
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raquel Cortes | + 2555 word(s) | 2555 | 2021-01-04 08:35:42 | | | |
| 2 | Camila Xu | Meta information modification | 2555 | 2021-03-18 03:59:47 | | |
Video Upload Options
Exosomes are lipid-bilayer-enclosed extracellular vesicles released by many cell types in both normal and pathological conditions, and which transport nucleic acids, lipids and proteins between cells. Due to their suitable proprieties, as well as their known therapeutic effects, exosome-based nanocarriers have a bright future as next-generation drug delivery vehicles.
1. Introduction
Autoimmune diseases are among the leading causes of morbidity and mortality associated with chronic disease worldwide, especially in women, who comprise more than 90% of affected patients [1][2]. Autoimmune diseases are classified in two types, the first of which is organ-specific and the second systemic, wherein the immune response attacks different organs and tissues simultaneously, as exemplified by diseases such as systemic lupus erythematosus (SLE) [3]. The pathogenesis of SLE hinges on loss of tolerance and sustained autoantibody production, characterized by the presence of autoreactive T cells and hyperactive B cells which produce autoantibodies; these later form immune complex deposits that damage different tissues on which autoantigens are expressed [3][4][5]. Although non-steroidal anti-inflammatory drugs such as glucocorticoids (GCs) and immunosuppressive therapies are often administered to SLE patients to relieve immunological inflammation during disease progression [6][7][8], these drugs can cause serious side effects due to their toxicity and the lack of target tissue disease. Several investigations point to cell-based therapies, such as stem cell transplantation, to treat SLE [9][10][11], but these are expensive and add to the long-term medical costs associated with the disease. These drawbacks underscore the urgent need to identify safe and effective therapies for SLE prevention and treatment. The development of drug delivery vehicles has recently emerged as a novel therapeutic approach in autoimmune diseases [12][13][14], being exosomes one of the most promising nanocarriers due to their various therapeutic advantages [15][16][17].
Exosomes are lipid-bilayer-enclosed extracellular vesicles released by many cell types in both normal and pathological conditions, and which transport nucleic acids, lipids and proteins between cells [18][19]. As drug and gene delivery carriers, exosomes have unique advantageous characteristics including encapsulating endogenous bioactive molecules, low immunogenicity, biodegradability, longer circulation time, smaller size than other nanocarriers and the ability to cross many biological barriers [15][17].
Current evidence shows that exosomes are able to modulate the immune system, as shuttles for antigen presentation [20][21][22][23], as immunosuppressive effectors of inflammation process and as immunologic agents for immunotherapy, emerging as promising tools for therapeutic delivery in autoimmune diseases [24][25]. In SLE, many studies have demonstrated that circulating exosomes are immunologically active and their levels correlate with disease activity [26][27]. In addition, through analysis of exosomal-derived microRNAs (miRNAs) levels, distinct miRNA have been identified to discriminate lupus nephritis (LN) [28], as predictors of early fibrosis [29][30][31], and response to therapy in LN [32]. Therefore, exosomes serve as novel biomarkers and predictors of SLE progress.
2. Biogenesis and Function of Extracellular Vesicles
Extracellular vesicles are small spherical lipid bilayer-coated vesicles, secreted by multiple cell types and present in many body fluids, which mediate intercellular communication [33][34]. Nowadays, interest in extracellular vesicles (EVs) centers mainly on their functions as component exchangers and as signaling transmitters under both normal and pathological conditions [35]. EVs display heterogeneity between different subtypes and are classified according to their size (small or large EVs), composition (CD63+/CD81+, cargos) or biogenesis (exosomes, microvesicles and apoptotic bodies) [18][33][34].
The most broadly studied EVs are exosomes, 40–150 nm in diameter, endosome-derived and originating from intraluminal vesicles (ILVs) that reside inside multivesicular bodies (MVBs) [18]. Nucleic acids and lipids are thought to be selectively and actively incorporated into ILVs [36]. Apart from the presence of membrane proteins, it is considered that inward budding of endosomal membranes integrates cytosolic proteins and other components to ILVs, after which MVBs fuse with plasma membrane, releasing ILVs as exosomes [18][37][38] (Figure 1A). Microparticles (MPs), microvesicles, also called ectosomes, are large EVs (100–1000 nm) shed directly by blebbing and budding mechanisms from the plasma membrane [39]. Apoptotic bodies are usually much larger (1000–5000 nm), composed of cellular and nuclear fragments, formed during the late stages of apoptosis [18][37]. Several molecular markers are rich in EV subtypes, such as alix, syntenin-1, TSG101 and CD81 for exosomes and metalloproteinases, integrins and phosphatidylserine for MVs [40][41].
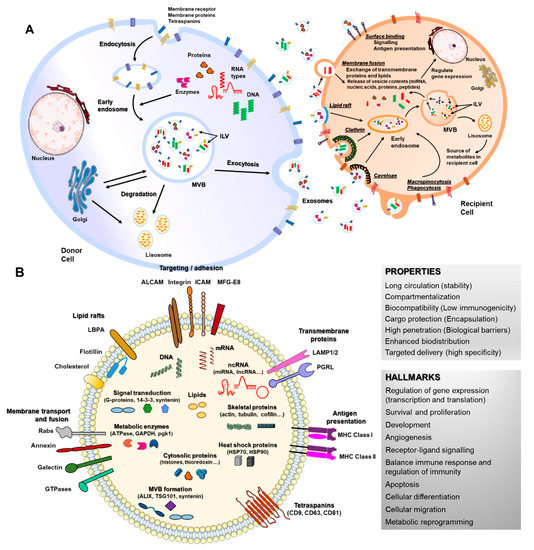
Figure 1. Biogenesis, secretion, uptake and molecular composition of exosomes. (A) exosomes from intraluminal vesicles (ILVs) in multivesicular bodies (MVBs) are secreted extracellularly by fusion with the cellular membrane. Next, exosomes interacting with recipient cells directly by surface binding, membrane fusion or internalization will target exogenous exosomes in the canonical endosomal pathway by lipid raft, clathrin, caveloae or micropinocytosis/phagocytosis processes. (B) exosomes can contain different types of cell surface proteins (tetraspanins, integrins, major histocompatibility complex (MHC), etc.), intracellular protein (skeletal proteins, heat shock proteins, etc.), nucleic acids (RNA, DNA, microRNAs (miRNA), long non-coding RNA (lncRNA), etc.), amino acids, lipids and metabolites. They are mediators of near and long-distance intercellular communication in health and disease, affecting several aspects of cell biology. This, together with their intrinsic features such as stability, biocompatibility, low immunogenicity and ability to overcome biological barriers, has prompted interest in using exosomes as drug delivery vehicles.
When released to the extracellular space, exosomes interact with their acceptor cells in different ways. Information transmission can occur at the cell surface without delivering any cargo (surface binding), as occurs during immune responses [20][42], but the most common way is internalization of exosomes or their content, through clathrin, caveolin or lipid rafts-mediated endocytosis, phagocytosis, macropinocytosis or direct membrane fusion [43][44][45][46][47][48] (Figure 1A). The function of EVs in recipient cells depends on their cargo and thus on the cell type from which they are released. Cargos transported by EVs are highly heterogeneous and include RNAs (coding and non-coding), DNAs, lipids and proteins that can be transported inside EVs or membrane-bound [35][38]. RNAs enclosed in EVs such as mRNAs can control cell differentiation processes, survival, repair and angiogenesis [49][50][51][52] (Figure 1B). MiRNA are the most studied RNAs present in exosomes, which exert their function through gene expression regulation in acceptor cells [36]. EVs-miRNAs have revealed a role in a wide range of cell processes, both beneficial and detrimental, such as immune response, angiogenesis, apoptosis and differentiation [53][54][55]. Lipids transported by EVs are asymmetrically distributed between the outer and inner EV membranes and include cholesterol, ceramides, prostaglandins, sphingolipids and phosphatidylserines. Their functions have been related to the EV biogenesis itself and more importantly, to an immunomodulatory role of bioactive lipids EV-transported in immune-related pathologies [56]. The protein content of EVs is highly enriched in cytoskeletal, cytosolic, heat shock and vesicular trafficking proteins that control different cell signaling pathways including calcium signaling, coagulation and inflammation [57][58][59]. Therefore, EVs and particularly exosomes modulate a variety of physiological processes related to cell homeostasis and regulation, also mediating detrimental processes during disease.
Indeed, due to their involvement in normal and pathological cellular physiology, EVs have been recognized as good biomarkers in a wide variety of diseases including cancer, cardiovascular diseases, nephropathies and autoimmune diseases [60][61][62][63][64]. Moreover, exosomes have been considered useful indicators of disease progression. In cancer, tumor-derived exosomes are pointed out as biomarkers of disease development through tumor progression monitoring [65][66]. In autoimmune diseases, recent works show exosome-associated miRNA profiles as a promising tool for disease monitoring [29][30][67] and a prognostic tool for therapy response [68].
3. Role of Extracellular Vesicles in Systemic Lupus Erythematosus
3.1. Modulation of Immune Response
Systemic lupus erythematosus is a prototypic autoimmune disease characterized by diverse immune disturbances. One of the serologic hallmarks of SLE is the production of antibodies to nuclear molecules (antinuclear antibodies (ANA)) [3][4]. These ANA can form circulating proinflammatory immune complexes (ICs) that trigger cytokine production by innate immune cells or deposition in the tissue (especially the kidney) to fix complement and incite inflammation [69]; EVs could be mediators in these processes.
Several reports indicate higher levels of circulating IC-carrying MPs in SLE; as an example, Ullal et al. demonstrated that MPs display DNA and nucleosomal molecules in an antigenic form and could represent a source of ICs in SLE [70]. Another study by Nielsen et al. demonstrated that plasma MPs carry antigens accessible to autoantibodies and that complement-activating ICs may form on MPs in SLE patients [71]. Additionally, Cloutier et al. showed that platelet-derived MPs from synovial fluid carried large quantities of ICs, composed primarily of anti-citrullinated protein antibodies (ACPAs) directed against vimentin and fibrinogen (well established autoantigens in rheumatoid arthritis) and not by MP Fc-receptor binding [72].
Given their composition and immune properties, EVs act as proinflammatory mediators in SLE [73]. In one study, healthy peripheral blood mononuclear cells (PBMCs) were stimulated with exosomes isolated from SLE patients, producing TNF-α, IL-1β, and IL-6. Investigators demonstrated that circulating exosomes are immunologically active and their levels correlate with disease activity in lupus [26]. Dieker et al. reported that circulating apoptotic MPs from SLE patients drive the activation of dendritic cell subsets and prime neutrophils [74]. In another study, Winber et al. demonstrated that SLE patients display increased ROS production and degranulation by polymorphonuclear leukocytes (PMNs) in response to MPs [75]. Recently, Burbano et al. highlighted the involvement of platelet-derived MPs during monocyte activation in patients with SLE, showing that MPs are one of the most representative sources of the total amount of circulating ICs-IgG+ in patients with SLE [76]. Another study revealed that macrovascular and microvascular endothelial cells exposed to MPs and MPs-ICs from patients with SLE increase expression of adhesion molecules, chemokine production and structural alterations [77]. Finally, Salvi et al. reported that exosomes isolated from the plasma of SLE patients can activate secretion of IFN-a by human blood plasmacytoid dendritic cells (pDCs) in vitro. They identified exosome-delivered miRNAs as potentially novel TLR7 endogenous ligands able to induce pDC activation in SLE patients [27]. These findings enhance EVs as novel mediators during onset of autoimmune reactions and potential therapeutic targets in the SLE treatment.
3.2. Biomarkers and Predictors of Disease Activity
Several studies reported EVs as reliable biomarkers of disease activity beyond their role in regulating immune responses, offering a valuable complement to classical laboratory markers [64][78]. Nielsen et al. demonstrated different concentrations and composition of MPs in SLE patients from those in healthy subjects [79]. Additionally, a deeper phenotypic analysis of MP from SLE showed that MP were more abundant in these patients and were associated with declining renal function [80]. A proteomic study revealed a specific SLE-MP with a particularly altered proteome, including diminished mitochondrial and platelet proteins and increased glycolytic and cytoskeletal proteins [81]. Investigators concluded that an abnormal generation of MPs may partake in the pathology of SLE and that new diagnostic, monitoring and treatment strategies targeting these processes may be advantageous. In a recent study, MPs isolated from platelet-poor plasma and urine from SLE patients were characterized by flow cytometry, the authors concluding that HMGB1 + MPs present in urine are hallmarks of nephritis in patients with SLE [82].
Many recent studies highlight exosomal-derived miRNAs levels to identify distinct miRNA to discriminate LN. Our group revealed increased urinary exosomal miRNAs levels in patients with SLE, discriminating LN [28], and in a recent study, we identified urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in LN [29]. Another study reported a unique miRNA expression profile of urinary exosome (miR-3135b, miR-654-5p and miR-146a-5p) as novel non-invasive diagnostic markers for type IV LN with cellular crescent [31]. In line with early renal fibrosis, Solé et al. identified urinary exosomal miR-29c as a novel non-invasive marker of early progression to fibrosis in patients with LN [83]. Moreover, a urinary exosomal multimarker panel composed of miR-21, miR-150 and miR-29c was provided as a non-invasive method to detect early renal fibrosis and predict disease progression in LN [30]. Finally, a recent study reported urinary exosomal miR-135b-5p, miR-107 and miR-31 as promising novel markers for clinical outcomes, regulating LN renal recovery by HIF1A inhibition [32].
In summary, quantity and phenotype of circulating EVs may be useful as novel biomarkers of activity and progression of SLE, providing a new therapeutic approach.
6. Exosome-Based Drug Delivery in Systemic Lupus Erythematosus
Both in themselves and as vehicles of drug and gene delivery, exosomes are under active development as therapeutic agents [17,186]. Exosomes loaded with endogenous and/or exogenous cargo have recently emerged as novel therapeutic effectors in immune therapy [24,187], targeting the autoimmune-mediated inflammatory pathology associated with SLE [188]. Exosomes can modify major components of innate and adaptive immune responses, including T-cells, B-cells and macrophages [189–191]. This immunomodulatory property makes exosomes an attractive tool for immunotherapy as well as tissue regeneration. The use of EVs as a cell-free therapeutic alternative offers several distinct advantages over parent stem cells. A major advantage is that EVs, depending on their source, may be less immunogenic than their parental cells, likely due to a lower abundance of transmembrane proteins such as MHC complexes [192]. Unlike live cells, EVs are highly stable and easily stored long-term. In addition, EVs do not replicate, thus avoiding risk of aneuploidy or other chromosomal abnormalities in tumor generation. Finally, exosomes are also able to cross biological barriers that MSCs cannot pass [85,106], a significant advantage in a systemic disease such as SLE which affect organs with physiological barriers as the brain and kidney (blood–brain and blood–urine). Immune-therapeutic exosomes include naturally occurring exosomes, exosomes secreted by modified cells, and exosomes loaded with exogenous cargos (Figure 3).
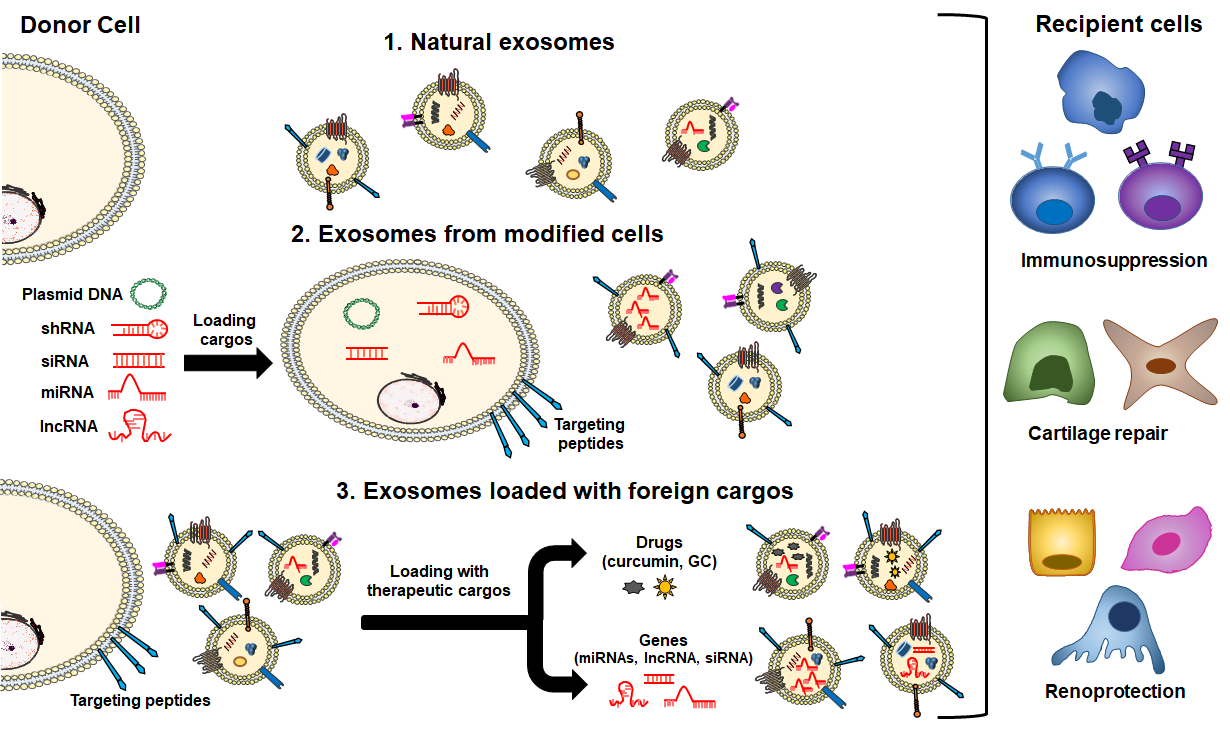
Several studies have shown that EVs from various cell sources have a therapeutic effect through their intrinsic content [193], which includes tumor-derived EVs, MSCs [93], activated antigen-presenting cells (APCs), natural killer (NK) cells and endothelial progenitor cells (EPCs) (Figure 3, route 1). Furthermore, cell-derived exosomes can improve immunosuppressive ability after cell pretreatment. With the stimulation by physical and chemical factors, exosomes can produce stronger immunosuppressive effects [191]. In autoimmune diseases, including SLE, the most studied naturally secreted EVs for therapeutic purposes derive from MSCs and EPCs. One major role of MSCs is to suppress proliferation and function of cells in both innate and adaptive immunity responses [194]. Loading therapeutic cargos into exosomes is based on their biological processes, cargo is loaded into donor cells or overexpressed certain gene products which are then packaged into exosomes (Figure 3, route 2). Exosome content of cells modified by pathological factors (physical/chemical factors) or transfection can be delivered to recipient cells as a therapeutic method [191]. Finally, exogenous cargos can be directly loaded into exosomes by different methods, and the cargo can be classified into three kinds, mainly including small molecule drugs, nucleic acids (miRNA, siRNA, lncRNA, etc.), proteins and peptides (Figure 3, route 3). However, using the properties of exosomes alone is insufficient to achieve specific delivery of exogenous cargos into diseased tissues. Therefore, as described before, different exosome engineering techniques to improve the therapeutic effect of exosomes are still being developed, such as surface modification of exosomes or loading magnetic nanoparticles [17,84,181,223].
In summary, these works illustrate the undeniable potential of exosomes as a therapeutic drug carrier in autoimmune diseases. Nevertheless, more studies are warranted for a clear understanding of the application of exosome-based drug delivery in autoimmune diseases, overall, in SLE. Exosome-based nanocarriers potentially have a bright future as next-generation drug delivery vehicles.
References
- Walsh, S.J.; Rau, L.M. Autoimmune diseases: A leading cause of death among young and middle-aged women in the United States. Am. J. Public Health 2000, 90, 1463–1466.
- Thomas, S.L.; Griffiths, C.; Smeeth, L.; Rooney, C.; Hall, A.J. Burden of mortality associated with autoimmune diseases among females in the United Kingdom. Am. J. Public Health 2010, 100, 2279–2287.
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730.
- Casciola-Rosen, L.A.; Anhalt, G.; Rosen, A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994, 179, 1317–1330.
- Colasanti, T.; Maselli, A.; Conti, F.; Sanchez, M.; Alessandri, C.; Barbati, C.; Vacirca, D.; Tinari, A.; Chiarotti, F.; Giovannetti, A.; et al. Autoantibodies to estrogen receptor alpha interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 778–787.
- Ruiz-Irastorza, G.; Danza, A.; Khamashta, M. Glucocorticoid use and abuse in SLE. Rheumatology 2012, 51, 1145–1153.
- Rostamzadeh, D.; Razavi, S.R.; Esmaeili, S.; Dolati, S.; Ahmahi, M.; Sadreddini, S.; Jadidi-Niaragh, F.; Yousefi, M. Application of nanoparticle technology in the treatment of Systemic lupus erythematous. Biomed. Pharmacother. 2016, 83, 1154–1163.
- Paley, M.A.; Strand, V.; Kim, A.H. From mechanism to therapies in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2017, 29, 178–186.
- Wang, D.; Zhang, H.; Liang, J.; Li, X.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Lu, L.; Gilkeson, G.S.; et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013, 22, 2267–2277.
- Gu, F.; Wang, D.; Zhang, H.; Feng, X.; Gilkeson, G.S.; Shi, S.; Sun, L. Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin. Rheumatol. 2014, 33, 1611–1619.
- Liang, J.; Zhang, H.; Kong, W.; Deng, W.; Wang, D.; Feng, X.; Zhao, C.; Hua, B.; Wang, H.; Sun, L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: A long-term retrospective study. Stem Cell Res. Ther. 2018, 9, 312.
- Neupane, Y.R.; Mahtab, A.; Siddiqui, L.; Singh, A.; Gautam, N.; Rabbani, S.A.; Goel, H.; Talegaonkar, S. Biocompatible nanovesicular drug delivery systems with targeting potential for autoimmune diseases. Curr. Pharm. Des. 2020.
- Diao, L.; Tao, J.; Wang, Y.; Hu, Y.; He, W. Co-Delivery Of Dihydroartemisinin And HMGB1 siRNA By TAT-Modified Cationic Liposomes through the TLR4 Signaling Pathway for Treatment of Lupus Nephritis. Int. J. Nanomed. 2019, 14, 8627–8645.
- Tao, S.; Song, P.; Zhang, X.; Zhang, L.; Chu, C.Q. Single-Stranded DNA Aptamers against TNF and Their Potential Applications. Methods Mol. Biol. 2020, 2108, 181–196.
- Zhang, M.; Zang, X.; Wang, M.; Li, Z.; Qiao, M.; Hu, H.; Chen, D. Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: Recent advances and challenges. J. Mater. Chem. B 2019, 7, 2421–2433.
- De Jong, B.; Barros, E.R.; Hoenderop, J.G.J.; Rigalli, J.P. Recent Advances in Extracellular Vesicles as Drug Delivery Systems and Their Potential in Precision Medicine. Pharmaceutics 2020, 12, 1006.
- Peng, H.; Ji, W.; Zhao, R.; Yang, J.; Lu, Z.; Li, Y.; Zhang, X. Exosome: A significant nano-scale drug delivery carrier. J. Mater. Chem. B 2020, 8, 7591–7608.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920.
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172.
- Lindenbergh, M.F.S.; Wubbolts, R.; Borg, E.G.F.; van ‘t Veld, E.M.; Boes, M.; Stoorvogel, W. Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation. J. Extracell. Vesicles 2020, 9, 1798606.
- Fu, C.; Peng, P.; Loschko, J.; Feng, L.; Pham, P.; Cui, W.; Lee, K.P.; Krug, A.B.; Jiang, A. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc. Natl. Acad. Sci. USA 2020, 117, 23730–23741.
- Muntasell, A.; Berger, A.C.; Roche, P.A. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007, 26, 4263–4272.
- Tran, T.H.; Mattheolabakis, G.; Aldawsari, H.; Amiji, M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immunol. 2015, 160, 46–58.
- Xu, H.; Jia, S.; Xu, H. Potential therapeutic applications of exosomes in different autoimmune diseases. Clin. Immunol. 2019, 205, 116–124.
- Lee, J.Y.; Park, J.K.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Circulating exosomes from patients with systemic lupus erythematosus induce an proinflammatory immune response. Arthritis Res. Ther. 2016, 18, 264.
- Salvi, V.; Gianello, V.; Busatto, S.; Bergese, P.; Andreoli, L.; D’Oro, U.; Zingoni, A.; Tincani, A.; Sozzani, S.; Bosisio, D. Exosome-delivered microRNAs promote IFN-alpha secretion by human plasmacytoid DCs via TLR7. JCI Insight 2018, 3, e98204.
- Perez-Hernandez, J.; Forner, M.J.; Pinto, C.; Chaves, F.J.; Cortes, R.; Redon, J. Increased Urinary Exosomal MicroRNAs in Patients with Systemic Lupus Erythematosus. PLoS ONE 2015, 10, e0138618.
- Perez-Hernandez, J.; Martinez-Arroyo, O.; Ortega, A.; Galera, M.; Solis-Salguero, M.A.; Chaves, F.J.; Redon, J.; Forner, M.J.; Cortes, R. Urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J. Nephrol. 2020.
- Sole, C.; Moline, T.; Vidal, M.; Ordi-Ros, J.; Cortes-Hernandez, J. An Exosomal Urinary miRNA Signature for Early Diagnosis of Renal Fibrosis in Lupus Nephritis. Cells 2019, 8, 773.
- Li, Y.; Xu, X.; Tang, X.; Bian, X.; Shen, B.; Zhao, H.; Luo, S.; Chen, Z.; Zhang, K. MicroRNA expression profile of urinary exosomes in Type IV lupus nephritis complicated by cellular crescent. J. Biol. Res. (Thessal.) 2018, 25, 16.
- Garcia-Vives, E.; Sole, C.; Moline, T.; Vidal, M.; Agraz, I.; Ordi-Ros, J.; Cortes-Hernandez, J. The Urinary Exosomal miRNA Expression Profile is Predictive of Clinical Response in Lupus Nephritis. Int. J. Mol. Sci. 2020, 21, 1372.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066.
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659.
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17.
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312.
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232.
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Thery, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977.
- Tian, J.; Casella, G.; Zhang, Y.; Rostami, A.; Li, X. Potential roles of extracellular vesicles in the pathophysiology, diagnosis, and treatment of autoimmune diseases. Int. J. Biol. Sci. 2020, 16, 620–632.
- Tkach, M.; Kowal, J.; Zucchetti, A.E.; Enserink, L.; Jouve, M.; Lankar, D.; Saitakis, M.; Martin-Jaular, L.; Thery, C. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017, 36, 3012–3028.
- Corbeil, D.; Santos, M.F.; Karbanova, J.; Kurth, T.; Rappa, G.; Lorico, A. Uptake and Fate of Extracellular Membrane Vesicles: Nucleoplasmic Reticulum-Associated Late Endosomes as a New Gate to Intercellular Communication. Cells 2020, 9, 1931.
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 2011, 11, 108.
- Svensson, K.J.; Christianson, H.C.; Wittrup, A.; Bourseau-Guilmain, E.; Lindqvist, E.; Svensson, L.M.; Morgelin, M.; Belting, M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 2013, 288, 17713–17724.
- Feng, D.; Zhao, W.L.; Ye, Y.Y.; Bai, X.C.; Liu, R.Q.; Chang, L.F.; Zhou, Q.; Sui, S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687.
- Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 124, 447–458.
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.L.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012, 119, 756–766.
- Lopatina, T.; Favaro, E.; Danilova, L.; Fertig, E.J.; Favorov, A.V.; Kagohara, L.T.; Martone, T.; Bussolati, B.; Romagnoli, R.; Albera, R.; et al. Extracellular Vesicles Released by Tumor Endothelial Cells Spread Immunosuppressive and Transforming Signals Through Various Recipient Cells. Front. Cell Dev. Biol. 2020, 8, 698.
- Yang, J.; Wu, S.; Hou, L.; Zhu, D.; Yin, S.; Yang, G.; Wang, Y. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol. Ther. Nucleic Acids 2020, 21, 512–522.
- Borges, F.T.; Melo, S.A.; Ozdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Gattone, V.H., 2nd; LeBleu, V.S.; Kalluri, R. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013, 24, 385–392.
- Tang, H.; He, Y.; Li, L.; Mao, W.; Chen, X.; Ni, H.; Dong, Y.; Lyu, F. Exosomal MMP2 derived from mature osteoblasts promotes angiogenesis of endothelial cells via VEGF/Erk1/2 signaling pathway. Exp. Cell Res. 2019, 383, 111541.
- Liu, Q.; Rojas-Canales, D.M.; Divito, S.J.; Shufesky, W.J.; Stolz, D.B.; Erdos, G.; Sullivan, M.L.; Gibson, G.A.; Watkins, S.C.; Larregina, A.T.; et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J. Clin. Investig. 2016, 126, 2805–2820.
- Yue, K.Y.; Zhang, P.R.; Zheng, M.H.; Cao, X.L.; Cao, Y.; Zhang, Y.Z.; Zhang, Y.F.; Wu, H.N.; Lu, Z.H.; Liang, L.; et al. Neurons can upregulate Cav-1 to increase intake of endothelial cells-derived extracellular vesicles that attenuate apoptosis via miR-1290. Cell Death Dis. 2019, 10, 869.
- Kovacs, A.F.; Fekete, N.; Turiak, L.; Acs, A.; Kohidai, L.; Buzas, E.I.; Pallinger, E. Unravelling the Role of Trophoblastic-Derived Extracellular Vesicles in Regulatory T Cell Differentiation. Int. J. Mol. Sci. 2019, 20, 3457.
- Sagini, K.; Costanzi, E.; Emiliani, C.; Buratta, S.; Urbanelli, L. Extracellular Vesicles as Conveyors of Membrane-Derived Bioactive Lipids in Immune System. Int. J. Mol. Sci. 2018, 19, 1227.
- Li, W.; Deng, M.; Loughran, P.A.; Yang, M.; Lin, M.; Yang, C.; Gao, W.; Jin, S.; Li, S.; Cai, J.; et al. LPS Induces Active HMGB1 Release From Hepatocytes Into Exosomes Through the Coordinated Activities of TLR4 and Caspase-11/GSDMD Signaling. Front. Immunol. 2020, 11, 229.
- Zhang, W.; Ou, X.; Wu, X. Proteomics profiling of plasma exosomes in epithelial ovarian cancer: A potential role in the coagulation cascade, diagnosis and prognosis. Int. J. Oncol. 2019, 54, 1719–1733.
- Chen, Z.; Dong, W.H.; Qiu, Z.M.; Li, Q.G. The Monocyte-Derived Exosomal CLMAT3 Activates the CtBP2-p300-NF-kappaB Transcriptional Complex to Induce Proinflammatory Cytokines in ALI. Mol. Ther. Nucleic Acids 2020, 21, 1100–1110.
- Li, S.; Yi, M.; Dong, B.; Tan, X.; Luo, S.; Wu, K. The role of exosomes in liquid biopsy for cancer diagnosis and prognosis prediction. Int. J. Cancer 2020.
- Masaoutis, C.; Al Besher, S.; Koutroulis, I.; Theocharis, S. Exosomes in Nephropathies: A Rich Source of Novel Biomarkers. Dis. Markers 2020, 2020, 8897833.
- Martinez-Arroyo, O.; Ortega, A.; Redon, J.; Cortes, R. Therapeutic Potential of Extracellular Vesicles in Hypertension-Associated Kidney Disease. Hypertension 2021, 77, 28–38.
- Hejrati, A.; Hasani, B.; Esmaili, M.; Bashash, D.; Tavakolinia, N.; Zafari, P. Role of exosome in autoimmunity, with a particular emphasis on rheumatoid arthritis. Int. J. Rheum. Dis. 2020.
- Perez-Hernandez, J.; Cortes, R. Extracellular Vesicles as Biomarkers of Systemic Lupus Erythematosus. Dis. Markers 2015, 2015, 613536.
- Maisano, D.; Mimmi, S.; Russo, R.; Fioravanti, A.; Fiume, G.; Vecchio, E.; Nistico, N.; Quinto, I.; Iaccino, E. Uncovering the Exosomes Diversity: A Window of Opportunity for Tumor Progression Monitoring. Pharmaceuticals 2020, 13, 180.
- Iaccino, E.; Mimmi, S.; Dattilo, V.; Marino, F.; Candeloro, P.; Di Loria, A.; Marimpietri, D.; Pisano, A.; Albano, F.; Vecchio, E.; et al. Monitoring multiple myeloma by idiotype-specific peptide binders of tumor-derived exosomes. Mol. Cancer 2017, 16, 159.
- Zakeri, Z.; Salmaninejad, A.; Hosseini, N.; Shahbakhsh, Y.; Fadaee, E.; Shahrzad, M.K.; Fadaei, S. MicroRNA and exosome: Key players in rheumatoid arthritis. J. Cell. Biochem. 2019, 120, 10930–10944.
- Manna, I.; Iaccino, E.; Dattilo, V.; Barone, S.; Vecchio, E.; Mimmi, S.; Filippelli, E.; Demonte, G.; Polidoro, S.; Granata, A.; et al. Exosome-associated miRNA profile as a prognostic tool for therapy response monitoring in multiple sclerosis patients. FASEB J. 2018, 32, 4241–4246.
- Pisetsky, D.S.; Lipsky, P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol 2020, 16, 565–579.
- Ullal, A.J.; Reich, C.F., 3rd; Clowse, M.; Criscione-Schreiber, L.G.; Tochacek, M.; Monestier, M.; Pisetsky, D.S. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J. Autoimmun. 2011, 36, 173–180.
- Nielsen, C.T.; Ostergaard, O.; Stener, L.; Iversen, L.V.; Truedsson, L.; Gullstrand, B.; Jacobsen, S.; Heegaard, N.H. Increased IgG on cell-derived plasma microparticles in systemic lupus erythematosus is associated with autoantibodies and complement activation. Arthritis Rheum. 2012, 64, 1227–1236.
- Cloutier, N.; Tan, S.; Boudreau, L.H.; Cramb, C.; Subbaiah, R.; Lahey, L.; Albert, A.; Shnayder, R.; Gobezie, R.; Nigrovic, P.A.; et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol. Med. 2013, 5, 235–249.
- Mobarrez, F.; Svenungsson, E.; Pisetsky, D.S. Microparticles as autoantigens in systemic lupus erythematosus. Eur. J. Clin. Investig. 2018, 48, e13010.
- Dieker, J.; Tel, J.; Pieterse, E.; Thielen, A.; Rother, N.; Bakker, M.; Fransen, J.; Dijkman, H.B.; Berden, J.H.; de Vries, J.M.; et al. Circulating Apoptotic Microparticles in Systemic Lupus Erythematosus Patients Drive the Activation of Dendritic Cell Subsets and Prime Neutrophils for NETosis. Arthritis Rheumatol. 2016, 68, 462–472.
- Winberg, L.K.; Jacobsen, S.; Nielsen, C.H. Microparticles from patients with systemic lupus erythematosus induce production of reactive oxygen species and degranulation of polymorphonuclear leukocytes. Arthritis Res. Ther. 2017, 19, 230.
- Burbano, C.; Villar-Vesga, J.; Orejuela, J.; Munoz, C.; Vanegas, A.; Vasquez, G.; Rojas, M.; Castano, D. Potential Involvement of Platelet-Derived Microparticles and Microparticles Forming Immune Complexes during Monocyte Activation in Patients with Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 322.
- Atehortua, L.; Rojas, M.; Vasquez, G.; Munoz-Vahos, C.H.; Vanegas-Garcia, A.; Posada-Duque, R.A.; Castano, D. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 34.
- Perez-Hernandez, J.; Redon, J.; Cortes, R. Extracellular Vesicles as Therapeutic Agents in Systemic Lupus Erythematosus. Int. J Mol. Sci. 2017, 18, 717.
- Nielsen, C.T.; Ostergaard, O.; Johnsen, C.; Jacobsen, S.; Heegaard, N.H. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3067–3077.
- Mobarrez, F.; Vikerfors, A.; Gustafsson, J.T.; Gunnarsson, I.; Zickert, A.; Larsson, A.; Pisetsky, D.S.; Wallen, H.; Svenungsson, E. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): Phenotypic characterization and clinical associations. Sci. Rep. 2016, 6, 36025.
- Ostergaard, O.; Nielsen, C.T.; Tanassi, J.T.; Iversen, L.V.; Jacobsen, S.; Heegaard, N.H.H. Distinct proteome pathology of circulating microparticles in systemic lupus erythematosus. Clin. Proteom. 2017, 14, 23.
- Burbano, C.; Gomez-Puerta, J.A.; Munoz-Vahos, C.; Vanegas-Garcia, A.; Rojas, M.; Vasquez, G.; Castano, D. HMGB1(+) microparticles present in urine are hallmarks of nephritis in patients with systemic lupus erythematosus. Eur. J. Immunol. 2019, 49, 323–335.
- Sole, C.; Cortes-Hernandez, J.; Felip, M.L.; Vidal, M.; Ordi-Ros, J. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol. Dial. Transplant. 2015, 30, 1488–1496.




