
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | I-Ta Lee | + 7241 word(s) | 7241 | 2021-01-11 05:00:36 |
Video Upload Options
Periodontal disease dominates as the most common inflammatory disease in the oral cavity. There is growing evidence on the involvement of oxidative stress, which is simply described as the imbalance between oxidants and antioxidants in favor of the former, in the initiation and progression of periodontal disease. Redox mechanistic insights that underlie immuno-inflammatory pathway may thus advance our understanding about periodontal pathogenesis, serving as the background for the development of efficient strategies for periodontal disease prevention and treatment.
1. Introduction
Periodontal disease comprises a group of all conditions, from inflammation to infections to tumors, that affect the well-being of the tooth-supportive apparatus, the so-called periodontium, including the gum (gingiva), periodontal ligament, cementum, and alveolar bone [1]. Among the wide variety of periodontal illnesses, gingivitis and periodontitis are the most common and have predominant relevance to both oral health and overall health. If left untreated, these diseases are highly likely to cause severe consequences for oral structures, such as bone resorption, tooth mobility, and eventually tooth loss, thereby compromising the functional integrity as well as appearance and social interaction. More seriously, the adverse outcomes of periodontal disease are not only restricted within the oral cavity but also even go beyond. It can negatively affect other organ systems and cause unexpected events in medically compromised patients [2]. Accumulating evidence regarding the relationship between periodontal disease and various systemic conditions, such as cardiovascular diseases, diabetes, arthritis, Alzheimer’s, pregnancy complications, and a growing list of other conditions, has been reported in the literature [3][4]. Despite such deleterious effects, periodontal disease has still been overlooked in many countries and regions, particularly in those with low socioeconomic status and a weak healthcare system, making it widespread in communities. It was reported that up to 90% of the global population have experienced some forms of periodontal disease in their life [5], and up to 50% have been affected by these illnesses [6]. According to the latest data from the Global Health Data Exchange database, periodontal disease is currently the 12th most prevalent condition worldwide. Furthermore, the cumulative prevalence has increased by 34% during the past three decades, and it is expected to increase in coming years [7]. Remarkably, periodontal disease is both preventable and treatable through adequate and proper approaches. One of the key fundamentals for such modalities is the management of causal factors. It is well established that the major etiologic factor of periodontal disease is periodontally pathogenic bacteria colonizing the dental plaque or biofilm, particularly concerning the subgingival area. Therefore, the control and eradication of such microorganisms as well as microbial biofilm, e.g., improving oral hygiene routine combined with periodically having professional dental cleaning, are considered as the most effective regimens for prevention and treatment of these illnesses [5]. Besides, the immuno-inflammatory responses involved in the onset and progression of periodontal disease have also been paid attention in recent years. It is well known that inflammation is one of the physiological events in response to offenses, such as stress, heat, chemical agents, infection, and injuries. Thus, the initial inflammation in the periodontal tissues can be considered as the physiological defense combating the microbial challenge and initiating the healing processes rather than the pathological response. However, if it is prolonged and severe, this inflammation might lead to tissue breakdown. Otherwise speaking, periodontal disease also arises from the dysregulation or dysfunction of the activation and resolution pathways of inflammation, subsequently resulting in chronic and destructive inflammation [8]. The underlying mechanisms are yet to be elucidated. A growing body of evidence has suggested that oxidative stress appears to be the culprit of both local (periodontal in this case) and systemic inflammation [9][10][11]. Oxidative stress represents the increase of oxidants and/or the decrease of antioxidants, leading to the damage of vital cellular components and the destruction of tissues via different paradigms, such as lipid peroxidation and protein inactivation [11]. Therefore, the oxidative stress-mediated inflammation is likely to be one of the plausible pathways contributing to the development of periodontal disease, and the in-depth knowledge regarding this scope is of interest.
2. Immuno-Inflammatory Pathogenesis of Periodontal Disease
In the context of this work, periodontal disease is defined as chronic inflammatory diseases, including gingivitis and periodontitis, that affect the tooth-supporting tissues. In general, gingivitis is described as a specific inflammation of gingiva (gum) mainly due to the accumulation of dental plaque or microbial biofilm. This condition is typically reversible upon the removal of the bacterial challenge, and causes no severe problems until it has become advanced. Unlike gingivitis, periodontitis is a destructive inflammation, which is characterized by the breakdown of periodontal tissues, such as periodontal ligament and alveolar bone, and it is irreversible [12]. The major etiology of periodontal disease is typically attributed to the invasion of microorganisms located within the oral microflora, particularly the subgingival dental plaque. In the past, gingivitis and periodontitis used to be considered as a continuum in which chronic gingivitis eventually progresses to destructive periodontitis over time. Nevertheless, in fact, this progression does not always occur, implicating that the microbial biofilm is essential to the etiology and pathogenesis of periodontal disease but is insufficient. Owing to the presence of periodontal microbes in individuals without evidence of disease development, the concern about the initiation and progression of these conditions has been dramatically focused on the net effect of the immune responses and the inflammatory processes in recent years [8]. This postulation is further supported by the fact that some diseases and systemic conditions or habits that modify the host response, such as diabetes mellitus, cardiovascular disease, obesity, medications, and tobacco smoking, among others, are risk factors or damage accelerators for periodontal disease [13]. Currently, the existing literature has indicated that both microbial biofilm and periodontal inflammation play pivotal roles in the onset and development of periodontal disease, and they mutually reinforce each other. Upon their invasion, the periodontally pathogenic microorganisms produce enzymes and other substances, which are able to cause the breakdown of periodontal tissues directly. Meanwhile, the cellular and humoral components of the host immune system, mainly associated to the periodontal immune responses, are activated. Typical examples of such protective aspects of the host response are the recruitment of neutrophils and macrophages, the production of cytokines and chemokines, and the activation of the complement cascade. If the immune defenses properly work, the periodontium is prevented from damages caused by the bacterial toxins and by-products. Thus, the periodontal inflammation can be considered as a local reaction against the microbial challenge in order to arrest or eliminate such causal factors. Otherwise speaking, the initial inflammation in the periodontal tissues may function as a physiological defense in response to the pathogens. Conversely, due to the incompetent immune or due to the persistent bacterial challenge, there is the failure of thoroughly eliminating the infection, and then the immune system is continuously stimulated, leading to chronic inflammatory responses. Such a perpetuation of inflammatory responses ultimately disrupts the homeostatic mechanisms and releases various mediators, such as proinflammatory cytokines, proteases, and prostanoids which can promote gingival breakdown and bone loss [8]. Therefore, it is increasingly obvious that the prospective strategies of periodontal healthcare will rely on a detailed map and perception at the molecular and cellular levels of the initiation and resolution of aberrant inflammation. To fill the gaps related to the immuno-inflammatory pathways in the pathogenesis of periodontal disease, there has been a growing body of publications providing panorama knowledge on the regulation of inflammatory responses in the periodontal tissues through the immunologic mechanisms [8][14][15]. Albeit the advances have been made in the immuno-inflammatory field of periodontal pathology, much more is still demanded for a complete understanding, particularly the role of oxidative stress.
3. The Role of Oxidative Stress in the Immuno-Inflammatory Paradigm of Periodontal Disease
3.1. Oxidants in Periodontal Tissues
Oxygen, or molecular oxygen (O2), is a key element for aerobic lives, including human. In eukaryotic cells, oxygen is completely reduced into water (H2O) through a four-step reduction given as the following: O2 → O2•− → H2O2 → •OH → H2O, via the electron transport chain (ETC) in mitochondria [16]. Such sequential reduction of O2 has arisen a matter of interest, which is referred to as the oxygen paradox. While the tetravalent reduction of O2 produces energy for aerobic organisms, its univalent reduction as a result of the leakage of electrons from the electronically reductive flow generates metabolites, the so-called reactive oxygen species (ROS), which can be divided into free radicals and non-radicals. A free radical is characterized by the presence of one or more unpaired electrons in its spatial configuration, making it highly unstable and reactive. Hence, free radicals can induce chain reactions through which other free radicals are consecutively generated. Free radicals of ROS that are of importance include superoxide anion radical (O2•−) and hydroxyl radical (•OH). The former is the most important widespread form as it leads to a cascade of other ROS production, whereas the latter represents the most reactive species. On the other hand, ROS also governs other non-radicals that are not true radicals but are capable of conversion into free radicals. Hydrogen peroxide (H2O2) is considered as the most significant non-radical of ROS since it converts into other ROS, particularly hydroxyl radical, in the presence of metals, such as iron, via Fenton reactions. Furthermore, hydrogen peroxide can generate hypochlorous acid (HOCl) in the presence of chloride anion, which is catalyzed by the unique enzyme in neutrophils namely myeloperoxidase (MPO). Hypochlorous acid is a strong reactive species, and it is grouped under the category of ROS as well [17]. Beyond the scope of the above species, nitric oxide (NO• or NO) also represents a crucial pathway associated to redox intermediate production. It has been well documented that NO plays a significant role as a physiological mediator in various tissues and organs in the human body. Following the characterization of the messenger molecule within the endothelial cells with vasodilating potential, termed endothelial-derived relaxing factor (EDRF), the fact that NO can function as EDRF was demonstrated [18]. In addition to its biological actions in the vasculature, the role of NO in neurotransmission and the cellular immune system has been appreciated as well. The understanding about the biological functions of NO was comprehensively reported in a recent review [19]. Remarkably, NO plays dual roles in biological systems. In particular, NO at low optimal concentrations can regulate numerous physiological processes, whereas the overproduction of NO may contribute to the pathogenesis of many diseases [20]. NO is typically generated through the metabolism of the amino acid L-arginine catalyzed by nitric oxide synthase (NOS) enzymes. Up to date, three different isoforms of NOS have been identified, including neuronal NOS (nNOS or NOS I), inducible NOS (iNOS or NOS II), and endothelial NOS (eNOS or NOS III). It has been widely accepted that two isoforms, nNOS and eNOS, are constitutively expressed, whereas iNOS isoform is only induced in response to infection, inflammation, or trauma [21]. Interestingly, NO generated by NOS located in different cellular and subcellular locations may elicit specific effects [22]. The generation of NO by iNOS has been indicated to function as a microbial killer and immune regulator. Besides, inflammatory cells, such as neutrophils and macrophages, which can generate high levels of ROS, also play critical roles in host defense and immunity [23]. Thus, the interplay between NO and ROS in cellular redox signaling may regulate the immuno-inflammatory responses. From the viewpoint of redox biology, NO was initially underestimated despite its radical nature. Nevertheless, owing to the discovery that NO can rapidly react with superoxide to produce highly reactive peroxide, namely peroxynitrite (ONOO−), the merging of ROS and NO pathways has occurred, highlighting the importance of NO in the redox paradigm. Peroxynitrite itself is highly toxic due to its powerful oxidant capacity. Moreover, it can further undergo reactions to generate hydroxyl radical and other reactive nitrogen species (RNS) [24]. Conversely, there have been a number of studies reporting the shifting from NO production into ROS generation by iNOS, which is regulated by ROS [23][25]. The decrease of iNOS-induced NO generation by ROS is presumed to result from the ROS-caused oxidation of the pterin cofactor tetrahydrobiopterin (BH4), which is required for NO production by iNOS. In the absence of BH4, iNOS fails to generate NO and becomes uncoupled, whereby the reduction of molecular oxygen driven by electron flow from the NOS reductase domain can generate superoxide or other ROS [25]. However, the precise mechanisms concerning the interaction of NO and ROS are not fully understood, requiring further investigations in the future. Taken together, the term ROS covers all kinds of chemical species derived from molecular oxygen, including radicals, molecules, and ions, that act as major pro-oxidants or oxidants in the biological system. The major generator of ROS is the mitochondrial ETC by which premature electron leakage of oxygen reduction may occur, liberating superoxide. Superoxide then dismutates into hydrogen peroxide spontaneously or by the catalysis of superoxide dismutase (SOD). In turn, hydrogen peroxide is further transformed into other species by three paths: (1) reduced into water by catalase (CAT) or glutathione peroxidase (GPX), (2) degraded into hydroxyl radical via Fenton and Haber–Weiss reactions, and (3) converted into hypochlorous acid by MPO in neutrophils. Besides, enzymatic reactions catalyzed by enzymes, including nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) and xanthine oxidases (XO), also play a significant role in ROS production. Other enzymes, such as cyclooxygenases (COX), lipoxygenases (LOX), and cytochrome P450 monooxygenase (P450s), have been proposed to generate ROS as well. However, none of these enzymes are typical ROS manufacturers, nor are superoxide and hydrogen peroxide primary reactive species produced during their catalytic activity [16]. Considering the oral cavity is one part of the whole body, the oral tissues, including periodontal tissues, are physiologically exposed to such sources of ROS. In addition to the physiological sources, ROS generation in periodontal tissues is enhanced upon the invasion of periodontal pathogens. As mentioned above, periodontal disease should be considered as the result of the interaction between pathogenic microorganisms and the immuno-inflammatory host responses. The lipopolysaccharides (LPSs) and other cell components from the microbes may lead to the production of various proinflammatory cytokines as well as the activation of signaling pathways, such as activator protein 1 (AP-1) and nuclear factor kappa B (NF-κB). Both routes are able to cause the recruitment and activation of immune cells, thus promoting ROS liberation. The former induces the recruitment and activation of hyper-responsive polymorphonuclear leukocytes (PMNs), whereas the latter results in the destruction of periodontal tissues via the activation of osteoclasts and the elevation of matrix metalloproteinase (MMP) concentrations. The overproduction of lipid peroxides, inflammatory mediators, and oxidized proteins from tissue breakdown further leads to the recruitment and activation of phagocytes, particularly macrophages and neutrophils [26]. These host defense cells, through phagocytosis using the oxygen-dependent pathway, induce a ‘respiratory burst’ or ‘oxidative burst’, which is characterized by an increase in oxygen consumption and enhancement of the production of ROS and other metabolic products [27]. ROS have multiple effects on periodontal tissues, which are determined by the redox state. Under the redox equilibrium, ROS may play a key role in the killing of periodontal pathogens. In particular, ROS can drive the disruption of the cellular oxidative environment, which is important for the survival of periodontal microorganisms, predominantly Gram-negative anaerobic or facultative bacteria. Furthermore, ROS may function as the second messenger that regulates signal transduction and transcription factor expression, providing the cytoprotective effects on periodontal tissues. Thus, ROS derived from respiratory burst caused by primary immune responses may represent one of the first lines of defense against the pathogenic microorganisms. Nonetheless, the overproduction of ROS, which comes from the prolonged recruitment and activation of immune cells due to persistent infection or dysregulated inflammation, possibly causes cytotoxic effects and periodontal tissue damage [26]. One of the considerable examples for such dysregulated inflammation is the bi-directional relationship between diabetes mellitus and periodontitis in which diabetes mellitus has an adverse impact on the progression of periodontitis and vice versa. Diabetes mellitus is well known as a chronic metabolic disease characterized by hyperglycemia that subsequently enhances the hyperinflammation and overproduction of oxidants. The consequences of the exacerbation of inflammatory responses further boost the breakdown of periodontal tissues in individuals suffering from both diabetes and periodontitis [9]. Mechanistically, the impact of ROS on the degradation of periodontal tissues may be attributed to its capacity to oxidize vital biomolecules, such as lipids, proteins, and nucleic acids, as well as to regulate the signal transduction and gene transcription involved in immuno-inflammatory responses and cell death. This further produces a vicious circle among the release of ROS and the breakdown of periodontal tissues via the immuno-inflammatory cascade. In addition to the endogenous sources, the periodontal tissues are likely to be exposed to ROS from exogenous stimuli, including heat, trauma, radiation, cigarette smoking, and dental materials used in clinical procedures, such as bleaching agents, among others [26]. In summary, ROS are produced in periodontal tissues from a wide range of both endogenous and exogenous sources under a variety of physiological and pathological conditions (Figure 1). Upon the concentrations, ROS may function as a good or bad weapon in the progression of periodontal disease (Figure 2).
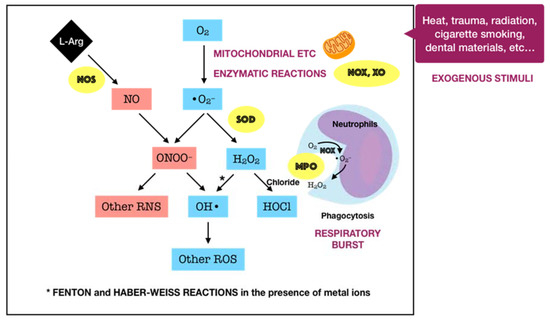
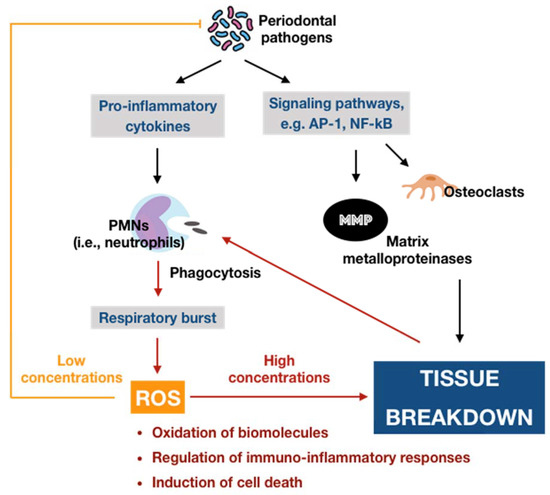
Figure 2. Schematic representation of the double-edged effects of ROS in periodontal disease. At physiological levels, ROS may contribute to the killing of periodontal pathogens and function as the second messenger that mediates biological processes, providing cytoprotective effects. In contrast, excessive ROS can induce many adverse effects, generating a vicious circle among ROS and tissue breakdown via the immuno-inflammatory cascade.
3.2. Innate Antioxidant Defense Systems in Periodontal Tissues
Considering the fact that ROS possibly causes a great harm to biological systems, humans have developed three major antioxidant defense systems, including non-enzymatic low-molecular defense system, enzymatic defense system, and repair system, in order to counteract the release as well as the impact of ROS, balancing the redox homeostasis. An antioxidant can be described as “any substance, that when present at low concentrations compared to those of an oxidizable substrate delays or prevents oxidation of that substrate” [28]. The non-enzymatic system, such as uric acid, albumin, glutathione, melatonin, bilirubin, and polyamines, among others, can interrupt and break the chain reactions caused by free radicals. Meanwhile, the enzymatic system, particularly superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GSR), and thioredoxin (TRX), can act as inhibitors or scavengers of ROS. For instance, SOD is one of the most significant cellular enzymatic antioxidants, which catalyzes the dismutation of superoxide to the less-reactive species hydrogen peroxide, whereas CAT located in peroxisome efficiently boosts the transformation of hydrogen peroxide into water and oxygen. Collectively, these two antioxidant systems function synergistically and interdependently to maintain the ROS concentration at an optimum level rather than completely eliminate ROS via two major modes of action. The primary mode is referred to as the preventive mode, which functions as the suppressor of free radical generation, the sequestrator of metal ions, and the quencher of singlet oxygen (1O2) or electronically excited oxygen, which may cause oxidative damage. The secondary mode is described as scavenging ROS or the chain breaking mode, by which the produced ROS are detoxified [27]. However, the equilibrium between ROS and antioxidants is relative in vivo. In fact, the balance may slightly shift toward ROS, leading to oxidative damage at a low degree. This demands a reparative category of the antioxidant defense system, including specific enzymes, proteolytic systems, phospholipases, peroxidases, and acyl transferases, which removes or repairs the impaired biomolecules [29]. Such a category may be interpreted as the third mode of action of the antioxidant defense system. The fundamental knowledge over the biosynthesis and the biological roles of endogenous antioxidants in the whole body has been relatively well presented in the literature [29][30][31].
Endogenous antioxidants are generally present in all body fluids and tissues in humans. Saliva, which is mostly secreted by three pairs of major salivary glands, including submandibular, parotid, and sublingual, as well as by hundreds of minor salivary glands to a lesser extent, is one of the most versatile and important body fluids [32]. Saliva typically comprises 99% water, and the remaining 1% consists of a variety of electrolytes and proteins. Such complexity in salivary constituents is responsible for the wide range of physiological functions attributed to saliva [33]. Remarkably, saliva is crucial for the innate oral immunity. The functions of the salivary defense proteins involved in both innate and acquired oral immunity were well presented. The authors summarized five primary defense networks of salivary proteins in the whole saliva. First, salivary proteins and peptides that bind to bacteria or to oral surfaces or both may be responsible for the microbial agglutination and/or surface exclusion. Second, salivary cationic peptides and lysozyme may be involved in the lysis of microbial membranes. The third and fourth networks may account for the antifungal and antiviral properties, respectively. Finally, the immune regulatory network, including all salivary proteins that function as immune activators or modulators, may be important for the fine regulation of the local action of the mucosal immune system. Importantly, almost all proteins are multi-functional, suggesting their ability to act together to result in an efficient molecular defense network in the oral cavity [34]. In regard to redox biology, saliva can be considered as a key reservoir of both non-enzymatic and enzymatic antioxidants, which also provide three lines of defense, including prevention, detoxification, and reparation. Since excess ROS is likely to be involved in the development of periodontal disease, protection from salivary antioxidants has been the subject of numerous biomedical investigations in the field of oral health. Of significant importance is the antioxidative role of oral peroxidase (OP). The peroxidase activity of human saliva is attributed to salivary lactoperoxidase (LPO) and, to a lesser extent, myeloperoxidase (MPO). LPO is an enzyme found in a variety of human tissues, glands, and secretions, and it is an important element of the non-specific immune response to microorganism challenge. The major action mechanism of LPO is based on the oxidation of thiocyanate ions (SCN−) in the presence of hydrogen peroxide to produce hypothiocyanite ions (OSCN−), which cause the dysfunction and inhibition of microorganisms by oxidizing the thiol group of amino acid residues of microbial proteins. Specific to the oral cavity, LPO present in saliva is one of the significant constituents maintaining oral health, for instance, by combating the pathogens in periodontal disease. The physiological roles of human salivary LPO were described in detail [35]. While salivary LPO is of salivary glandular origin, MPO present in saliva is produced by neutrophils migrating into the oral cavity via the gingival crevices. Thus, the salivary MPO activity might indicate the infection or inflammation of oral tissues. Similar to LPO, MPO can also catalyze the oxidation of SCN− ions in the presence of hydrogen peroxide to produce much more bactericidal agent, namely hypothiocyanite ions [36]. In our work, the term OP is utilized to indicate the total activity of both peroxidase species, LPO and MPO. OP detoxifies hydrogen peroxide, the major ROS produced by bacterial metabolism, through the peroxidase-catalyzed oxidation of SCN− to yield potent hypothiocyanite ions that exert antimicrobial activity by oxidizing structural and functional components of microbes. In addition to the antimicrobial capacity, hypothiocyanite can also disrupt the production of hydrogen peroxide by oral microorganisms [37]. SCN− is a pseudohalide thiolate existing in secreted biological fluids, such as saliva, plasma, and urine, among others [38]. There is significant variation in terms of SCN− levels amongst such fluids. In a recent review, saliva was reported to exhibit the highest SCN− levels, with a range from 0.5 up to 2 mM, whereas plasma or urine SCN− concentrations were detected at the μM degree [39]. The remarkable difference of SCN− levels in saliva from those in others may be due to the presence of LPO along with other antimicrobial defenses in saliva [40]. Owing to the large pool of salivary SCN−, the oral cavity thus provides a favorable environment for oral peroxidase activity in the presence of H2O2, underlining the importance of the OP/SCN−/H2O2 system in the host defense. On the other hand, saliva is rich in non-enzymatic antioxidants, such as uric acid, albumin, and glutathione, among others. It is well established that uric acid is one of the predominant salivary antioxidants, which is responsible for more than 70% of the total antioxidant capacity of saliva. Uric acid acts as a sweeping agent that eradicates free radicals [41]. In addition to saliva, gingival crevicular fluid (GCF) is also likely to contain a number of antioxidants, which are locally derived from microbiofilm, neutrophils, and crevicular epithelium. The levels of SOD and glutathione represent the most important enzymatic and non-enzymatic antioxidants in GCF, respectively [27]. Taken together, it is clear to postulate that periodontal tissues overcome the oxidative damage caused by exaggerated ROS mainly through the locally endogenous antioxidants (Figure 3). Thus, a deficiency or depletion in such protection may induce oxidative damage and tissue breakdown, eventually leading to the advanced progression of periodontal disease. Indeed, there has been a growing body of evidence consistently indicating a decrease of the antioxidant status in patients with periodontitis in comparison to periodontally healthy individuals despite the diversity of analytical approaches. Many observational studies were comprehensively summarized in a recent review, indicating that the antioxidant status in blood was negatively correlated with the severity of periodontal disease. The more severe the periodontitis, the more powerful the association. Consistently, it has also been confirmed that the total antioxidant capacity was diminished either systemically in plasma or serum, or locally in saliva or GCF, in patients with any forms of periodontal disease [42]. These findings suggest the important roles of antioxidants in the pathogenesis of periodontal disease. Despite the advances of research concerning this scope, there are still gaps that require elucidation. In particular, the paucity of powerful evidence, such as in vivo data, that explores the dynamics of antioxidants during the progression of periodontal disease seems to be a considerable obstacle.
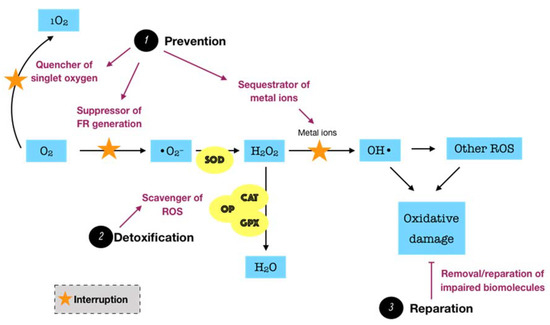
Figure 3. Schematic representation of the functions of endogenous antioxidant defense systems. Endogenous antioxidants, which present in all body fluids and tissues, consist of enzymatic and non-enzymatic systems. They counteract ROS and maintain redox balance via three major modes of action. First, the preventive mode (prevention) functions as the quencher of singlet oxygen, suppressor of free radical (FR) production, and sequestrator of metal ions. Second, the detoxifying mode (detoxification) involves the scavenger of ROS or interruption of chain reactions. Third, the reparative mode (reparation) functions to remove or repair the biomolecules suffering from oxidative damage. In the oral cavity, saliva and gingival crevicular fluid represent important local reservoirs of endogenous antioxidants.
3.3. Oxidative Stress and Its Involvement in the Development of Periodontal Disease via the Immuno-Inflammatory Pathway
Description of oxidative stress. As mentioned above, ROS are physiologically generated during mitochondrial oxidative metabolism and other cellular processes. They are mostly believed to be detrimental entities due to their potency to cause oxidative damage to vital biomolecules. Paradoxically, accumulating evidence has shown that ROS at low concentrations also serve as critical signaling molecules in a variety of cellular processes, suggesting their necessity in biological systems. Thus, ROS should be considered as double-edged swords in which the concentration is a key element determining which edge is displayed [43]. Other factors, such as the species of ROS as well as the subcellular location and cellular source of their generation, may also be related to the effects of ROS [44]. In order to control the ROS under an acceptable threshold, the human body is equipped with a defensive network known as intrinsic antioxidants. The maintenance of a physiological level of oxidants, particularly ROS, is greatly important for biological systems through redox signaling. In contrast, an excessive oxidative status due to the overproduction of ROS and/or the deficiency or incompetence of antioxidant defenses may cause damage to the biomolecules that govern living processes, termed oxidative stress [45]. It is widely accepted that oxidative stress is “an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage” [46]. According to this definition, it is obvious to perceive that oxidative stress is inextricably involved in the disturbance of redox homeostasis, the interruption of signaling pathways, and the impairment of biomolecules, in turn, significantly contributing to the promotion of the aging process as well as the development of numerous diseases [47].
The causal relationship between ROS-mediated oxidative stress and periodontal disease. The causal relationship between oxidative stress mediated by ROS with periodontal disease can be established based on four lines of evidences. Firstly, it has been found that excessive ROS and subsequent oxidative damage are present at the site of injury. A number of studies have directly demonstrated that the levels of reactive oxygen metabolites in the serum of patients with periodontal disease, particularly chronic periodontitis, were significantly higher than those of periodontally healthy individuals [48][49][50]. Furthermore, there have been observations of the destruction of biomolecules induced by ROS as indirect evidence concerning the association between oxidative stress and periodontal disease. Most publications have focused on biomarkers of lipid peroxidation, such as malondialdehyde (MDA). The findings have consistently suggested that patients suffering from periodontal disease display higher levels of lipid peroxidation than periodontally healthy individuals [51]. Besides, the biomarkers of protein damage and DNA damage have been investigated as well. For instance, the levels of 8-hydroxydeoxyguanosine (8-OHdG), a marker of oxidative DNA damage, in subjects with chronic periodontitis have been demonstrated to be significantly elevated in comparison to those in controls with healthy periodontium [52]. Meanwhile, the evidence on protein oxidation was mostly obtained through the elevation of protein carbonyl, which reflects the oxidative damage to proteins. This biomarker has shown high levels in saliva, GCF, and even serum in individuals suffering from advanced periodontal disease [52][53][54]. Secondly, it has been demonstrated that the time course of production of ROS or oxidative damage may occur previously or simultaneously as tissue injury. One representative of this second evidence line is the experimental research that investigated the liberation of ROS (H2O2) and oxidative DNA damage biomarker (8-OHdG) using rat models with or without application of periodontal pathogens as the experimental groups and control group, respectively. The authors only observed remarkable expression of H2O2 in the junctional epithelium and the subepithelial connective tissue in periodontitis-induced rats. Moreover, both 8-OHdG expression in gingival fibroblasts and 8-OHdG levels in the plasma of the periodontitis groups were significantly higher than those of the control group. The positive correlation between plasma 8-OHdG and 8-OHdG-positive fibroblasts was demonstrated as well. These findings suggested that ROS were released by gingival fibroblasts in response to pathogens, and the densities and levels of 8-OHdG appeared to reflect the status of periodontal health [55]. Thirdly, experimentally, direct application of ROS at concentrations over a relevant time course to tissues should reproduce similar damage in vivo to that observed in tissues suffering from injury [56]. For instance, a previous study indicated that the intradermal injection of xanthine and xanthine oxidase as a superoxide-generating system in rats may cause significant infiltration of neutrophils in vivo as determined by histological observations. Furthermore, the intravenous administration of superoxide dismutase (SOD) along with the superoxide-generating system was found to suppress the inflammatory responses and inhibit leukocyte infiltration into the treated sites, further supporting the superoxide-dependent accumulation of inflammatory cells in vivo [57]. However, due to the high reactivity and short lifetime of ROS, it is challenging to design a ROS-generating system for in vivo application. Moreover, the direct measurement of free radical generation in an in vivo model detected very low concentrations of ROS [58], making the direct application of ROS more difficult. Thus, almost all existing reports have investigated the direct effects of ROS using in vitro models that may be implicated in tissue damage in vivo. Periodontal disease, especially in advanced stages, is characterized by the degradation and subsequent loss of ligamentous support and alveolar bone, ultimately leading to tooth loss [59]. A number of available data have shown that excessive ROS may cause non-selective damage to the components of the extracellular matrix of periodontal tissues. It has been demonstrated that ROS can damage proteoglycans, hyaluronan, and collagen, leading to the breakdown of connective tissue [56]. Furthermore, although adverse effects of ROS on bone resorption have not been directly investigated yet, they have been determined to probably hinder alveolar bone formation by inhibiting osteoblastic differentiation and promoting osteoclastogenesis [60][61], suggesting their potential role in bone resorption in periodontal disease. Such a role in bone resorption was further supported by the finding that direct exposure of gingival fibroblasts to hydroxy radical and, to a lesser extent, hydrogen peroxide can degrade alveolar bone proteoglycans in vitro [62]. While these findings have important implications for periodontal pathogenesis, the lack of evidence that in vivo levels of ROS production in periodontal tissues cause such damage still needs to be addressed in the future. Finally, it has been documented that the removal or inhibition of ROS generation may subside tissue damage to a degree similar to the antioxidant action in vivo. The typical case of this evidence line comes from the effects of SOD known as an essential scavenger of ROS. By using rat models, SOD was demonstrated to provide therapeutic effects on periodontal pathogen-induced inflammation and to promote the healing process [63]. Similarly, another study using periodontitis-induced Beagle dogs found that adjunctive SOD significantly improved the clinical symptoms and inhibited the inflammation in comparison to mechanical treatment alone [64]. Taken together, the suggestion with respect to the causal role of oxidative stress mediated by ROS in the pathology of periodontal disease can be drawn. Therefore, it is reasonable to postulate that periodontal disease is initially launched by the interaction between microbial challenge and host response but is further progressed by the oxidative stress paradigm via excessive ROS activities and the aberrant inflammatory state.
The oxidative stress-mediated immunoinflammatory pathway in periodontal disease. Recently, the great interest into the relationship between oxidative stress with immuno-inflammatory responses and inflammation has provided new perspectives regarding the initiation and progression of diseases, particularly chronic conditions [65][66][67]. Considering periodontal disease is defined as a chronic inflammatory condition in the context of this work, the discovery of the role of oxidative stress-mediated inflammation should be considered as important as the detection of the pathogenic microorganisms in periodontal pathology. As mentioned above, the levels of ROS are elevated in periodontal tissues during the primary immune responses following the periodontally microbial challenge via respiratory burst. Albeit their destructive properties can function as an antimicrobial killer to benefit the whole periodontal apparatus, ROS not only harass the pathogens but also non-selectively damage surrounding cells and even tissues in the periodontium. Such periodontal tissue breakdown may enhance the synthesis of proinflammatory cytokines followed by the recruitment and activation of immune cells, leading to abnormal ROS production and subsequent oxidative stress. In this way, an infinite circuit among immuno-inflammatory responses, ROS-mediated oxidative stress occurrence, and periodontal tissue destruction may occur. The burden of ROS in periodontal tissues can be amplified in the presence of risk factors, particularly diabetes mellitus and tobacco smoking, which have been demonstrated to associate with oxidative stress as well [68][69]. Thus, oxidative stress caused by excessive ROS may act as the culprit of chronic inflammation in periodontal tissues, leading to the development of periodontal disease. Now, the more complex matter is the underlying paradigm by which ROS-induced oxidative stress regulates the immuno-inflammatory responses to cause periodontal tissue damage. To our knowledge, there are three major routes relevant to this issue (Figure 4). Nevertheless, the detailed mechanisms are yet to be fully elucidated.
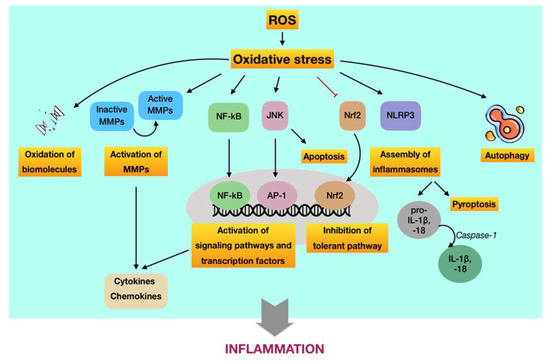
Figure 4. Schematic representation of the underlying pathways by which ROS-induced oxidative stress regulates inflammation, contributing to the progression of periodontal disease. First, oxidative stress may induce oxidation of vital biomolecules and activate matrix metalloproteinases MMPs, which are regulators of inflammation. Second, oxidative stress may enhance the production and expression of proinflammatory cytokines, chemokines, among others, through the activation of NF-κB-, JNK-, and NLRP3-dependent pathways. Moreover, the JNK route may induce apoptosis, whereas the NLRP3 route may cause pyroptosis. Besides, severe oxidative stress may also hinder the tolerant mechanism via suppression of the Nrf2 paradigm. Finally, oxidative stress may function as an inducer or inhibitor of autophagy activities.
(i) ROS-induced oxidative stress may cause damage at molecular and cellular levels. Aside from the destruction of biomolecules, this route is featured by the oxidative activation of matrix metalloproteinases (MMPs), which are calcium-dependent zinc-containing enzymes involved in the degradation of the extracellular matrix [69]. From the viewpoint of periodontal disease, MMPs are essential proteases that are associated with the destructive process of periodontium. Among members of the MMP family, MMP-2, MMP-8, MMP-9, and MMP-13 are of great importance in the pathology of periodontal disease [70]. MMPs are synthesized as inactive pro-enzymes in the body. During the development of periodontal disease, they can be activated by independent or cooperative proteolytic cascades, leading to widespread tissue destruction and pathological progression. On the other hand, oxidative non-proteolytic MMP activation seems to play an important role as well [71]. Evidence has figured out that ROS are able to activate MMPs in periodontal tissues via direct oxidation. It was found that MMP-2 and MMP-9 were activated in different cell systems, particularly periodontal ligament fibroblasts, by exposure to ROS, such as hydrogen peroxide [72][73]. Besides, other studies indicated that oxidative stress may increase the extracellular matrix turnover mediated by MMP-2, MMP-8, MMP-9, and MMP-13 [74][75]. The pivotal role of MMPs as regulators of periodontal inflammation was well reported in a recent review. Active MMPs are likely involved in the process and cleavage of signaling molecules, such as cytokines, chemokines, and growth factors, among others, thus modulating their biological functions and/or bioavailability, eventually resulting in destructive and prolonged inflammation [76].
(ii) ROS-induced oxidative stress may regulate signaling pathways and transcription factors involved in immuno-inflammatory responses. The predominant route by which ROS-induced oxidative stress involved in aberrant periodontal inflammation is the activation or inhibition of signaling pathways and transcription factors that modulate the immuno-inflammatory responses. First, it has been reported that ROS may activate the NF-κB signaling pathway [77][78]. Such activation may initiate the transcription of a wide range of genes, including proinflammatory cytokines, chemokines, MMPs, and other inflammatory mediators, eventually resulting in breakdown of periodontal tissues via the sustained inflammatory responses and osteoclastic differentiation [79]. In addition, it has been indicated that ROS may trigger the c-Jun N-terminal kinase (JNK) signaling pathway [80]. It is well-known that JNK is a member of a subfamily of mitogen-activated protein kinases (MAPKs), which play crucial roles in signal transduction of extracellular hormones, growth factors, cytokines, bacterial antigens, and environmental stresses, as well as in immune-mediated inflammatory responses [79]. As an oxidative stress-activated protein kinase, JNK can lead to ROS-induced apoptosis, which may play role in the pathology of periodontal disease [81]. Furthermore, ROS-activated JNK can induce the activation of downstream transcription factors, such as c-Jun/AP-1, in turn operating the expression of different genes in response to cytokines [82]. Inhibitors of JNK have also been demonstrated to efficiently hinder the production of proinflammatory mediators [83][84]. The overproduction of ROS and occurrence of oxidative stress not only exacerbates the periodontal damage by promoting the release and aggregation of proinflammatory cytokines and mediators via the activation of signaling pathways but also by the assembly of inflammasomes. The inflammasome is part of the innate immune system that counters microorganisms or stress challenge via the activation of caspase-1 and the induction of inflammation [85]. In regard to periodontal disease, the expression of the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome has been demonstrated to be increased in patients with periodontitis compared to periodontally healthy subjects [86][87]. Following the infection of the periodontal pathogen, namely P. gingivalis, it was found that the release of IL-1β, IL-6, and IL-18; the gene expression of pro-IL-1β and pro-IL-18; and the activity of caspase-1 in wild-type mice were significantly enhanced in comparison to NLRP3-deficient mice [88]. The activation of caspase-1 is considered as necessary element for the transformation of IL-1β and IL-18 into active cytokines as well as for the onset of a highly inflammatory form of programmed cell death, so-called pyroptosis. Thus, NLRP3 appeared to associate with the production and maturation of proinflammatory cytokines, such as IL-1β and IL-18, and the initiation of pyroptosis, leading to chronic inflammation [89]. There was evidence indicating that ROS may induce the activation of NLRP3 in periodontal tissues [90]. In addition to the activating aspect, ROS-induced oxidative stress has also been shown to inhibit key transcription factors involved in the tolerance of periodontal disease. Of significance, nuclear factor erythroid 2-related factor 2 (Nrf2), a basic leucine zipper transcription factor, is a crucial element that regulates the transcription of a large group of genes of antioxidants and detoxifying enzymes. Under physiological conditions, Nrf2 is bound to Kelch-like ECH-associated protein 1 (Keap1), which functions as a mediator for Nrf2 degradation, thereby maintaining acceptable levels of Nrf2 and preventing unnecessary transcription of antioxidant genes. In contrast, Nrf2 is dissociated from Keap1 during oxidative stress to upregulate Nrf2-associated antioxidants and detoxifying enzymes, providing important protective effects, such as a reduction of inflammatory signaling pathways and oxidative damage in periodontal tissues. However, the downregulation of the Nrf2 pathway followed by the decreased production of antioxidants were observed to associate with the elevated recruitment of oral PMNs and with the advanced progression of periodontitis. These findings suggested that the overwhelmed oxidative stress may blunt the Nrf2 signaling [91].
(iii) ROS-induced oxidative stress may regulate autophagic activities. Autophagy can be defined as a cellular self-degradative process by which long-lived proteins and damaged organelles are recycled to maintain energy homeostasis. Despite the original classification as a form of programmed cell death, autophagy has been widely considered as a survival mechanism to cope with the external challenges [92]. There have been few evidence regarding the role of autophagy induced by ROS in the pathology of periodontal disease; however, they still remain controversial. It may have a protective impact on periodontal tissues through the selective elimination of specific periodontal pathogens, the regulation of immune and inflammatory responses, and the antagonist of apoptosis. Meanwhile, excessive ROS may function as either an inducer or inhibitor of autophagy activities by targeting autophagy-related genes and/or upstream signaling pathways [93]. Owing to the lack of sufficient evidence regarding the interdependence between ROS-mediated oxidative stress and autophagy in the development of periodontal disease, many more investigations about the redox regulation of autophagy involved in this ailment are required.
4. Conclusion
Periodontal disease remains a major burden for oral healthcare workers worldwide. Despite the myriad of possible mechanisms involving in the periodontal disease, oxidative stress-mediated immunoinflammatory pathway has become an area of intensive research associated to this ailment. Unveiling such oxidative pattern of periodontal pathology may be highly beneficial for the prevention and treatment of periodontal disease, particularly through the development and application of antioxidant-based strategies.
References
- Satish S. Kumar; Evidence-Based Update on Diagnosis and Management of Gingivitis and Periodontitis. Dental Clinics of North America 2019, 63, 69-81, 10.1016/j.cden.2018.08.005.
- Richard G Watt; Poul E. Petersen; Periodontal health through public health - the case for oral health promotion. Periodontology 2000 2012, 60, 147-155, 10.1111/j.1600-0757.2011.00426.x.
- Abiodun O. Arigbede; B. Osagbemiro Babatope; M. Kolude Bamidele; Periodontitis and systemic diseases: A literature review. Journal of Indian Society of Periodontology 2012, 16, 487-91, 10.4103/0972-124X.106878.
- Artur Falcao; Pedro Bullon; A review of the influence of periodontal treatment in systemic diseases. Periodontology 2000 2019, 79, 117-128, 10.1111/prd.12249.
- Periodontal Disease . Treasure Island (FL): StatPearls Publishing. Retrieved 2021-1-11
- N.J. Kassebaum; A.G.C. Smith; E. Bernabé; T.D. Fleming; A.E. Reynolds; T. Vos; C.J.L. Murray; W. Marcenes; GBD 2015 Oral Health Collaborators; G.Y. Abyu; et al.U. AlsharifH. AsayeshH. BenzianL. DandonaA. KasaeianY.S. KhaderY.H. KhangY. KokuboG.A. KotsakisR. LallooA. MisganawP. MonteroM. NourzadehC. PinhoM. QorbaniM.J. Rios BlancasM. SawhneyC. SteinerJ. TraebertS. TyrovolasK.N. UkwajaS.E. VollsetN. Yonemoto Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. Journal of Dental Research 2017, 96, 380-387, 10.1177/0022034517693566.
- Riccardo Nocini; Giuseppe Lippi; Camilla Mattiuzzi; Periodontal disease: the portrait of an epidemic. Journal of Public Health and Emergency 2020, 4, 10-10, 10.21037/jphe.2020.03.01.
- Ali Cekici; Alpdogan Kantarci; Hatice Hasturk; Thomas E. Van Dyke; Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 2013, 64, 57-80, 10.1111/prd.12002.
- Ruby Ramya Vincent; Devapriya Appukuttan; P.S.G.Pra Kash; Dhayanand John Victor; Oxidative stress: role in pathogenesis of periodontal disease. International Journal of pharma and Bio Sciences 2017, 8, 1033-1041, 10.22376/ijpbs.2017.8.3.b1033-1041.
- Yue Wang; Oleh Andrukhov; Xiaohui Rausch-Fan; Oxidative Stress and Antioxidant System in Periodontitis. Frontiers in Physiology 2017, 8, 910, 10.3389/fphys.2017.00910.
- Pınar Pınargümüş; Burcu Huseyinalemdaroglu; Nurcan Buduneli; The role of oxidative stress in the interaction of periodontal disease with systemic diseases or conditions. Oxidants and Antioxidants in Medical Science 2016, 5, 33-38, 10.5455/oams.310516.rv.024.
- Thomas E. Van Dyke; P. Mark Bartold; Eric C. Reynolds; The Nexus Between Periodontal Inflammation and Dysbiosis. Frontiers in Immunology 2020, 11, 511, 10.3389/fimmu.2020.00511.
- Yousef A. Aljehani; Risk Factors of Periodontal Disease: Review of the Literature. International Journal of Dentistry 2014, 2014, 1-9, 10.1155/2014/182513.
- Immunopathogenesis of Chronic Periodontitis . IntechOpen. Retrieved 2021-1-11
- Nasi Huang; Frank C. Gibson; Immuno-Pathogenesis of Periodontal Disease: Current and Emerging Paradigms. Current Oral Health Reports 2014, 1, 124-132, 10.1007/s40496-014-0017-8.
- Balaraman Kalyanaraman; Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biology 2013, 1, 244-257, 10.1016/j.redox.2013.01.014.
- Alugoju Phaniendra; Dinesh Babu Jestadi; Latha Periyasamy; Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian Journal of Clinical Biochemistry 2014, 30, 11-26, 10.1007/s12291-014-0446-0.
- Robert F. Furchgott; Endothelium-Derived Relaxing Factor: Discovery, Early Studies, and Identification as Nitric Oxide. Bioscience Reports 1999, 19, 235-251, 10.1023/a:1020537506008.
- Martina Antosova; Jana Plevkova; Anna Strapkova; Tomas Buday; Martina Antosova; Jana Plevkova; Strapkova Anna; Buday Tomas; Nitric oxide—Important messenger in human body. Open Journal of Molecular and Integrative Physiology 2012, 2, 98-106, 10.4236/ojmip.2012.23014.
- E. Änggård; Nitric oxide: mediator, murderer, and medicine. The Lancet 1994, 343, 1199-1206, 10.1016/s0140-6736(94)92405-8.
- Carl Nathan; Qiao-Wen Xie; Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915-918, 10.1016/0092-8674(94)90266-6.
- Cleva Villanueva; Cecilia Giulivi; Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radical Biology and Medicine 2010, 49, 307-316, 10.1016/j.freeradbiomed.2010.04.004.
- Eileen McNeill; Mark J. Crabtree; Natasha Sahgal; Jyoti Patel; Surawee Chuaiphichai; Asif J. Iqbal; Ashley B. Hale; David R. Greaves; Keith Channon; Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radical Biology and Medicine 2015, 79, 206-216, 10.1016/j.freeradbiomed.2014.10.575.
- Rafael Radi; Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proceedings of the National Academy of Sciences 2018, 115, 5839-5848, 10.1073/pnas.1804932115.
- Jian Sun; Lawrence J. Druhan; Jay L. Zweier; Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Archives of Biochemistry and Biophysics 2010, 494, 130-137, 10.1016/j.abb.2009.11.019.
- Parveen Dahiya; Rohit Bhardwaj; Karun Chaudhary; Reet Kamal; Rajan Gupta; Simerpreet Kaur; Reactive oxygen species in periodontitis. Journal of Indian Society of Periodontology 2013, 17, 411-6, 10.4103/0972-124x.118306.
- C. Rudrakshi; M. L. V. Prabhuji; S. Parween; S. Jyothsna; Relationship between Antioxidants and the Development of the Periodontal Disease. Cytology & Tissue Biology 2017, 4, 1-9, 10.24966/ctb-9107/100016.
- Barry Halliwell; Antioxidants: The Basics-what they are and how to Evaluate them. Studies in Surface Science and Catalysis 1996, 38, 3-20, 10.1016/s1054-3589(08)60976-x.
- Borut Poljsak; Dušan Šuput; Irina Milisav; Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Medicine and Cellular Longevity 2013, 2013, 1-11, 10.1155/2013/956792.
- Peter Patlevič; Janka Vašková; Pavol Švorc; Ladislav Vaško; Reactive oxygen species and antioxidant defense in human gastrointestinal diseases. Integrative Medicine Research 2016, 5, 250-258, 10.1016/j.imr.2016.07.004.
- Nonenzymatic Exogenous and Endogenous Antioxidants, Free Radical Medicine and Biology . IntechOpen. Retrieved 2021-1-11
- D. P. Lima; D. G. Diniz; S. A. Moimaz; D. H. Sumida; A. C. Okamoto; Saliva: reflection of the body. International Journal of Infectious and Diseases 2010, 14, e184-188, https://doi.org/10.1016/j.ijid.2009.04.022.
- Luciana Reis Azevedo; Antônio Adilson Soares De Lima; Maria Ângela Naval Machado; Ana Maria Trindade Grégio; Patricia Del Vigna De Almeida; Saliva Composition and Functions: A Comprehensive Review. The Journal of Contemporary Dental Practice 2008, 9, 72-80, 10.5005/jcdp-9-3-72.
- Tibor Károly Fábián; Péter Hermann; Anita Beck; Pál Fejérdy; Gábor Fábián; Salivary Defense Proteins: Their Network and Role in Innate and Acquired Oral Immunity. International Journal of Molecular Sciences 2012, 13, 4295-4320, 10.3390/ijms13044295.
- Marcin Magacz; Karolina Kędziora; Jacek Sapa; Wirginia Krzyściak; The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products.. International Journal of Molecular Sciences 2019, 20, 1443, 10.3390/ijms20061443.
- Denise Schlorke; Jorg Flemmig; Jana Gau; Paul G. Furtmüller; Christian Obinger; Jürgen Arnhold; New insights into thiocyanate oxidation by human myeloperoxidase. Journal of Inorganic Biochemistry 2016, 162, 117-126, 10.1016/j.jinorgbio.2016.06.019.
- J. Carlsson; Salivary peroxidase: an important part of our defense against oxygen toxicity. Journal of Oral Pathology & Medicine 2007, 16, 412-416, 10.1111/j.1600-0714.1987.tb02077.x.
- Joshua D. Chandler; Brian J. Day; THIOCYANATE: A potentially useful therapeutic agent with host defense and antioxidant properties. Biochemical Pharmacology 2012, 84, 1381-1387, 10.1016/j.bcp.2012.07.029.
- Han Shi; Yuyang Liu; Angie L. Schroder; Hans Zoellner; Belal Chami; The Role of Thiocyanate in Modulating Myeloperoxidase Activity during Disease. International Journal of Molecular Sciences 2020, 21, 6450, 10.3390/ijms21176450.
- Michael T. Ashby; Hypothiocyanite. Computational Chemistry 2012, 64, 263-303, 10.1016/b978-0-12-396462-5.00008-8.
- Ivan Minic; Antioxidant Role of Saliva. Journal of Otolaryngology Research 2019, 2, 124.
- L'ubomíra Tóthová; Peter Celec; Oxidative Stress and Antioxidants in the Diagnosis and Therapy of Periodontitis. Frontiers in Physiology 2017, 8, 1055, 10.3389/fphys.2017.01055.
- Aristidis S. Veskoukis; Aristidis Tsatsakis; Dimitrios Kouretas; Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress and Chaperones 2011, 17, 11-21, 10.1007/s12192-011-0293-3.
- Theodore Kalogeris; Yimin Bao; Ronald J. Korthuis; Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs preconditioning. Redox Biology 2014, 2, 702-714, 10.1016/j.redox.2014.05.006.
- Helmut Sies; Carsten Berndt; Dean Jones; Oxidative Stress. Annual Review of Biochemistry 2017, 86, 715-748, 10.1146/annurev-biochem-061516-045037.
- Helmut Sies; Oxidative stress: a concept in redox biology and medicine. Redox Biology 2015, 4, 180-183, 10.1016/j.redox.2015.01.002.
- Gabriele Pizzino; Natasha Irrera; Mariapaola Cucinotta; Giovanni Pallio; Federica Mannino; Vincenzo Arcoraci; Francesco Squadrito; Domenica Altavilla; Alessandra Bitto; Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity 2017, 2017, 1-13, 10.1155/2017/8416763.
- Anders Gustafsson; H. Ito; B. Asman; K. Bergström; Hyper-reactive mononuclear cells and neutrophils in chronic periodontitis. Journal of Clinical Periodontology 2006, 33, 126-129, 10.1111/j.1600-051x.2005.00883.x.
- J. B. Matthews; H. J. Wright; A. Roberts; P. R. Cooper; I. L. C. Chapple; Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clinical & Experimental Immunology 2006, 147, 255-264, 10.1111/j.1365-2249.2006.03276.x.
- Martin R Ling; Iain L. C. Chapple; John B. Matthews; Neutrophil superoxide release and plasma C-reactive protein levels pre- and post-periodontal therapy. Journal of Clinical Periodontology 2016, 43, 652-658, 10.1111/jcpe.12575.
- Mengmeng Chen; Wenjin Cai; Shufan Zhao; Lixi Shi; Yang Chen; Xumin Li; Xiaoyu Sun; Yixin Mao; Bing He; Yubo Hou; et al.Yu ZhouQiaozhen ZhouJianfeng MaShengbin Huang Oxidative stress‐related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology 2019, 46, 608-622, 10.1111/jcpe.13112.
- Haixiang Su; Mervyn Gornitsky; Ana M. Velly; Hanling Yu; Michael Benarroch; Hyman M. Schipper; Salivary DNA, lipid, and protein oxidation in nonsmokers with periodontal disease. Free Radical Biology and Medicine 2009, 46, 914-921, 10.1016/j.freeradbiomed.2009.01.008.
- Avani R. Pradeep; M. V. Ramchandraprasad; Pavan Bajaj; Nishanth S. Rao; Esha Agarwal; Protein carbonyl: An oxidative stress marker in gingival crevicular fluid in healthy, gingivitis, and chronic periodontitis subjects. Contemporary Clinical Dentistry 2013, 4, 27-31, 10.4103/0976-237X.111589.
- Esra Baltacıoğlu; Ferda Alev Akalın; Ahmet Alver; Orhan Değer; Erdem Karabulut; Esra Baltacioglu; Protein carbonyl levels in serum and gingival crevicular fluid in patients with chronic periodontitis. Archives of Oral Biology 2008, 53, 716-722, 10.1016/j.archoralbio.2008.02.002.
- Daisuke Ekuni; Takaaki Tomofuji; Naofumi Tamaki; Toshihiro Sanbe; Tetsuji Azuma; Reiko Yamanaka; Tatsuo Yamamoto; Tatsuo Watanabe; Mechanical stimulation of gingiva reduces plasma 8-OHdG level in rat periodontitis. Archives of Oral Biology 2008, 53, 324-329, 10.1016/j.archoralbio.2007.10.005.
- Iain L. C. Chapple; John B. Matthews; The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160-232, 10.1111/j.1600-0757.2006.00178.x.
- W. F. Petrone; D. K. English; K. Wong; J. M. Mccord; Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma.. Proceedings of the National Academy of Sciences 1980, 77, 1159-1163, 10.1073/pnas.77.2.1159.
- Howard P. Grill; Jay L. Zweier; Periannan Kuppusamy; Myron L. Weisfeldt; John T. Flaherty; Direct measurement of myocardial free radical generation in an in vivo model: Effects of postischemic reperfusion and treatment with human recombinant superoxide dismutase. Journal of the American College of Cardiology 1992, 20, 1604-1611, 10.1016/0735-1097(92)90457-x.
- Marja L. Laine; Wim Crielaard; Bruno G. Loos; Genetic susceptibility to periodontitis. Periodontology 2000 2011, 58, 37-68, 10.1111/j.1600-0757.2011.00415.x.
- Aleksandar Jakovljevic; Miroslav Andric; Maja Miletic; Katarina Beljic-Ivanovic; Aleksandra Knezevic; Slavko Mojsilovic; Jelena Milasin; Epstein-Barr virus infection induces bone resorption in apical periodontitis via increased production of reactive oxygen species. Medical Hypotheses 2016, 94, 40-42, 10.1016/j.mehy.2016.06.020.
- Hiroyuki Kanzaki; Satoshi Wada; Tsuyoshi Narimiya; Yuuki Yamaguchi; Yuta Katsumata; Kanako Itohiya; Sari Fukaya; Yutaka Miyamoto; Yoshiki Nakamura; Pathways that Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis. Frontiers in Physiology 2017, 8, 351, 10.3389/fphys.2017.00351.
- R. Moseley; Rachel J. Waddington; G. Embery; S. G. Rees; The Modification of Alveolar Bone Proteoglycans by Reactive Oxygen SpeciesIn Vitro. Connective Tissue Research 1998, 37, 13-28, 10.3109/03008209809028897.
- Hiroki Misaki; Makoto Suzuki; H. Yoshie; Kohji Hara; The effect of superoxide dismutase on the inflammation induced by periodontal pathogenic bacteria and wound healing of gingival incision.. Nihon Shishubyo Gakkai Kaishi (Journal of the Japanese Society of Periodontology) 1990, 32, 93-110, 10.2329/perio.32.93.
- Milan Petelin; Zlatko Pavlica; Teodora Ivanuša; Marjeta Šentjurc; Uroš Skalerič; Local delivery of liposome-encapsulated superoxide dismutase and catalase suppress periodontal inflammation in beagles. Journal of Clinical Periodontology 2000, 27, 918-925, 10.1034/j.1600-051x.2000.027012918.x.
- N. D. Vaziri; Bernardo Rodríguez-Iturbe; Mechanisms of Disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nature Clinical Practice Nephrology 2006, 2, 582-593, 10.1038/ncpneph0283.
- David P. Hajjar; Antonio M. Gotto; Biological Relevance of Inflammation and Oxidative Stress in the Pathogenesis of Arterial Diseases. The American Journal of Pathology 2013, 182, 1474-1481, 10.1016/j.ajpath.2013.01.010.
- Jérôme Lugrin; Nathalie Rosenblatt-Velin; Roumen Parapanov; Lucas Liaudet; The role of oxidative stress during inflammatory processes. Biological Chemistry 2014, 395, 203-230, 10.1515/hsz-2013-0241.
- Ullah Asmat; Khan Abad; Khan Ismail; Diabetes mellitus and oxidative stress—A concise review. Saudi Pharmaceutical Journal 2016, 24, 547-553, 10.1016/j.jsps.2015.03.013.
- Ian M. Fearon; Gary Phillips; Tony Carr; Mark Taylor; D. Breheny; Stephen P. Faux; The Role of Oxidative Stress in Smoking-Related Diseases. Mini-Reviews in Organic Chemistry 2011, 8, 360-371, 10.2174/157019311797440317.
- M. P. Caley; Vera L. C. Martins; Edel A. O'toole; Metalloproteinases and Wound Healing. Advances in Wound Care 2015, 4, 225-234, 10.1089/wound.2014.0581.
- Dragana Stanisic; Radmila Obradovic; Sanja Vujovic; Milica Jovanovic; Vladimir Zivkovic; The Connection of Periodontal Disease and Diabetes Mellitus: The Role of Matrix Metalloproteinases and Oxidative Stress. Serbian Journal of Experimental and Clinical Research 2019, 10.2478/sjecr-2019-0051, 10.2478/sjecr-2019-0051, 10.2478/sjecr-2019-0051.
- Cavalla Franco; Patricia Hernández-Ríos; Timo Sorsa; Biguetti Claudia; M. Hernández; Matrix Metalloproteinases as Regulators of Periodontal Inflammation. International Journal of Molecular Sciences 2017, 18, 440, 10.3390/ijms18020440.
- Joan M. Cook-Mills; Hydrogen peroxide activation of endothelial cell-associated MMPs during VCAM-1-dependent leukocyte migration.. Cellular and Molecular Biology 2006, 52, 8-16.
- Carlos G Osorio; Franco Cavalla; Andrea C Paulalima; Guillermo Díaz Araya; Rolando Vernal; P. Ahumada; Jorge Gamonal; M. Hernández; H2 O2 activates matrix metalloproteinases through the nuclear factor kappa B pathway and Ca2+ signals in human periodontal fibroblasts. Journal of Periodontal Research 2015, 50, 798-806, 10.1111/jre.12267.
- Patricia Hernández-Ríos; Pirkko J. Pussinen; Rolando Vernal; Marcela Hernández; Oxidative Stress in the Local and Systemic Events of Apical Periodontitis. Frontiers in Physiology 2017, 8, 869-869, 10.3389/fphys.2017.00869.
- Hitesh Desarda; Subodh Gaikwad; Matrix Metalloproteinases & Implication in Periodontitis- A Short Review. Journal of Dental and Allied Sciences 2013, 2, 66, 10.4103/2277-4696.159288.
- Chong-Hou Sam; Hsein-Kun Lu; The role of hypochlorous acid as one of the reactive oxygen species in periodontal disease. Journal of Dental Sciences 2009, 4, 45-54, 10.1016/s1991-7902(09)60008-8.
- Michael J Morgan; Zheng-Gang Liu; Crosstalk of reactive oxygen species and NF-κB signaling. Cell Research 2010, 21, 103-115, 10.1038/cr.2010.178.
- João A.C. Souza; Carlos Rossa; Gustavo Pompermaier Garlet; Andressa V.B. Nogueira; Joni Augusto Cirelli; Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease.. Journal of Applied Oral Science 2012, 20, 128-138.
- Hiroyasu Nakano; A Nakajima; S Sakon-Komazawa; J-H Piao; X Xue; K Okumura; Reactive oxygen species mediate crosstalk between NF-κB and JNK. Cell Death & Differentiation 2005, 13, 730-737, 10.1038/sj.cdd.4401830.
- S.W. Kang; H.J. Park; J.Y. Ban; J.H. Chung; G.S. Chun; J.O. Cho; Effects of nicotine on apoptosis in human gingival fibroblasts. Archives of Oral Biology 2011, 56, 1091-1097, 10.1016/j.archoralbio.2011.03.016.
- Karine Z. Oben; Sara S AlHakeem; Mary K. McKenna; Jason A. Brandon; Rajeswaran Mani; Sunil K. Noothi; Liu Jinpeng; Shailaja Akunuru; Sanjit K. Dhar; Inder P. Singh; et al.Ying LiangChi WangAhmed Abdel-LatifHarold F. Stills JrDaret K. St. ClairHartmut GeigerNatarajan MuthusamyKaoru TohyamaRamesh C. GuptaSubbarao Bondada Oxidative stress-induced JNK/AP-1 signaling is a major pathway involved in selective apoptosis of myelodysplastic syndrome cells by Withaferin-A. Oncotarget 2017, 8, 77436-77452, 10.18632/oncotarget.20497.
- Danielle Morse; Soeren E. Pischke; Zhihong Zhou; Roger J. Davis; Richard A. Flavell; Torsten Loop; Sherrie L. Otterbein; Leo E. Otterbein; Augustine M.K. Choi; Suppression of Inflammatory Cytokine Production by Carbon Monoxide Involves the JNK Pathway and AP-1. Journal of Biological Chemistry 2003, 278, 36993-36998, 10.1074/jbc.m302942200.
- Michelle Melino; Charles S Hii; Shaun R. McColl; Antonio Ferrante; The effect of the JNK inhibitor, JIP peptide, on human T lymphocyte proliferation and cytokine production.. The Journal of Immunology 2008, 181, 7300-7306, 10.4049/jimmunol.181.10.7300.
- Haitao Guo; Justin B. Callaway; Jenny Ting; Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature Medicine 2015, 21, 677-687, 10.1038/nm.3893.
- Pei-Hui Ding; Meng-Xin Yang; Na-Na Wang; Li-Jian Jin; Yan Dong; Xia Cai; Li-Li Chen; Porphyromonas gingivalis-Induced NLRP3 Inflammasome Activation and Its Downstream Interleukin-1β Release Depend on Caspase-4. Frontiers in Microbiology 2020, 11, 1881, 10.3389/fmicb.2020.01881.
- Julie T. Marchesan; Mustafa Saadat Girnary; Kevin Moss; Eugenia Timofeev Monaghan; Grant Joseph Egnatz; Yizu Jiao; Shaoping Zhang; Jim Beck; Karen V. Swanson; Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontology 2000 2019, 82, 93-114, 10.1111/prd.12269.
- Yohei Yamaguchi; Tomoko Kurita-Ochiai; Ryoki Kobayashi; Toshihiko Suzuki; Tomohiro Ando; Regulation of the NLRP3 inflammasome in Porphyromonas gingivalis-accelerated periodontal disease. Inflammation Research 2016, 66, 59-65, 10.1007/s00011-016-0992-4.
- Luigi Franchi; Tatjana Eigenbrod; Raúl Muñoz-Planillo; Gabriel Nuñez; The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature Immunology 2009, 10, 241-247, 10.1038/ni.1703.
- Dawei Lian; Linfeng Dai; Zhaoyu Xie; Xing Zhou; Xiaohong Liu; Yang Zhang; Yi Huang; Yang Chen; Periodontal ligament fibroblasts migration injury via ROS/TXNIP/Nlrp3 inflammasome pathway with Porphyromonas gingivalis lipopolysaccharide. Molecular Immunology 2018, 103, 209-219, 10.1016/j.molimm.2018.10.001.
- A.V. Chiu; M. Al Saigh; C.A. McCulloch; M. Glogauer; The Role of NrF2 in the Regulation of Periodontal Health and Disease. Journal of Dental Research 2017, 96, 975-983, 10.1177/0022034517715007.
- Amber N. Hale; Dan J. Ledbetter; Thomas R. Gawriluk; Edmund B. Rucker; Autophagy. Autophagy 2013, 9, 951-972, 10.4161/auto.24273.
- Maria Greabu; Francesca Giampieri; Marina Melescanu Imre; Maria Mohora; Alexandra Totan; Silviu Mirel Pituru; Ecaterina Ionescu; Autophagy, One of the Main Steps in Periodontitis Pathogenesis and Evolution. Molecules 2020, 25, 4338, 10.3390/molecules25184338.




