly suitable for correlating the behaviour of TCs/CD34+SCs in in vivo and in vitro studies and for following the changes of these cells in early stages of repair to granulation tissue. Thus, the contributions in this section confirm the previous observations of our group supporting the hypothesis of TCs/CD34+SCs as precursor cells [24][25].
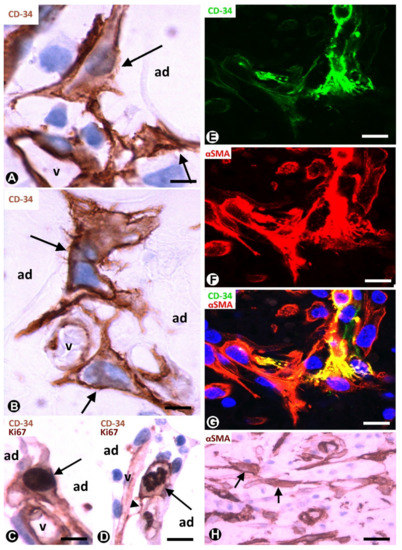
Figure 2. TCs/CD34+SCs of the adipose tissue in early (A–G) and most advanced (H) stages of repair through granulation tissue. (A,B): bulky TCs/CD34+SCs (arrows) with enlarged nuclei are observed around small vessels (v) and adipocytes (ad). (C): the co-expression of CD34 and Ki-67 in a TC/CD34+SC (CD34 express in the membrane and in the periphery of cytoplasm, and Ki-67 in the nucleus) (arrow). (D): the co-expression of CD34 (in the membrane and peripheral cytoplasm) and Ki 67 (in chromosomes) is shown in a TC/CD34+SC in mitosis (arrow). Note in this cell a thin process (telopode, arrowhead) in contact with a capillary (v). (E–G) the co-expression of CD34 (green) and αSMA (red) in stromal cells. H: stromal cells expressing αSMA are present in an advanced stage of repair. A and B: immunochemistry for CD34. (C,D): sections are doubly immunostained with CD34 and Ki-67 (CD34 stained the cell membrane and peripheral cytoplasm, and Ki-67 the nucleus). (E–G): confocal microscopy, frontal view, immunofluorescent label with anti-CD34 (green) and anti-αSMA (red). (H): immunochemistry for αSMA. Haematoxylin contrast in A–D and H. DAPI (blue) counterstain in G. Bar: (A,B): 10 µm; (C–G): 20 µm; (H): 40 µm.
3.2. TCs/CD34+SCs in Adipose Tissue with Iatrogenic Insulin-Amyloid Type Amyloidosis
A particularly good example of TCs/CD34+SCs in the adipose tissue pathology of endocrine origin is the involvement of subcutaneous adipose tissue in iatrogenic insulin-amyloid type amyloidosis. Indeed, insulin-derived amyloidosis can occur in adipose tissue at sites of repeated insulin administration (insulin injection amyloidosis, iatrogenic insulin-amyloid type, injected-localised insulin amyloid, insulin balls, amyloidoma).
In a study of this rare process using morphological and immunohistochemical procedures, we observed numerous TCs/CD34+SCs in differently sized amyloidotic nodules in subcutaneous adipose tissue (Figure 3A) (unpublished observations). TCs/CD34+SCs are present around thick deposits (with a sheet aspect) containing collagen and amyloid (see below) and vessels in the amyloid nodules, especially in the smallest. TC telopodes surrounding amyloid deposits have been described in the human heart (isolated atrial amyloidosis) [41]. Amyloidosis in the heart differs from insulin-derived amyloidosis because of the association of amyloid material with collagen in the latter. Then, we will examine amyloid deposits, and the behaviour of TCs/CD34+SCs in amyloidogenic nodules in adipose tissue.
In the nodules (Figure 3A, insert), the amyloid deposits are located near adipocytes (Figure 3A) and show immunostaining for anti-insulin (Figure 3B) and amyloid P (Figure 3C), as well as Congo red positivity (Figure 3D), with yellow-green birefringence (Figure 3E). The amyloid deposits are negative for anti-A-amyloid and anti-transthyretin. Under confocal microscopy, the colocalisation of collagen I and insulin is demonstrated in the amyloid deposits (Figure 3F–H). TCs/CD34+SCs are increased in number and size, and are arranged in close association with these extracellular deposits formed by amyloid and collagen I. Indeed, TCs/CD34+SCs show ovoid or elongated nuclei and bipolar or multipolar cytoplasmic processes (telopodes), which surround the amyloid deposits (Figure 3I,J) and adipocytes (Figure 3K). Telopodes encircling the amyloid deposits can be related to the role of TCs in organising and controlling the extracellular matrix. Thus, the ability of these cells to limit amyloid spread has been suggested in atrial amyloidosis [41]. TCs/CD34+SCs are negative for anti-CD31, anti-CD45, anti-αSMA and anti-h-caldesmon. In the vessels, TCs/CD34+SCs are also located around amyloid and collagenous materials, which are deposited in the transition between the media layer and the adventitia (Figure 3L). In the central areas of the largest nodules, in which amyloid and collagenous deposits converge, the number of adipocytes, vessels and TCs/CD34+SCs decreases dramatically. Insulin amyloid has toxic action [42] and the decline in adipocytes, vessels and TCs/CD34+SCs in the centre of large nodules may be due to this action. Isolated CD68+ macrophages are present, some with a signet ring aspect. The association of TCs/CD34+SCs and macrophages is also observed (the last two observations are shown). In this section, we therefore contribute the presence of TCs/CD34+SCs (in high numbers) in insulin-derived amyloid type amyloidosis, and their possible role as regulators of local homeostasis.
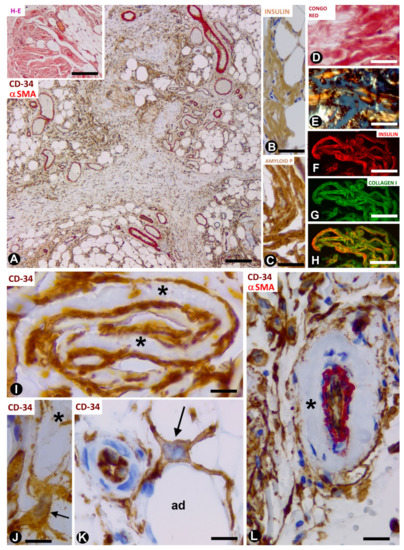
Figure 3. TCs/CD34+SCs in adipose tissue with iatrogenic insulin-amyloid type amyloidosis. (A): numerous TCs/CD34+SCs in adipose lobules are arranged between fibrous tracts. Double immunochemistry labelling for CD34 (brown) and αSMA (red). Insert: a nodule with haematoxylin staining. (B–H): amyloid deposits showing immunochemistry labelling for insulin (B) and amyloid P (C), Congo red positivity (D) with yellow-green birefringence under polarised light (E), and the colocalisation of insulin and collagen I (F: insulin—red; G: collagen I—green and H: the colocation of both). (I): projections of TCs/CD34+SCs (brown) around folded amyloid deposits (asterisks). (J–L): TCs/CD34+SCs (brown, arrows) with projections around an amyloid deposit (J, asterisk), adipocytes (K, ad) and amyloid components in the adventitia (asterisk) of a vessel with double immunochemistry labelling for CD34 (brown) and αSMA (red). Bar: ((A) and insert): 160 µm; (B–H): 50 µm; (I–K): 20 µm; (L): 60 µm.
3.3. TCs/CD34+SCs in Non-Adipose Pathologic Processes Growing in Adipose Tissue
In this section, we present examples of adipose tissue infiltration by pathologic processes in which resident TCs/CD34+SCs behave differently. These processes include neuromas and neurogenic hyperplasia, signet ring carcinoma with Krukenberg tumour and peritoneal dissemination, and colon adenocarcinoma.
3.3.1. TCs/CD34+SCs in Neuromas and Hyperplastic Neurogenic Processes Affecting Adipose Tissue
Neuromas can extend to adipose tissue, with the presence of nerve fascicles and isolated nerve fibres between adipocytes. These nervous components in adipose tissue can be very numerous. Using immunofluorescence in confocal microscopy, and semithin and ultrathin sections, we observe TCs/CD34+SCs around and within nerve fascicles (Figure 4A,B and inserts). Telopodes of these cells were seen near nerve fibres (Figure 4A,B and inserts 1 and 2).
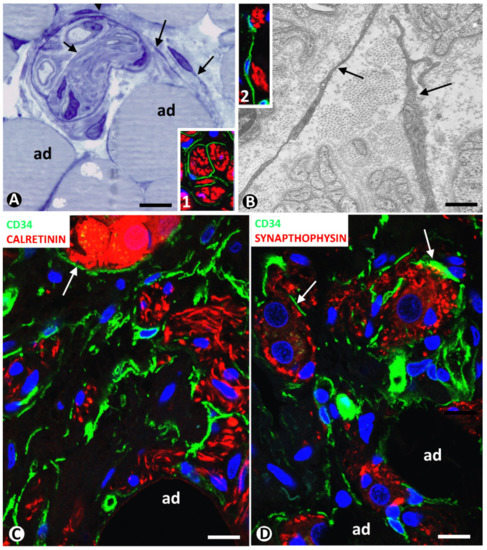
Figure 4. (A,B) TCs/CD34+SCs in neuromas. (A): a nerve fascicle between adipocytes (ad), TCs (arrows) are observed around and within the fascicle in a semithin section stained with toluidine blue. (B): ultrastructural image showing TC telopodes (arrows) around nerve fibres. Ultrathin section. Uranyl acetate and lead citrate. Inserts in A and B, TCs/CD34+SCs (green) and Schwann cells (red) shown by double immunofluorescence labelling for CD34 (green) and S100 protein (red). (C,D): TCs/CD34+SCs (green) around nerve fibres and neuro-glial units (arrows) (red) between adipocytes (ad) in a hyperplastic neurogenic process. Double immunofluorescence labelling for CD34 (green) and calretinin (C, red) or synaptophysin (D, red). Bar: (A,C,D): 40 µm; (B): 4 µm; inserts of A and B: 50 µm.
In a previous review on TCs/CD34+SCs in the peripheral nervous system, we highlighted the behaviour of these cells in neuropathies of the appendix and gallbladder [43]. Using confocal microscopy, we then studied the involvement of adipose tissue in appendicular hyperplastic neurogenic processes. TCs/CD34+SCs and their telopodes were observed around nerve fibres and their accompanying neurons (neural–glial units) in adipose tissue (Figure 4C,D). Neuromas and hyperplastic neurogenic processes are therefore examples of TC/CD34+SC activation conserving CD34 expression.
3.3.2. CD34+ and αSMA+ SCs in Tumours Infiltrating Adipose Tissue
We chose two types of infiltrating malignant epithelial neoplasms in adipose tissue as examples of the different behaviour of tissue resident TCs/CD34+SCs: with the conservation or loss of CD34 expression, and with the gain of αSMA expression. Signet ring cell carcinoma with Krukenberg tumour and peritoneal dissemination, and colon adenocarcinoma are taken into consideration below.
We observed an important increase in adipose resident TCs/CD34+SCs in signet ring cell carcinoma with Krukenberg tumour and peritoneal dissemination (Figure 5A) (original observation). A striking finding is that TCs/CD34+SCs completely surround each cell or groups of two or three neoplastic cells (Figure 5A,B,D) (unpublished observation), which show intracytoplasmic PAS (Periodic acid–Schiff) positive vacuoles (Figure 5C) and cytokeratin expression (Figure 5D). Further research is required to clarify whether this encircling action represents a nurse cell behaviour, tumour cell isolation or both (see below).
All stromal cells around neoplastic glands in the adipose tissue infiltrated by adenocarcinoma of the colon show αSMA expression (Figure 5E). The changes from TCs/CD34+SCs to stromal cells expressing αSMA in this type of advanced neoplasm cannot be well demonstrated, unlike in the early stages of repair through granulation tissue, in which follow up is possible.
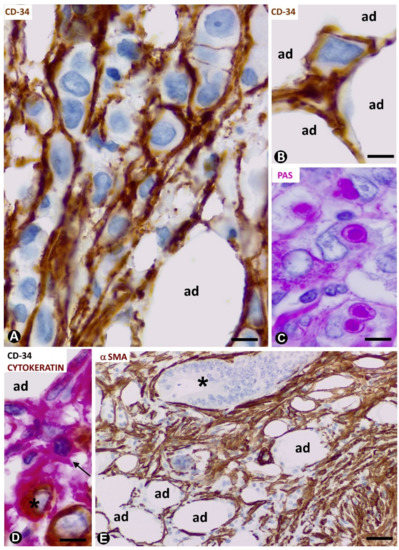
Figure 5. CD34+ and αSMA+ stromal cells in tumours infiltrating adipose tissue. (A–D): signet ring carcinoma with Krukenberg tumour and peritoneal dissemination. TCs/CD34+SCs are observed around neoplastic cells (A,B,D), which show PAS+ vacuoles (C) and express cytokeratin AE1/AE3 (D). A, B: CD34 immunochemistry; haematoxylin counterstain. C: PAS staining. D: double immunochemistry labelling for CD34 (red) and cytokeratin AE1/AE3 (brown). (E): neoplastic glands (asterisk) of adenocarcinoma of colon infiltrating adipose tissue. αSMA cells (brown) are observed in the stroma. αSMA immunochemistry. Haematoxylin counterstain. Bar: (A–D): 20 µm; (E): 100 µm.
References
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab. 2018, 27, 68–83.
- Braun, J.; Kurtz, A.; Barutcu, N.; Bodo, J.; Thiel, A.; Dong, J. Concerted regulation of CD34 and CD105 accompanies mesenchymal stromal cell derivation from human adventitial stromal cell. Stem Cells Dev. 2013, 22, 815–827.
- Lin, G.; Garcia, M.; Ning, H.; Banie, L.; Guo, Y.L.; Lue, T.F.; Lin, C.S. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008, 17, 1053–1063.
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385.
- Peng, Q.; Alipour, H.; Porsborg, S.; Fink, T.; Zachar, V. Evolution of ASC immunophenotypical subsets during expansion in vitro. J. Mol. Sci. 2020, 21, 1408.
- Faussone-Pellegrini, M.S.; Popescu, L.M. Telocytes. Concepts 2011, 2, 481–489.
- Popescu, L.M.; Faussone-Pellegrini, M.S. Telocytes—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to telocytes. Cell. Mol. Med. 2010, 14, 729–740.
- Bani, D.; Formigli, L.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. Cell. Mol. Med. 2010, 14, 2531–2538.
- Cretoiu, S.M.; Popescu, L.M. Telocytes revisited. Concepts 2014, 5, 353–369.
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L., Jr.; Madrid, J.F. Uptake and intracytoplasmic storage of pigmented particles by human CD34+ stromal cells/telocytes: Endocytic property of telocytes. Cell. Mol. Med. 2014, 18, 2478–2487.
- Marini, M.; Ibba-Manneschi, L.; Rosa, I.; Sgambati, E.; Manetti, M. Changes in the telocyte/CD34+ stromal cell and α-+ myoid cell networks in human testicular seminoma. Acta Histochem. 2019, 121, 151442.
- Nicolescu, M.I.; Rusu, M.C.; Voinea, L.M.; Vrapciu, A.D.; Bâră, R.I. Lymphatic lacunae of the human eye conjunctiva embedded within a stroma containing CD34+ J. Cell. Mol. Med. 2020, 24, 8871–8875.
- Romano, E.; Rosa, I.; Fioretto, B.S.; Lucattelli, E.; Innocenti, M.; Ibba-Manneschi, L.; Matucci-Cerinic, M.; Manetti, M. A Two-Step immunomagnetic microbead-based method for the isolation of human primary skin telocytes/CD34+ stromal cells. J. Mol. Sci. 2020, 21, 5877.
- Rosa, I.; Marini, M.; Guasti, D.; Ibba-Manneschi, L.; Manetti, M. Morphological evidence of telocytes in human synovium. Rep. 2018, 8, 3581.
- Rusu, M.C.; Mănoiu, V.S.; Creţoiu, D.; Creţoiu, S.M.; Vrapciu, A.D. Stromal cells/telocytes and endothelial progenitors in the perivascular niches of the trigeminal ganglion. Anat. 2018, 218, 141–155.
- Zhou, Q.; Wei, L.; Zhong, C.; Fu, S.; Bei, Y.; Huică, R.I.; Wang, F.; Xiao, J. Cardiac telocytes are double positive for CD34/PDGFR-α. Cell. Mol. Med. 2015, 19, 2036–2042.
- Zhang, H.; Yu, P.; Zhong, S.; Ge, T.; Peng, S.; Guo, X.; Zhou, Z. Telocytes in pancreas of the Chinese giant salamander (Andrias davidianus). Cell. Mol. Med. 2016, 20, 2215–2219.
- Estève, D.; Boulet, N.; Belles, C.; Zakaroff-Girard, A.; Decaunes, P.; Briot, A.; Veeranagouda, Y.; Didier, M.; Remaury, A.; Guillemot, J.C.; et al. Lobular architecture of human adipose tissue defines the niche and fate of progenitor cells. Commun. 2019, 10, 2549.
- Cretoiu, D.; Xu, J.; Xiao, J.; Cretoiu, S.M. Telocytes and their extracellular vesicles-evidence and hypotheses. J. Mol. Sci. 2016, 17, 1322.
- Cretoiu, D.; Roatesi, S.; Bica, I.; Plesca, C.; Stefan, A.; Bajenaru, O.; Condrat, C.E.; Cretoiu, S.M. Simulation and modeling of telocytes behavior in signaling and intercellular communication processes. J. Mol. Sci. 2020, 21, 2615.
- Ibba-Manneschi, L.; Rosa, I.; Manetti, M. Telocyte implications in human pathology: An overview. Cell. Dev. Biol. 2016, 55, 62–69.
- Nicolescu, M.I.; Bucur, A.; Dinca, O.; Rusu, M.C.; Popescu, L.M. Telocytes in parotid glands. Anat Rec. 2012, 295, 378–385.
- Veress, B.; Ohlsson, B. Spatial relationship between telocytes, interstitial cells of Cajal and the enteric nervous system in the human ileum and colon. Cell. Mol. Med. 2020, 24, 3399–3406.
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González, M.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L., Jr.; Madrid, J.F. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of αSMA+ cells during repair. Histopathol. 2015, 30, 615–627.
- Díaz-Flores, L.; Gutiérrez, R.; Litzarza, K.; González-Gómez, M.; García, M.P.; Saez, F.J.; Díaz-Flores, L., Jr.; Madrid, J.F. Behavior of in situ human native adipose tissue CD34+ stromal/progenitor cells during different stages of repair. Tissue-resident CD34+ stromal cells as a source of myofibroblasts. Rec. 2015, 298, 917–930.
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Díaz-Flores, L., Jr.; Álvarez-Argüelles, H.; Carrasco, J.L. Presence/Absence and specific location of resident CD34+ stromal cells/telocytes condition stromal cell development in repair and tumors. Cell. Dev. Biol. 2020, 8, 544845.
- Vannucchi, M.G.; Bani, D.; Faussone-Pellegrini, M.S. Telocytes contribute as cell progenitors and differentiation inductors in tissue regeneration. Stem Cell Res. Ther. 2016, 11, 383–389.
- Bani, D.; Nistri, S. New insights into the morphogenic role of stromal cells and their relevance for regenerative medicine. lessons from the heart. Cell. Mol. Med. 2014, 18, 363–370.
- Ceafalan, L.; Gherghiceanu, M.; Popescu, L.M.; Simionescu, O. Telocytes in human skin--are they involved in skin regeneration? Cell. Mol. Med. 2012, 16, 1405–1420.
- Gherghiceanu, M.; Popescu, L.M. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: Electron microscope images. Cell. Mol. Med. 2010, 14, 871–877.
- Gibbons, S.J.; De Giorgio, R.; Faussone Pellegrini, M.S.; Garrity-Park, M.M.; Miller, S.M.; Schmalz, P.F.; Young-Fadok, T.M.; Larson, D.W.; Dozois, E.J.; Camilleri, M. et al. Apoptotic cell death of human interstitial cells of Cajal. Motil. 2009, 2, 85–93.
- Popescu, L.M.; Manole, E.; Serboiu, C.S.; Manole, C.G.; Suciu, L.C.; Gherghiceanu, M.; Popescu, B.O. Identification of telocytes in skeletal muscle interstitium: Implication for muscle regeneration. Cell. Mol. Med. 2011, 15, 1379–1392.
- Popescu, L.M.; Gherghiceanu, M.; Suciu, L.C.; Manole, C.G.; Hinescu, M.E. Telocytes and putative stem cells in the lungs: Electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011, 345, 391–403.
- Manetti, M.; Tani, A.; Rosa, I.; Chellini, F.; Squecco, R.; Idrizaj, E.; Zecchi-Orlandini, S.; Ibba-Manneschi, L.; Sassoli, C. Morphological evidence for telocytes as stromal cells supporting satellite cell activation in eccentric contraction-induced skeletal muscle injury. Rep. 2019, 9, 14515.
- Popescu, L.M.; Nicolescu, M.I. Telocytes and stem cells. In Resident Stem Cells and Regenerative Therapy; dos Santos Goldenberg, R.C., de Carvalho, A.C.C., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Oxford, UK, 2013; pp. 205–231.
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Res. 2014, 114, 333–344.
- Suciu, L.; Popescu, L.M.; Gherghiceanu, M.; Regalia, T.; Nicolescu, M.I.; Hinescu, M.E.; Faussone-Pellegrini, M.S. Telocytes in human term placenta: Morphology and phenotype. Cells Tissues Organs 2010, 192, 325–339.
- Vannucchi, M.G.; Traini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini, M.S. Telocytes express PDGFRα in the human gastrointestinal tract. Cell. Mol. Med. 2013, 17, 1099–1108.
- Wang, F.; Song, Y.; Bei, Y.; Zhao, Y.; Xiao, J.; Yang, C. Telocytes in liver regeneration: Possible roles. Cell. Mol. Med. 2014, 18, 1720–1726.
- Zhou, J.; Wang, Y.; Zhu, P.; Sun, H.; Mou, Y.; Duan, C.; Yao, A.; Lv, S.; Wang, C. Distribution and characteristics of telocytes as nurse cells in the architectural organization of engineered heart tissues. China Life Sci. 2014, 57, 241–247.
- Mandache, E.; Gherghiceanu, M.; Macarie, C.; Kostin, S.; Popescu, L.M. Telocytes in human isolated atrial amyloidosis: Ultrastructural remodelling. Cell. Mol. Med. 2010, 14, 2739–2747.
- Iwasa, S.; Enomoto, A.; Onoue, S.; Nakai, M. Chromatographic analysis of conformationally changed insulin and its cytotoxic effect on PC12 cells. Health Sci. 2009, 55, 825–831.
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Gayoso, S.; Gutiérrez, E.; Díaz-Flores, L., Jr.; Carrasco, J.L. Telocytes in the normal and pathological peripheral nervous system. J. Mol. Sci. 2020, 21, 4320.




