| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Parvaneh Mehrbod | + 10398 word(s) | 10398 | 2020-12-31 04:38:27 | | | |
| 2 | Dean Liu | -3930 word(s) | 6468 | 2021-01-11 09:56:22 | | |
Video Upload Options
This manuscript was provided as a comprehensive review of the anti-influenza virus effect of quercetin and its derivatives with critical evaluation. We provided different classifications and focused on viral pathogenesis, animal models, human studies, in silico and docking studies and molecular pathways of quercetin and derivatives effects comprehensively which is not included in similar articles. This review is a multidisciplinary collection of cell biology, biotechnology, drug development, and virus investigation.
1. Introduction
1.1 Influenza Virus
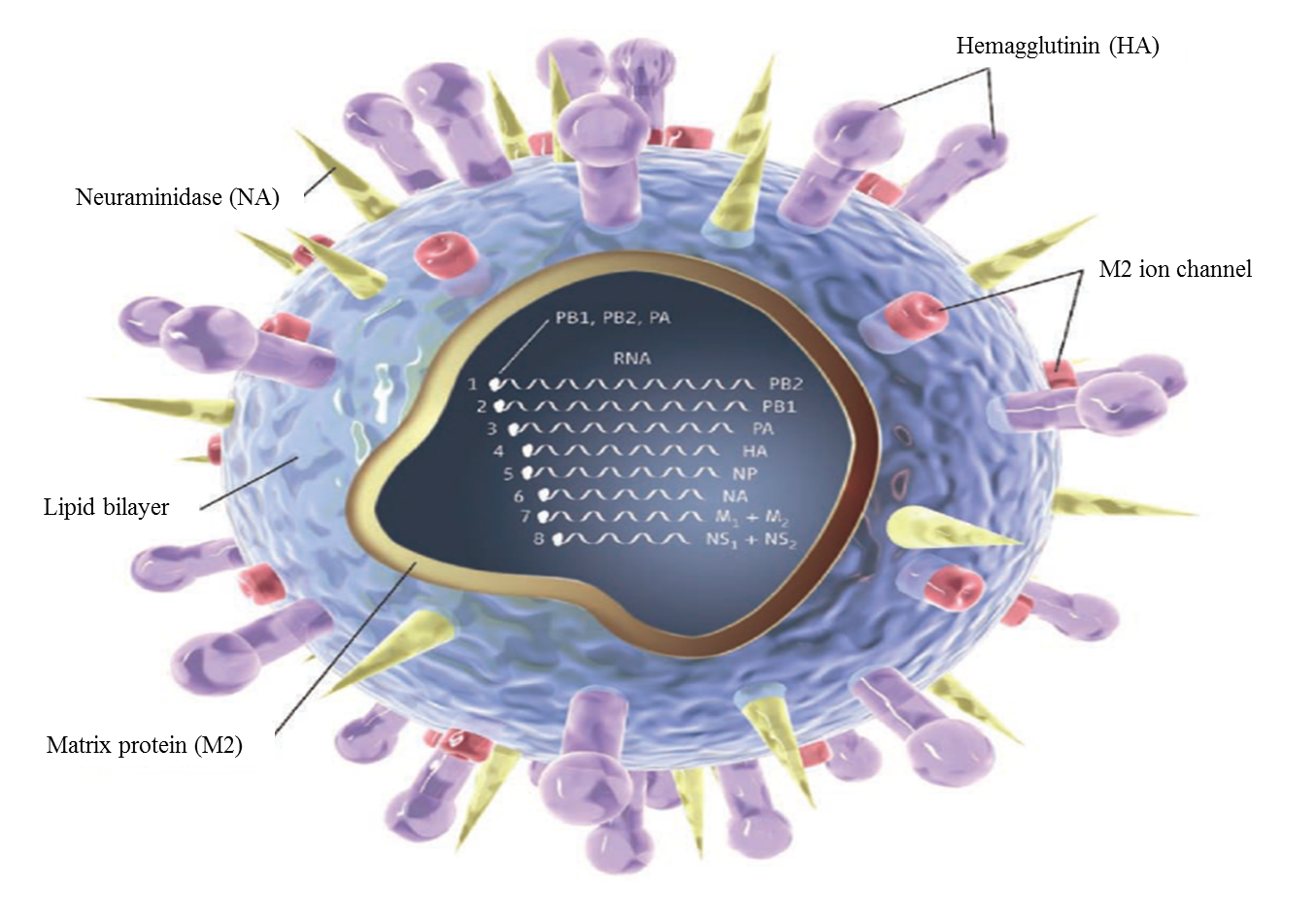
Influenza A virus (IAV) is the underlying cause for human and animal flu infections[1]. It has an eight-segment negative-sense, single-stranded RNA genome, which code several functional, structural, and non-structural proteins (Figure 1)[2]. Several antigenic drifts and shifts in its genetic structure, especially in HA and NA, have created different types and subtypes of this virus. Among them, three HA (H1, H2, and H3) and two NA (N1 and N2) subtypes have been responsible for human epidemics and pandemics for hundreds of years[3]. These viral proteins play essential roles in viral life cycle steps including attachment and entry, synthesis of viral RNA and proteins, packaging, budding, and release[2].
Figure 1. Structure of influenza virus
Influenza afflicts about 5%-10% of adults and 20%-30% of children annually[4]. Severe disease could result in the deaths of about 290 000 to 650 000 people worldwide in one season[5]. Beside deaths, influenza may cause respiratory, diabetic, cardiovascular, renal and neurological complications[6]. Additionally, the estimated cost of influenza infection in the US alone amounts to about $11.2 billion annually[7]. In 2019/2020, the ‘influenza-season’ coincided with the COVID-19 pandemic outbreak and the current (2020/2021) ‘influenza-season’ is coinciding with the peak of COVID-19 epidemics in many countries, increasing the risk of co-infection with both viruses. To date, at least 13 cases of co-infection of Covid-19 and influenza have been reported in PubMed-listed articles. A larger prevalence of co-infection is suspected, though still under investigation due to the symptoms similarities, as reviewed by H. Khorramdelazad et al. (2020)[8]. In some of the reviewed cases, the co-infection was not more severe than infection with COVID-19 alone[9]. One report suggested a protective effect of influenza infection against infection with COVID-19[10]. Co-infection was also investigated in an animal model study using golden Syrian hamsters[11]. The study results indicated more ‘clinically’ severe disease in the case of co-infection with both viruses. In September 2020 a British SAGE (Scientific Advisory Group for Emergencies) report was released concerning co-infection of COVID-19 and influenza in hospitalized patients. About 18% of hospitalized Covid-19 patients that were tested for influenza also carried influenza virus. Only 3,9% of all COVID-19 positive cases were also cross-tested for influenza, and only 0.7% of them were influenza–positive [12]. The consequences of co-infections were an increased number of critical care admissions, longer hospitalization time and a greater need for oxygen and mechanical ventilation[12]. As both diseases produce similar symptoms, it is highly recommended to use all possible means to prevent the infection with influenza, as the COVID-19 vaccine is still not widely available.
Yearly vaccination is the primary plan to control this infection; however, annual vaccination has limitations[13][14].
Current therapeutic strategies are primarily used for prophylaxis and treatment. One category of anti-influenza drugs, neuraminidase inhibitors (NAI), inhibits influenza virus neuraminidase reducing viral shedding within the respiratory tract[15]. Another category, which includes M2 channel blockers, functions by blocking the viral RNA uncoating within infected cells and preventing its replication by disrupting the function of the transmembrane domain of the viral M2 protein[15].
Despite the improved development of conventional antiviral agents, their effectiveness against influenza viruses is limited[16] and their clinical efficacy is also ambiguous[17][18][19]. Moreover, dramatic increases in resistance of influenza virus against these drugs have been reported[20][21][22][23][24], although resistance to neuraminidase inhibitors has remained at low levels[25]. Severe cases of influenza infection are associated with the risk of death, especially in children, the elderly, and in immunocompromised patients[26]. Therefore, efficient control of influenza outbreaks requires the discovery and development of novel antiviral drugs affecting different aspects of the viral infection pathway. For this purpose, nature might be promising as a reference for targetable pathways and modes.
1.2. Viral Pathogenesis
The interactions between virus and host cell proteins are strong determinants of influenza A virus pathogenicity. Once influenza virus gains access to the human respiratory tract, it attaches to and penetrates the epithelial cells via the HA protein and uses the host cell system for its replication. The newly emerging progeny viruses bud from cell membrane and released with the assistance of NA and infect adjacent epithelial cells. The NS1 protein inhibits IFN-I production in virus-infected cells by interfering with the RIG-I signaling pathway. Pulmonary macrophages then induce epithelial cell apoptosis, which is the underlying mechanism of viral pathogenesis and is mediated by the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-DR6 in the infected lung cells. After shedding, it causes local inflammatory responses as well as systemic toxic symptoms such as high fever, pain and a decreased white blood cells (WBC) count. The infected cells can also produce excessive interferon (IFN), which may be related to the systemic symptoms. However, no viremia has been reported[27][28].
Oda et al. (1989) indicated that oxygen radicals produced by the host’s delayed response affect the mortality of virus-infected mice and are important for the pathogenesis of influenza virus infection[29]. The effect of influenza virus on the pro-/antioxidant balance in the host cells has been investigated and the virus has been shown to be able to generate reactive oxygen species (ROS) and pro-oxidant cytokines such as TNF from phagocytes [30]. Other studies did not support a direct role for ROS-induced tissue damage in the pathogenesis of influenza virus infection in the lungs of infected mice[31]. It was concluded that excessive production of NO, mediated by IFN-γ, together with O2-, which forms more reactive peroxynitrite, may be the most important pathogenic factors in influenza virus-induced pneumonia in mice[32]. High influenza viral load activates a burst in phagocytic cells. The infected mice lung cells exhibited oxidative stress via enhanced superoxide and xanthine oxidase (XO) generation, as well as decreased capacity of small molecular antioxidants. Cytokines produced in the lungs may contribute to the systemic effects of influenza. ROS may activate viral replication via activation of NFκB, while oxidants decrease the CD4+ T cell count by inducing apoptosis in these cells[33]. Furthermore, it has been suggested that the influenza virus activates phagocytes by the generation of metabolites derived from superoxide (O2) and nitric oxide (NO) after interaction between the viral surface glycoproteins and the phagocyte’s plasma membrane. The virus may take advantage of these potentially virucidal metabolites to promote infection. Murphy et al. (1998), showed that experimental influenza virus infection increased oral NO, but not nasal exhaled NO levels, suggesting that NO does not directly contribute to the respiratory tract symptoms after infection with influenza A virus[34].
With regards to surface glycoproteins, neuraminidase of the virus plays an essential role in the release of virus from the infected cells by cleaving the terminal sialic acid residue from the host receptor and helps newly synthesized progeny viruses to release. This protein has a highly conserved active site in both type A and type B of influenza viruses and is the target of neuraminidase inhibitors. Resistance to neuraminidase inhibitors, which is accompanied by mutations in hemagglutinin, could affect the antigenicity of influenza viruses[35].
Influenza infection has been shown to increase ROS production and decrease antioxidant levels in the host by dysregulation of T helper (Th)1/Th2 cytokine balance and an increase in proinflammatory cytokines production. After influenza infection, IFN-a/b, IFN-a, and IL-2 appear to have protective roles against influenza infection, while IL-1, TNF-α, and IL-6 seem to be involved in the inflammatory phase of the infection[36]. IFN-a/b induces an antiviral state in cells by stimulating the transcription of genes coding for antiviral proteins such as 20,50-oligoadenylate synthetase (2-5 OAS), double-stranded RNA-dependent protein kinase and the Mx proteins[37]. The protein 20,50-oligoadenylate, produced by the activity of 2-5 OAS, activates cellular endoribonuclease which is responsible for single-stranded viral and cellular RNA degradation[37]. Protein kinase P1/eIF2 blocks viral protein synthesis and limits viral spread[38] .Human MxA protein inhibits influenza viral replication at posttranscriptional and translational levels [39]. IFNa/b also stimulates memory cells[40]. IFN-b, which is produced by Th1 and NK cells, stimulates macrophages and NK cell activity, up-regulates the expression of major histocompatibility complex (MHC) molecules, and controls immunoglobulin class switching[41]. Meanwhile, IL-2, also produced by Th1, stimulates a rapid proliferation of T cells, which arrange many immunologic events to induce antibody responses[42]. The IL-1 family includes of IL-1a, IL-1b, and IL-1 receptor antagonist (Ra). IL-1a and IL-1b can induce fever, anorexia, hypotension and sleepiness. IL-1Ra provides some protection against IL-1 by blocking the binding of IL-1 to its cell surface receptors[43]. Because of its cytotoxic and proinflammatory effects, TNF-a plays a dual role (beneficial and harmful to the patient) during influenza infection. It is produced by several immune cells, including monocytes, NK, T, B, and neutrophils[44]. In vitro TNF-a treatment leads to infectious virus decrease, viral protein synthesis inhibition and cytopathic effect of virus reduction [45]. Systemic TNF-a administration produced fever and anorexia, which regulate body temperature and appetite. Other functions of TNF-a include induction of other cytokines (such as IL-1 and IL-6), induction of apoptosis in mature T cells and increased phagocytic activity[44]. IL-6, produced by lymphoid and non-lymphoid cells, is a multifunctional cytokine that regulates immune and acute phase responses and hemopoiesis through its involvement in T cell activation, growth and differentiation, as well as B cell differentiation[46].
In a case study by Jacoby et al. (2002), it was discussed that influenza virus infection may cause an increase in airway inflammation and bronchoconstriction, particularly during asthma attacks. Inflammatory mediators which are produced by epithelial cells and increase in response to influenza virus infection include IL-6 and IL-8, regulated on activation, normal T lymphocytes expressed (RANTES), Eotaxin, oxygen radicals and antioxidant enzymes[47].
It has been suggested that the cell cycle would be arrested under stress conditions by malondialdehyde (MDA), the endogenous product of lipid peroxidation. MDA can trigger p53 and p21 induction, and may induce G1, S, and G2/M cell cycle arrest irreversibly[48]. Exposure of the vascular smooth muscle cell (VSMC) to HNE (the aldehyde component of oxidized low-density lipoprotein) causes mitogenesis by ERK, JNK and p38 MAP kinase activation, induction of c-fos and c-jun gene expression, as well as activator protein-1 (AP-1) activity. ERK is involved in cellular proliferation and differentiation, whereas JNK and p38 MAP kinases perform powerful responses to cellular stress such as proinflammatory cytokines. Activation of JNK induces apoptosis in response to stimulating cell proliferation and transformation, while c-fos and c-jun activate the transcription of several genes controlling cellular growth. AP-1, which is encoded by c-fos and c-jun, is also believed to regulate genes involved in the control of cell growth and differentiation. Indeed, HNE induces VSMC growth, which is consistent with other observations. These observations are consistent with the role of lipid peroxidation products in vascular smooth muscle cell growth[49].
Palamara et al. (2005) also showed that influenza A virus infection causes the activation of various MAPK pathways, such as the p38MAPK and JNK pathways which play roles in the inflammatory and apoptotic responses, and the Raf/MEK/ERK cascade which results in nuclear transport of vRNPs. Phosphorylation events also play critical roles in virus penetration into the cell, efficient nuclear export of NP, and progeny viruses budding[50]. Influenza viruses use several strategies to recruit host cell machinery for their replication and infection. Among these, the imbalance of intracellular redox state plays an important role in modifying the activity of different signaling pathways. In particular, mild oxidative imbalance[51], ligand/receptor binding[52] and cytokines[53] cause localized oxidation of reactive cysteine residues of “redox sensitive” proteins, which reversibly activate or deactivate protein functionality[54].
It has been reported that influenza virus is propagated in the epithelial cells lining the respiratory tract. The virus infected cells are lysed by the cytocidal action of CTLs and NK cells in the local mucosal membrane. One of the mechanisms by which CTL exerts cytolytic activity is via Perforin, a pore-forming protein which is a main constituent of cytotoxic proteins involved in the granule exocytosis pathway. It plays a crucial role in the host defense mechanism against influenza virus infection, especially in its early stage, as the local respiratory defense by inducing apoptosis of virus-infected cells[55].
The effect of different models of oxidative stress, such as immobilization, cold and cold-restraint stress, was investigated on liver monooxygenase activity and lipid peroxidation in influenza virus-infected mice. These models, in combination with influenza virus infection, were associated with graduated oxidative disturbances in the livers of mice, which might be related to the production of hepatotoxic mediators outside of liver and their transportation and diffusion to the liver. Also, these models were accompanied with a significant increase in lipid peroxidation products, such as thiobarbituric acid reactive substances (TBARS), a decrease of natural antioxidants (including vitamin E, glutathione) and cytochrome P-450, a cytochrome c reductase and liver monooxygenases[56]. At a later stage, following influenza A virus (A/Aichi/2/68/H3N2) infection several disorganizations were recorded in lung and blood: Increase in total MDA in lung, progressive damage of the alveolar cells, acute inflammatory reactions, development of bronchitis and pneumonia, lung disorder associated with release of cytokines and massive infiltration of polymorphonuclear leukocytes into the alveolar space, conversion of xanthine dehydrogenase to xanthine oxidase, decrease of PaO2 and development of hypoxia, increase of PaCO2 and development of metabolic acidosis, eicosanoids and prostaglandin E2 and enhanced immune response, oxidative stress in lung tissue and blood, accumulation of methemoglobin in blood and nitric oxide in lung, free radicals production, increase in conjugated dienes, decrease in blood vitamin E, as well as intensive generation of free radicals mostly in the early stage of influenza virus infection in blood and lung. The promising effect of vitamin E supplementation was dose-dependent in blood, but dose-independent in lung[57].
Valyi-Nagy et al. (2012) highlighted that oxidative damage is a mechanism activated by viral infection which may result from direct effects of virus on cells or indirect effects of host inflammatory responses. Inflammatory responses of the host cells augmented by viral infection may cause for O2- and other ROS production by infiltrating phagocytic cells and XO-mediated humoral responses. Inflammatory cytokines such as IFN-b lead to the induction of inducible nitric oxide synthase (iNOS) during influenza virus infection. ROS, reactive nitrogen species (RNS) and secondary lipid peroxidation products like HNE and MDA may affect viral replication through modulation of the activation state of cells, regulation of host immune responses, and oxidative damage to host tissues and viral components[58].
ROS production is an important host defense mechanism against influenza virus infection. However, excessive superoxide production may stimulate the release of proinflammatory cytokines and chemokines, such as TNF-a IL-6, IFN-γ, IL-8, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 2 (MIP-2) and MIP-1a, as well as inflammatory cells such as macrophages and neutrophils into the lung. This induces activation of the innate immune system, which is essential for viral clearance and resolution of inflammation, but might be harmful to the cell. Persistent trafficking of these inflammatory factors causes a cytokine storm, which may have a reverse effect on viral clearance and causes excessive damage. Suppression of superoxide production by targeting the enzymatic major source of superoxide and ROS in mammalian inflammatory cells, NADPH oxidase 2 (Nox2), reduces influenza A virus-induced lung injury and virus replication[59]. Superoxide anion, produced by macrophages infiltrated into the virus-infected organs, is used for the development of severe influenza-associated complications. Influenza-induced oxidative stress increases vascular permeability and breakability resulting in the airway mucosa and lung tissue edema, as well as multiple hemorrhages in the alveolar area, interstitial lung cells and all internal organs. Therefore, the combination of antioxidants with antiviral drugs could synergistically reduce the lethal effects of influenza virus infections[60].
1.3. Medicinal plants
Medicinal plants are increasingly regaining popularity in modern society as natural alternatives to synthetic medicines and they offer great potential as potentially effective new antiviral agents[61]. Traditional herbs are generally cheaper, more accessible, or readily available, and sometimes more culturally acceptable. Furthermore, some adverse effects of synthetic drugs[62][63] have made researchers focus on natural herbal substances as complementary therapies and preventive medicine.
A crude extract is not safe to prescribe, for two reasons: first, some phytochemicals may exist at toxic levels in crude extracts[64], and secondly, the bioactivity may be suboptimal if maximal activity requires specific combinations of phytochemicals[65]. Therefore, WHO has recommended DNA Barcoding for identification of medicinal plants and to facilitate the detection and quantification of the required chemical claimed compounds[66].
1.4. Quercetin and its derivatives
Quercetin (3,3’,4’,5,7-pentahydroxyflavone) is a natural compound from the flavonoid family of the glycoside rutin [67], which all share a similar flavone backbone (3-ringed molecule with OH groups attached). Several other substitutions have generated subclasses of flavonoids with different compounds within these subclasses. Quercetin has been isolated from more than twenty plant materials from the USA, European and Asian countries, and South Africa[68][69][70]. It is well known for its range of therapeutic properties, which include anti-inflammatory[70][71], anti-proliferative [72], anti-oxidative[73], anti-bacterial[74], anti-cancer[75], neuroprotective[76] hepatoprotective[77] and antiviral [69][78][79]activities.
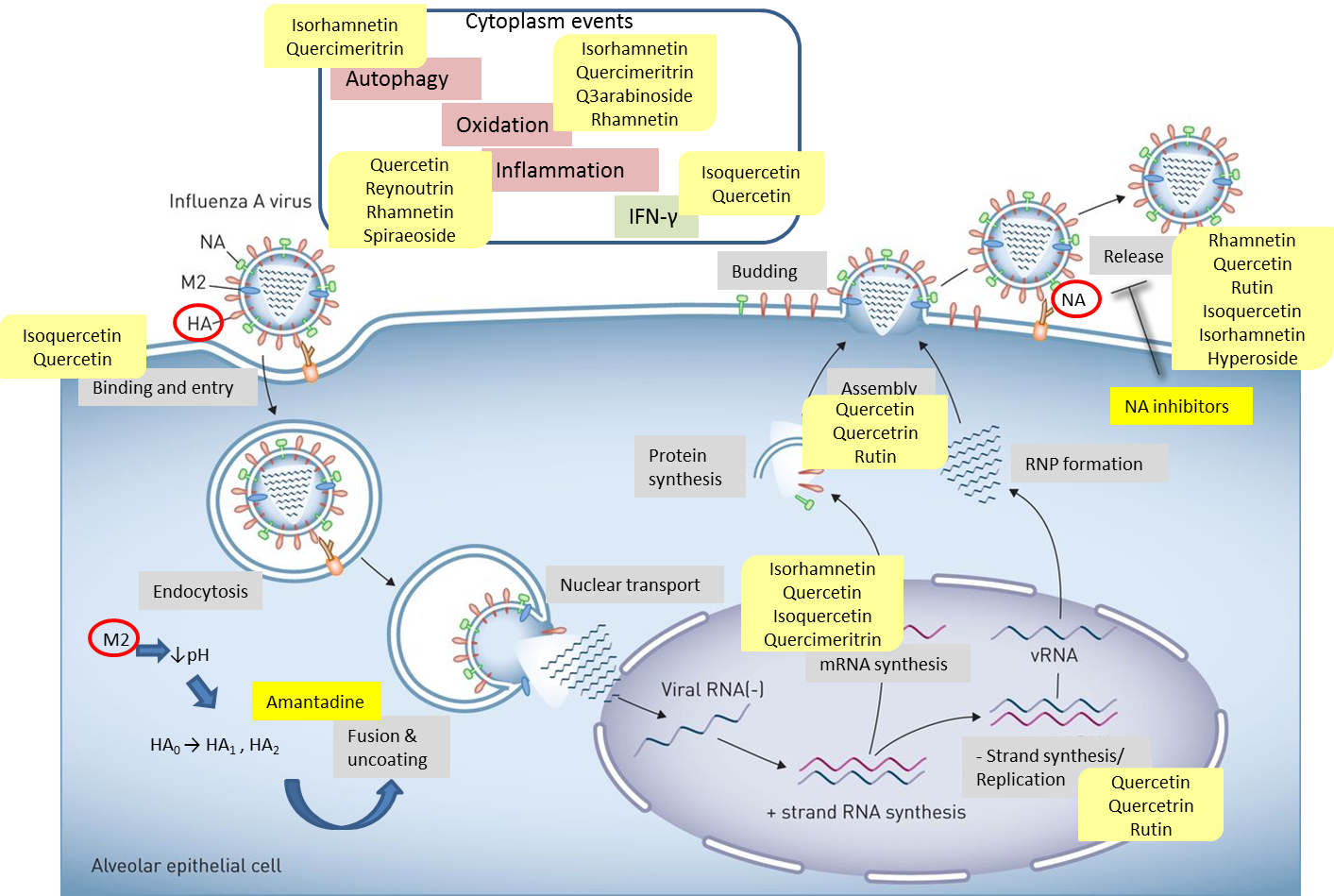
Quercetin, the most abundant flavonoid molecule[67], is itself the aglycone form of rutin, but flavonoids are bound to sugars in their natural state and quercetin is thus also found naturally in its glycoside forms. Glycosylation can occur at any hydroxyl group of the compound[80]. The compound possesses 2 benzene rings (ring A and B), which are linked by a 3-carbon chain that forms a closed pyran ring (C ring) with variously distributed hydroxyl and methyl groups[81]. It is worth noting that Pawlikowska-Pawlęga et al. (2007), [82] in their study highlighted modifications in the phospholipid membranes of human skin fibroblasts (HSF) after exposure to quercetin, which was found to localize in the polar head regions of the membrane. It could be detected on membranes in the cytoplasm, around the nuclear envelope and inside the nucleus and nuclei by increasing the exposure time. This property affects its pharmacological activities quality. Figure 2 shows the schematic mode of attack of quercetin and its derivatives on the influenza virus life cycle in different parts of the cell.
Figure 2. Schematic of the influenza virus life cycle and the pathways affected by quercetin and its derivatives. Influenza virus uses HA receptor for binding and entry. M2 ion channel facilitates fusion & uncoating, and NA induces the release of progeny virus. Quercetin and its derivatives impact different steps of viral life cycle, such as binding, uncoating, mRNA synthesis, negative-strand synthesis, assembly and release. They can also affect cytoplasm events to influence the virus indirectly.
2. Methods
2.1. In vitro and in Silico Studies Against Influenza Virus
2.1.1. General Overview
Choi et al (2009) showed that quercetin 3-rhamnoside (Q3R) isolated from Houttuynia cordata has antiviral activity against influenza A/WS/33 virus in vitro[78]. The antiviral activity of Tetrastigma hemsleyanum Diels et Gilg, a widely used medicinal plant in China, containing quercetin and isoquercetin, was characterized against the recombinant influenza virus PR8-NS1-Gluc in MDCK cells. [83].
In accordance with previously described findings, isoquercetin inhibited the replication of both influenza A and B viruses at the lowest 50% effective concentration (EC50). Isoquercetin, in combination with amantadine, exhibited synergistic effects on viral replication reduction in vitro, as well as suppressing the emergence of the resistant virus [84]. Capparis sinaica Veill methanol polyphenol-rich extract containing quercetin, isoquercetin, and rutin showed 68.13%, 79.66%, and 73.22% reduction in viral titer at concentration of 1 ng/ml, respectively[85].
Yang et al. (2014) investigated the in vitro antiviral activity of tea polyphenol compounds against influenza A/PR/8/34 and B/Lee/1940 viruses. Tea polyphenols mainly contain catechins, proanthocyanidins and theaflavins as active compounds, as well as flavonols such as kaempferol, quercetin and myricetin, which are similar to catechins but have more planar chemical structure. This study did not show a strong anti-influenza effect of quercetin in MDCK cells as infection model compared to the other polyphenols, which may be due to different study designs and starting points[86].
Abdal Dayem et al. (2015)[81] showed anti-influenza activity of some flavonoid compounds such as diosmetin, eriodictyol, kaempferol, isorhamnetin along with quercetin isolated from polyphenol extracts. They evaluated the effects of isorhamnetin and quercetin both in 50 µM concentration in pre- and co-treatments. Quercetin led to a significant decrease in virus titer [81].
The procyanidin B2 and ferulic acid derivatives, ferulic acid O-hexosides, quercetin-3-O-rhamnoside and quercetin-O-pentoside-O-rhamnoside compounds were identified in Vaccinium oldhamii. Quercetin glycosides showed weak activity in this study.
To investigate the effect of structural changes on the quercetin activity, various C3, C3’, and C5 hydroxyl group substituted quercetin derivatives were synthesized with various phenolic ester, alkoxy and aminoalkoxy moieties, and their anti-H1N1 activities investigated against A/swine/OH/511445/2007[H1N1], Oh7. The results imply that further modification at C3 functional group may play an essential role in improving the compound efficacy[87].
The structure-activity relationship (SAR) of quercetin was analyzed in the Yang et al. study (2014) [86]. Two mechanisms were assumed for the antiviral activity of quercetin on both influenza A/PR/8/34 and B/Lee/1940 viruses: one is at the early stage by blocking the viral binding to the cell receptors, and the other is after the entry by attenuation of viral replication. It has been shown for in vitro NA inhibition assay that rigid flavonols (kaempferol, quercetin and myricetin) have stronger inhibitory effects than flexible ones (catechin and epicatechin)[88]. One hydroxyl at C-4′ position in the B ring, a double bond at C-2–C-3, and a ketone at the C-4 position is essential for inhibiting influenza virus as NA inhibitor. While the presence of more hydroxyl in the B ring, such as quercetin and myricetin, causes steric interference and disfavors the anti-influenza effect[88]. It seems that dimeric compounds are more potent antivirals against both influenza A and B viruses than the monomers such as catechin and quercetin [86].
2.1.2. No Activity Against Influenza Virus
Several compounds including 3-O-methylquercetin and quercetin in acetone extract of the shoots of Helichrysum melanacme, showed no activity against influenza virus A/Panama/1/68 [H3N2][89].
Quercetin-3-O-b-D-glucouronide, along with some other flavonoids (rumpictuside A, apigenin 7-O-b-Dglucoside, vitexin, orientin, and isorientin), isolated from Rumex pictus found to be inactive against influenza A virus infection [90].
Preparations with antioxidant and/or anti-hypoxant properties, such as hypoxene, reduced glutathione, dihydroquercetin, trolox, coenzyme Q10, and the enzymatic preparation of superoxide-dismutase, alone or in combination with rimantadine, were investigated against influenza A (H3N2) infection. They did not show protective effect and in some cases, even enhanced the production of viral particles and decreased the antiviral action of rimantadine[91].
2.1.3. Anti-inflammatory and Immunomodulatory Effects
Shikimic acid alone and in combination with quercetin were evaluated in comparison with oseltamivir in an in vitro model in terms of their ability to modulate the expression of IL-6 and IL-8 as immunomodulatory cytokines from peripheral blood mononuclear cells (PBMCs)[92]. PBMCs isolated from six healthy volunteers were treated with different doses of shikimic acid with or without quercetin. Shikimic acid in combination with quercetin, even at low doses, effectively modulated innate immunity by increasing the IL-8 and IL-6 levels[92].
Chaenomeles speciosa (Sweet) Nakai is a traditional herb in China. Studies have confirmed the presence of glycosides, flavones, phenolics, tannins and organic acids in this fruit, including quercetin. As a powerful antioxidant, quercetin from this fruit was suggested as a potential candidate antiviral and anti-inflammatory agent [93].
The aqueous extract of Epimedium koreanum Nakai, a traditional Korean and Chinese plant, was evaluated against influenza A virus (PR/8/34). The primary component of this extract was found to be quercetin. Application of the extract induced the secretion of type I IFN and proinflammatory cytokines, stimulating the antiviral activity in the cells. [94].
Choi et al. (2017)[95]observed induction of immunomodulatory signaling molecules (IL-6, IFN-b and TNF-a and antiviral activity of aqueous extract of Eupatorium fortunei including quercetin, psoralen, and quercitrin against influenza A virus, Newcastle disease virus, and vesicular stomatitis virus[95]. This extract and its active components were introduced as immunomodulators of the innate immune response in murine macrophages as a basis for the development of prophylactic or treatments against viruses[95].
Rapanea melanophloeos, a South African medicinal plant, was investigated as a source of quercetin-3-O-a-L-rhamnopyranoside and for its immunomodulatory properties against influenza A virus. This plant has traditionally been used to treat respiratory ailments[70]. A previous preliminary report described anti-influenza activity exhibited by a methanol extract of this plant, protecting cell viability and viral titer decrement[96]. Quercetin-3-O-a-L-rhamnopyranoside was found to decrease the viral titer by 6 log fold, reduced viral genome expression (P<0.01) and affected cytokine levels significantly[70].
Further research focused on the specific mechanism of virus-host interactions. The expression of IL-6 and CCL-2 at genome and protein levels were significantly affected by quercetin-3-O-a-L-rhamnopyranoside, especially upon co-penetration treatment [70].
2.1.4. Influence on Molecular Pathways
Several studies have investigated the potential of quercetin and its derivatives to counteract signaling pathways used for influenza virus propagation.
Quercetin and oseltamivir were evaluated for their effect on the Toll-like receptor 7 (TLR7) signaling pathway in dendritic cells and macrophages infected with H1N1. Human bronchial epithelial cells (16HBE) were infected with H1N1 and co-cultured with the immunological cells, following which quercetin and oseltamivir were added into the medium. Quercetin and oseltamivir increased cell viability and inhibited the TLR7 signaling pathway induced by viral infection[97].
Wan et al. (2013) investigated the effect of quercetin on cyclin-dependent kinase 4 (CDK4) at mRNA and protein level in A549 human lung epithelial cells infected by influenza A H1N1 (A1/Qian fang/166/85) in parallel with ribavirin. The CDK4 expression increased significantly in the H1N1 group (P<0.01), but decreased significantly in response to treatment with quercetin and ribavirin (P<0.01). It seems that quercetin can induce DNA repair mechanisms in A549 cells infected with H1N1, contributing to host cell proliferation. Quercetin was not as potent as ribavirin in promoting cell survival, but it was less toxic and inhibited CDK4 expression induced by H1N1 infection[98].
However, it has been shown that influenza A virus is able to inhibit autophagy[99], which causes the accumulation of autophagosomes by blocking autophagosome union with lysosomes using ion channel M2 protein, which is necessary for fusion[99][100]. Therefore, managing this process can inhibit IAV proliferation and may be a strategy for developing new anti-IAV agents.
Quercetin-7-O-glucoside isolated form Dianthus superbus, a medicinal plant used in Mongolia and China for its anti-inflammatory properties, was evaluated for efficacy against virus-induced symptoms, ROS production and autophagy induction. Quercetin-7-O-glucoside reduced ROS and autophagy formation by affecting cellular pathways but not the virus particles directly. Molecular docking analysis showed an inhibitory effect of quercetin-7-O-glucoside on viral RNA polymerase by occupying the binding site of m7GTP on viral PB2 protein[73].
Vaidya et al. (2016) evaluated the antiviral potential of biochemical components present in Kimchi, a traditional fermented food commonly consumed in Korea[101], with particular focus on quercetin against influenza A/PR/8/34. Significantly higher cell viability, lower expression of the influenza PA gene, reduced apoptosis and reduced cell death were observed in cells subjected to periodic treatment compared with cells at the pre-treatment stage. Comparative proteomics analysis showed differentially modulation by quercetin treatment in influenza-infected cells on 56 proteins and post-translational modifications in 68 proteins. The periodic treatment of quercetin was influential in the regulation of key protein expression. The major regulatory proteins identified in this study included FN1, HSPs, PHB, TUBA1C and TUBA4A, all of which are associated with cell and IAV interaction network. Moreover, post-translational modification of CCT4, EIF3A, HSPA2 and TUBB2B could also be involved in controlling IAV replication [102].
The frequency of apoptosis was examined in response to simultaneous combination treatment with quercetin-3-O-a-L-rhamnopyranoside and influenza A/PR/8/34 by detecting RhoA GTPase protein and caspase-3 activity. This combination increased caspase-3 activity while decreasing RhoA activation[79].
Choi et al. (2019)[103] investigated the antiviral activity of Aloe vera ethanolic extract and its components quercetin, catechin hydrate and kaempferol, against H1N1 and H3N2 IAV strains in vitro [103]. The antiviral activity of aloe-emodin against H1N1 strain was related to the restoration of NS1-inhibited STAT1-mediated antiviral responses in the transfected cells[104]. Choi et al. (2019)[103] also showed that this extract inhibited autophagy which was induced by influenza A virus.
Quercetin 3-glucoside, isolated from Dianthus superbus L, was evaluated against influenza A and B virus infection. [105]. It exhibited potent antiviral activity. Quercetin 3-glucoside blocked influenza A/PR/8/34 replication and decreased expression of the related genes M, Atg-5 and LC3-b. Virus-induced ROS production and autophagy were reduced by quercetin 3-glucoside[106].
2.1.5. Neuraminidase Inhibitory Activity
The inhibitory potency of several flavonoid derivatives has been evaluated on neuraminidase activity (NA) of different types of influenza virus. The neuraminidase inhibitory activity of flavonoids was investigated against NA of A/California/7/09 (A/H1N1/pdm09), A/Perth/16/09 (A[H3N2])) and B/Brisbane/60/08 compared to the oseltamivir. The experimental findings and molecular dynamics simulations confirmed moderate inhibition of the flavonoids on NA activity. However, the sites of glycosylation of the flavonoids showed no significant influence on their inhibitory potency[107].
The compounds quercetin 3-O-b-D-galactopyranoside, quercetin 3-O-a-L-rhamnopyranoside, kaempferol 3-O-a-L-hamnopyranoside were obtained from Zanthoxylum piperitum and investigated against influenza A/NWS/33 [H1N1] virus in vitro. Although their effect was not so strong as oseltamivir, they have the capacity as antiviral agents against the influenza A virus[108].
Six flavonoids (quercetin-3-sophoroside, kaempferol-3-sophoroside, kaempferol-3-sambubioside, kaempferol-3-neohesperidoside, kaempferol-3-glucoside and luteolin) and one alkaloid (chelianthifoline) isolated from bee pollen methanol extract of Korean Papaver rhoeas plant[109]. Lee et al. (2016)[109] suggested that a bulky sugar moiety on quercetin-3-sophoroside and other flavonoids decreased their NA inhibitory activity compared to the aglycone compounds.
Dianthus superbus ethyl acetate, butanol and distilled water extracts were evaluated. The butanol extract showed potent antiviral activity against both influenza A and B viruses (A/PR/8/34 and B/LEE/40). Both isolated quercetin-3-rutinoside and isorhamnetin-3-glucoside showed high NA inhibitory activity[110].
2.1.6. In Silico and Docking Studies
Quercetin, baicalein, chlorogenic acid and oleanolic acid in molecular docking study showed high binding potential with NA of H7N9 which even exceeded that of oseltamivir. The mutation R294K in NA structure induced oseltamivir resistance, while other compounds showed stable binding with mutated NA, which renders them potential agents to overcome the drug resistance of the mutant virus[111].
Quercetin and chlorogenic acid, potential lead compounds derived from traditional Chinese medicine, were tested in molecular docking against influenza A virus H1N1 (A/PR/8/34). They showed strong binding abilities (−10.23 and −11.05 kcal/mol for quercetin and chlorogenic acid, respectively) with NA of this virus which were comparable with oseltamivir (−11.24 kcal/mol).[112].
Naïve Bayesian, recursive partitioning and CDOCKER methods as virtual screening systems to predict compound-protein interaction, were used to predict active compounds in Yizhihao, one of the common Chinese herbal formulas used to treat influenza and cold symptoms. It consists of Radix isatidis, Folium isatidis and Artemisia rupestris. The results from CPE reduction assay in this study provided scientific information for its usage and showed that quercetin, along with other compounds such as acacetin, indirubin, tryptanthrin, luteolin, emodin and apigenin, had protective effects against wild-type strains A/PR/8/34 [H1N1] and A/Minfang/151/2000 [H3N2] with lower IC50 values and stronger activities than oseltamivir and ribavirin. Quercetin supports the activity of the compounds by reducing H1N1 viral activity or impairing viral adsorption[113].
In a docking study by Sadati et al. (2019), the flavonoids quercetin, vitexin, chrysin, catechin, luteolin, naringenin, kaempferol and hispidulin were evaluated for their potential inhibitory effect on influenza H1N1 virus by looking for their binding energy to the NA active site structure along with oseltamivir. These compounds showed high potential and affinity for binding to the active site of NA domain N1 with lower binding energies (‑6.8 kcal/mol for quercetin, ‑7.2 kcal/mol for catechin, ‑6.9 kcal/mol for naringenin, ‑7.1 kcal/mol for luteolin, ‑6.8 kcal/mol for dinatin, ‑7.5 kcal/mol for vitexin, ‑6.8 kcal/mol for chrysin and ‑6.8 kcal/mol for kaempferol) than oseltamivir (‑5.8 kcal/mol). They were suggested to block the NA active site effectively[114].
In computational modeling, strong binding of quercetin-3-O-a-L-rhamnopyranoside with M2 transmembrane and NA was observed for 2009 pandemic H1N1, N1 and H1 of PR/8/1934 and human RhoA proteins, with docking energies of -10.81, -10.47, -9.52, -9.24 and -8.78 kcal/mol, respectively, which makes quercetin-3-O-α-L-rhamnopyranoside a potent anti-influenza candidate[79].
Docking simulation using AutoDock Vina Software indicated a higher binding affinity of quercetin (-5.35 kcal/mol) as well as catechin hydrate (-5.48 kcal /mol) isolated from Aloe vera ethanolic extract for the M2 protein than amantadine (-4.52 kcal/mol)[103]. Another molecular docking study revealed higher binding activities towards influenza polymerase and surface glycoproteins for flavonol glycosides[110]. Molecular docking studies highlighted the blockade of the cap-binding domain of polymerase basic protein subunit, showing higher binding affinity (-8.0 kcal/mal) than GTP (-7.0 kcal/mol)[106].
With respect to mutant viruses, A/HebeiXinhua/SWL1106/2017 (oseltamivir & amantadine-resistant H1N1) and mutant A/FujianXinluo/SWL2457/2014 (amantadine-resistant H1N1), quercetin, luteolin and apigenin could more effectively reduce viral activity or impair the adsorption of viruses on the cells compared to amantadine and oseltamivir[115].
2.1.7. Hemagglutinin Inhibitory Activity
Quercetin has been reported to counteract influenza infection in the early stages by interacting with the HA2 subunit of influenza HA protein and inhibited virus-cell fusion. Quercetin exhibited an apparent inhibitory effect against both H1N1 and H3N2 virus strain infections in a dose-dependent manner. It also reduced the HA mRNA transcription and NP protein synthesis in a dose-dependent manner in MDCK cells. A time-of-addition assay was performed to identify the effective stage by measuring HA mRNA and protein levels at the stages of virus entry (0–2 hr), replication and translation (2–8 hr) and/or release (8–10 hr). The best performance was observed in the entry and whole life cycle steps including attachment, endocytosis and fusion. It was more effective during the process of virus infection rather than post-virus infection and was more effective on virus particles rather than the host cell. The binding affinity between HA protein and quercetin was confirmed by surface plasmon resonance (SPR) assay, which showed a medium-binding affinity of quercetin to HA protein. For further confirmation, the microscale thermophoresis (MST) assay was conducted, which showed that quercetin interacted with influenza HA protein in a dose-dependent manner. Using the pseudovirus-based drug screening system, quercetin also found to inhibit the entry of the H5N1 virus, which confirmed the specific interference with the function of the H5N1 influenza HA envelope protein[116].
2.2. In Vivo and Human Studies Against Influenza Virus
The therapeutic effects of quercetin and derivatives have been evaluated in animal models as well as humans and have been studied from different aspects. The key notes of in vivo studies are categorized in Table 1.
Table 1. Animal model and human studies of quercetin and derivatives
|
Compound studied |
Human/Animal study |
Study |
Reference |
|
Aqueous extract of propolis containing rutin and quercetin |
In mice infected with A/PR8/34 (H1N1) |
Experimental post-infection of the extract, decrease HA titer in the lung suspensions, slight decrease in mortality and increase in survival length |
[117] |
|
Quercetin |
1 mg/ml administered orally. In adult male Swiss albino mice infected with influenza virus (A/Hong Kong/8/68) |
Decreased oxidative stress by decreasing lipid peroxidation, and anti-oxidant enzymes; superoxide dismutase and catalase |
[118] |
|
Quercetin |
In mice infected with A/Udorn/317/72 (H3N2) influenza virus |
Decreased lipid peroxidation, protected lungs by maintaining the levels of endogenous antioxidant enzymes |
[119] |
|
Rutin and quercetin |
In mice infected with A/Aichi/2/68 (H3N2) |
Quercetin showed stronger activity and caused oxidative damage in healthy mice but antioxidant activity in infected animals |
[120] |
|
Combination of selenium, vitamin E, N-acetyl-cysteine /glutathione, resveratrol and quercetin |
Against H5N1 influenza in humans |
Antagonized the pathogenic processes of H5N1 influenza in humans |
[121] |
|
Short-term quercetin feeding before viral challenge |
In four-week-old male ICR mice forced to run on a treadmill for 3 consecutive days to fatigue, inoculated with A/PR8/34 (H1N1) |
Reduced the susceptibility to infection |
[122] |
|
Isoquercetin |
In four-week-old female BALB/c mice infected with human influenza A virus |
Decreased IFN-γ, RANTES and iNOS as indicators of lung inflammation and virus titers |
[123] |
|
Quercetin-3-O-β-D-glucuronide isolated form Polygonum perfoliatum L. |
In 3 and 6 mg/kg concentrations. In mice infected with influenza virus |
it could suppress lung edema caused by virus at 28% and 20%, respectively |
[124] |
|
quercetin 3‐rhamnoside (Q3R) |
In mice infected with A/WS/33 influenza virus |
Decreased weight loss, necrosis, inflammation, mortality and lung virus titer |
[125] |
|
Epimedium koreanum Nakai aquous extract containing quercetin |
In BALB/c mice infected with 5MLD50 of H1N1, H5N2, H7N3 and H9N2 influenza viruses |
Reduced viral titers and body weight loss. Inhibited viral replication and promoted the survival |
[94] |
|
Quercetin and chlorogenic acid |
In BALB/c female mice infected with A/PR8/34 (H1N1) |
improved lung index and survival rate |
[126] |
3. Conclusion
Quercetin and its derivatives are flavonol compounds predominantly found in fruits and vegetables and they have a strong reputation for inflammatory diseases treatment. They have long been recognized for their therapeutic effects and used as traditional medicines in different parts of the world, typically as herbal drinks to treat this and several other types of diseases. They have recently gained increasing interest in different treatment protocols. This review introduces the most recent developments in terms of in vitro, in silico and in vivo evaluation of quercetin and its derivatives against influenza infection, which may highlight experimental parameters and bioavailability issues with regards to these compounds.
Although the research outcomes are somewhat contradictory and indeed some information regarding adverse effects may not be disclosed on scientific websites, this natural compound may nevertheless possess great therapeutic value and is worthy of further investigation. DNA barcoding should also be applied in a complementary manner for the accurate identification of plant species with well-characterized chemical properties, thus improving the quality of medicines.
4. Future Perspective
Regarding the importance and efficacy of quercetin and other flavonols against influenza virus infection, flavonoids have attracted increasing attention as a potential therapeutic to combat infection with SARS-CoV-2, from human coronavirus family, which has been responsible for a rapid worldwide pandemic starting from 2020. Few in silico and computational studies have evaluated libraries of natural products and phenolic compounds against SARS-CoV-2. As a result, quercetin was found among the top 5 most potent compounds to bind strongly to the Spike Protein (S) receptor of the virus[127], and with even stronger affinity for the COVID-19 main protease active site than hydroxy-chloroquine[128]. Also rutin was identified to have the potential to bind to its main protease active site[129] with notable inhibitory activity against SARS-CoV-2 main protease[130]. It should be noted that oral administration of quercetin would not achieve drug contact with viral S Protein, because of its glycosylation for biotransformation in the gastrointestinal tract[131]. Therefore, using nasal or throat spray containing quercetin in a suitable form was recommended to deliver proper volume to the virus active sites[132] and be effective in clinical trials[133]. Therefore, the flavonoid quercetin appears to be the most promising natural antiviral compound on the basis of its antiviral activity against influenza A virus and SARS-CoV-2 infections and is a noteworthy candidate for further in vivo studies.
References
- Rajasekaran, D., Palombo, E.A., Chia Yeo, T., Lim Siok Ley, D., Lee Tu, C., Malherbe, F., Grollo, L. Identification of traditional medicinal plant extracts with novel anti-influenza activity. PLoS ONE, 2013. 8:
- Bouvier, N.M., Palese, P. The biology of influenza viruses. Vaccine, 2008. 26 Suppl 4: D49-D53.
- Palese, P., Shaw, M., Fields Virology. Orthomyxoviridae: The Viruses and their Replication, ed. Knipe D; Howley P. 2007, Philadelphia: Lippincott Williams & Wilkins.
- WHO, Vaccines against influenza WHO position paper – November 2012, in The Weekly Epidemiological Record p. 461-476.
- Iuliano A.D., R.K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., Wu P., Kyncl J., Ang L.W., Park M., Redlberger-Fritz M., Yu H., Espenhain L., Krishnan A., Emukule G., van Asten L., Pereira da Silva S., Aungkulanon S., Buchholz U., Widdowson M.A., Bresee J.S. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. The Lancet, 2018. 391(10127): 1285–1300.
- Macias AE, M.J., Chaves SS, Nealon J, Nunes MC, Samson SI, Seet BT, Weinke T, Yu H. The disease burden of influenza beyond respiratory illness. Vaccine, 2020. S0264-410X(20)31209-3.
- Wayan C.W.S. Putri, D.J.M., Melissa S. Stockwell, Anthony T. Newall. Economic burden of seasonal influenza in the United States. Vaccine, 2018. 36: 3960-3966.
- Hossein Khorramdelazad, M.H.K., Alireza Najafi, Maryam Keykhaee, Reza Zolfaghari Emameh, Reza Falak. Immunopathological similarities between COVID-19 and influenza: Investigating the consequences of Co-infection. Microbial Pathogenesis, 2020. 104554.
- Ding, Q., Lu, P, Fan, Y, Xia, Y, Liu, M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan. Journal of Medical Virology, 2019. 92: 1549– 1555.
- Wang Ge, X.M., Ma Jingdong, Guan Jialun, Song Yang, Wen Yue, Fang Dan, Wang Muru, Tian De-an, Li Peiyuan, . Is Co-Infection with Influenza Virus a Protective Factor of COVID-19? The Lancet Infectious Diseases, 2020.
- Zhang AJ, L.A., Chan JF, Liu F, Li C, Chen Y, Chu H, Lau SY, Wang P, Chan CC, Poon VK, Yuan S, To KK, Chen H, Yuen KY. Co-infection by severe acute respiratory syndrome coronavirus 2 and influenza A(H1N1)pdm09 virus enhances the severity of pneumonia in golden Syrian hamsters. Clinical Infectious Diseases, 2020. ciaa1747.
- Thomas Drake, C.F., Antonia Ho, Lance Turtle, Clark Russell, Ewen Harrison, Calum Semple, Influenza infection in patients hospitalized with COVID-19: rapid report from CO-CIN data, in 59 SAGE Meeting
- Hayden, F.G. Respiratory viral threats. Current Opinion in Infectious Disease, 2006. 19: 169-178.
- Soema, P.C., Kompier, R., Amorij, J.-P., Kersten, G.F.A. Current and next generation influenza vaccines: Formulation and production strategies. Journal of Pharmaceutical Sciences, 2015. 94: 251-263.
- Barik, S. New treatments for influenza. BMC Medicine, 2012. 10:
- Fedson, D.S. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infectious Diseases, 2008. 8: 571-576.
- Rodriguez, W.J., Hall, C.B., Welliver, R., Simoes, E.A., Ryan, M.E., Stutman, H., Johnson, G.E., Van Dyke, R., Groothuis, J.R., Arrobio, J. Efficacy and safety of aerosolized ribavirin in young children hospitalized with influenza: a double-blind, multicenter, placebo-controlled trial Journal of Pediatrics, 1994. 125: 129-135.
- Gubareva, L.V., Webster, R.G., Hayden, F.G. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrobial Agents and Chemotherapy, 2001. 45: 3403-3408.
- Treanor, J.J., Hayden, F.G., Vrooman, P.S., Barbarash, R., Bettis, R., Riff, D., Singh, S., Kinnersley, N., Ward, P., Mills, R.G. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza; A randomized controlled trial. Journal of the American Medical Association, 2000. 283: 1016-1024.
- Pathumwadee, I., Chittima, L., Thanyada, R., Arthorn, L., Maturos, M., Panita, D., Ornjira, A., Krit, C., Nopphorn, K., Pornthep, S., et al. How amantadine and rimantadine inhibit proton transport in the M2 protein channel. Journal of Molecular Graphics and Modelling, 2008. 27 342-348.
- Bouvier, N.M., Lowen, A.C., Palese, P. Oseltamivir-resistant influenza A viruses are transmitted efficiently among guinea pigs by direct contact but not by aerosol. Journal of Virology, 2008. 82: 10052-10058.
- Bouvier, N.M., Rahmat, S., Pica, N. Enhanced mammalian transmissibility of seasonal influenza A/H1N1 viruses encoding an oseltamivir-resistant neuraminidase. Journal of Virology, 2012. 86: 7268-7279.
- Ginting, T.E., Shinya, K., Kyan, Y., Makino, A., Matsumoto, N., Kaneda, S., Kawaoka, Y. Amino acid changes in hemagglutinin contribute to the replication of Ooeltamivir-resistant H1N1 influenza viruses. Journal of Virology, 2012. 86: 121-127.
- Hussain, M., Galvin, H.D., Haw, T.Y., Nutsford, A.N., Husain, M. Drug resistance in influenza A virus: the epidemiology and management. Infection and Drug Resistance, 2017. 10: 121-134.
- Lampejo, T. Influenza and antiviral resistance: an overview. European Journal of Clinical Microbiology & Infectious Diseases, 2020. 39: 1201-1208.
- Global Influenza Strategy 2019-2030. Wkly. Prevention, Control and Preparation 2019; 31 p.]. Available from: https://apps.who.int/iris/handle/10665/311184.
- Wang, J., Pathogenesis of influenza, in Radiology of Influenza: A Practical Approach, Li H, Editor. 2016, Springer Netherlands: Dordrecht. p. 9-9.
- Fukuyama, S., Kawaoka, Y. The pathogenesis of influenza virus infections: the contributions of virus and host factors. Current Opinion in Immunology, 2011. 23: 481-486.
- Oda, T., Akaike, T., Hamamoto, T., Suzuki, F., Hirano, T., Maeda, H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science, 1989. 244: 974-976.
- Buffington, G.D., Christen, S., Peterhans, E., Stocker, R. Oxidative stress in lungs of mice infected with influenza A virus. Free Radical Research Communications, 1992. 16: 99-110.
- Schwarz, K.B. Oxidative stress during viral infection: a review. Free Radical Biology and Medicine, 1996. 21: 641-6499.
- Akaike, T., Noguchi, Y., Ijiri, S., Setoguchi, K., Suga, M., Zheng, Y.M., Dietzschold, B., Maeda, H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proceedings of the National Academy of Sciences of USA, 1996. 93: 2448-2453.
- Peterhans, E. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. Journal of Nutrition, 1997. 127: 962S-965S.
- Murphy, A.W., Platts-Mills, T.A., Lobo, M., Hayden, F. Respiratory nitric oxide levels in experimental human influenza. Chest, 1998. 114: 452-456.
- Cox, N.J., Hughes, J.M. New options for the prevention of influenza. New England Journal of Medicine, 1999. 341: 1387-1388.
- Han, S.N., Meydani, S.N. Antioxidants, cytokines, and influenza infection in aged mice and elderly humans. Journal of Infectious Diseases, 2000. 182 Suppl 1: S74-S80.
- De Maeyer, E., De Maeyer-Guignard, J., Interferons. 2nd ed ed. The cytokine handbook, ed. Thomson AW e. 1994, London: Academic Press. 265–288.
- Samuel, C.E. Antiviral actions of interferon interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology, 1991. 183: 1-11.
- Pavlovic, J., Haller, O., Staeheli, P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. Journal of Virology, 1992. 66: 2564–2569.
- Tough, D.F., Borrow, P., Sprent, J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science, 1996. 272: 1947-1950.
- Boehm, U., Klamp, T., Groot, M., Howard, J.C. Cellular responses to interferon-gamma. Annual Review of Immunology, 1997. 15: 749-495.
- Marrack, P., Kappler, J. Subversion of the immune system by pathogens. Cell, 1994. 76: 323-332.
- Dinarello, C.A., Wolff, S.M. The Role of Interleukin-1 in Disease. New England Journal of Medicine, 1993. 328: 106-113.
- Zhang, M., Tracey, K., cytokine handbook. 3rd ed. ed. Tumor necrosis factor, ed. Thomson AW e. 1998, San Diego: Academic Press. 517–548.
- Van Campen, H. Influenza A virus replication is inhibited by tumor necrosis factor-α in vitro. Archives of Virology, 1994. 136: 439-446.
- Hirano, T., The cytokine handbook. Interleukin-6, ed. Thomson A e. 1994, London: Academic Press.
- Jacoby, D.B. Virus-Induced Asthma Attacks. Journal of the American Medical Association, 2002. 287: 755-761.
- Ji, C., Rouzer, C.A., Marnett, L.J., Pietenpol, J.A. Induction of cell cycle arrest by the endogenous product of lipid peroxidation, malondialdehyde. Carcinogenesis, 1998. 19: 1275-1283.
- Kakishita, H., Hattori, Y. Vascular smooth muscle cell activation and growth by 4-hydroxynonenal. Life Sciences, 2001. 69: 689-697.
- Palamara, A.T., Nencioni, L., Aquilano, K., De Chiara, G., Hernandez, L., Cozzolino, F., Ciriolo, M.R., Garaci, E. Inhibition of influenza A virus replication by resveratrol. Journal of Infectious Diseases, 2005. 191: 1719-1729.
- Fraternale, A., Paoletti, M.F., Casabianca, A., Oiry, J., Clayette, P., Vogel, J.U., Cinatl, J., Jr., Palamara, A.T., Sgarbanti, R., Garaci, E., et al. Antiviral and immunomodulatory properties of new pro-glutathione (GSH) molecules. Current Medicinal Chemistry, 2006. 13: 1749-1755.
- Akhand, A.A., Du, J., Liu, W., Hossain, K., Miyata, T., Nagase, F., Kato, M., Suzuki, H., Nakashima, I. Redox-linked cell surface-oriented signaling for T-cell death. Antioxidants & Redox Signaling, 2002. 4: 445-454.
- Nakamura, H., Nakamura, K., Yodoi, J. Redox regulation of cellular activation. Annual Review of Immunology, 1997. 15: 351-369.
- Saladino, R., Barontini, M., Crucianelli, M., Nencioni, L., Sgarbanti, R., Palamara, A.T. Current advances in anti-influenza therapy. Current Medicinal Chemistry, 2010. 17: 2101-2140.
- Liu, B., Mori, I., Jaber Hossain, M., Dong, L., Chen, Z., Kimura, Y. Local immune responses to influenza virus infection in mice with a targeted disruption of perforin gene. Microbial Pathogenesis, 2003. 34: 161-167.
- Mileva, M., Bakalova, R., Tancheva, L., Galabov, S. Effect of immobilization, cold and cold-restraint stress on liver monooxygenase activity and lipid peroxidation of influenza virus-infected mice. Archives of Toxicology, 2002. 76: 96-103.
- Mileva, M., Bakalova, R., Tancheva, L., Galabov, A., Ribarov, S. Effect of vitamin E supplementation on lipid peroxidation in blood and lung of influenza virus infected mice. Comparative Immunology, Microbiology and Infectious Diseases, 2002. 25: 1-11.
- Valyi-Nagy, T., Dermody, T.S. Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histology and Histopathology, 2005. 20: 957-967.
- Vlahos, R., Stambas, J., Selemidis, S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends in Pharmacological Sciences, 2012. 33: 3-8.
- Mileva, М. Oxidative stress as a target for medication of influenza virus infection. Acta Microbiologica Bulgarica, 2016. 3-9.
- Lin, L.T., Hsu, W.C., Lin, C.C. Antiviral natural products and herbal medicines. Journal of Traditional and Complementary Medicine, 2014. 4: 24-35.
- Aqil, F., Ahmad, I., Mehmood, Z. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turkish Journal of Biology, 2006. 30: 177-183.
- Karimi, A., Majlesi, M., Rafieian-Kopaei, M. Herbal versus synthetic drugs; beliefs and facts. Journal of Nephropharmacology, 2015. 4: 27-30.
- Zink, T., Chaffin, J. Herbal health products: what family physicians need to know. American Academy of Family Physicians, 1998. 58: 1133-1140.
- Milugo, T.K., Omosa, L.K., Ochanda, J.O., Owuor, B.O., Wamunyokoli, F.A., Oyugi, J.O., Ochieng, J.W. Antagonistic effect of alkaloids and saponins on bioactivity in the quinine tree (Rauvolfia caffra sond.): further evidence to support biotechnology in traditional medicinal plants. BMC Complementary Medicine and Therapies, 2013. 13:
- Palhares, R.M., Gonçalves Drummond, M., Dos Santos Alves Figueiredo Brasil, B., Pereira Cosenza, G., das Graças Lins Brandão, M., Oliveira, G. Medicinal plants recommended by the world health organization: DNA barcode identification associated with chemical analyses guarantees their quality. PloS ONE, 2015. 10:
- Gansukh, E., Muthu, M., Paul, D., Ethiraj, G., Chun, S., Gopal, J. Nature nominee quercetin's anti-influenza combat strategy—Demonstrations and remonstrations. Reviews in Medical Virology, 2017. 27:
- Anand David, A.V., Arulmoli, R., Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacognosy Reviews, 2016. 10: 84-89.
- Chun, O.K., Chung, S.J., Claycombe, K.J., Song, W.O. Serum C-Reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. Journal of Nutrition, 2008. 138: 753-760.
- Mehrbod, P., Abdalla, M.A., Fotouhi, F., Heidarzadeh, M., Aro, A.O., Eloff, J.N., McGaw, L.J., Fasina, F.O. Immunomodulatory properties of quercetin-3-O-α-L-rhamnopyranoside from Rapanea melanophloeos against influenza a virus. BMC Complementary Medicine and Therapies, 2018b. 18: 1-11.
- Lin, C.-F., Leu, Y.-L., Al-Suwayeh, S.A., Ku, M.-C., Hwang, T.-L., Fang, J.-Y. Anti-inflammatory activity and percutaneous absorption of quercetin and its polymethoxylated compound and glycosides: The relationships to chemical structures. European Journal of Pharmaceutical Sciences, 2012. 47: 857-864.
- Delgado, L., Fernandes, I., González-Manzano, S., de Freitas, V., Mateus, N., Santos-Buelga, C. Anti-proliferative effects of quercetin and catechin metabolites. Food & Function, 2014. 5: 797-803.
- Gansukh, E., Kazibwe, Z., Pandurangan, M., Judy, G., Kim, D.H. Probing the impact of quercetin-7-O-glucoside on influenza virus replication influence. Phytomedicine, 2016. 23: 958-967.
- Li, M., Xu, Z. Quercetin in a lotus leaves extract may be responsible for antibacterial activity. Archives of Pharmacal Research, 2008. 31: 640-644.
- Atashpour, S., Fouladdel, S., Komeili Movahhed, T., Barzegar, E., Ghahremani, M.H., Ostad, S.N., Azizi, E. Quercetin induces cell cycle arrest and apoptosis in CD133+ cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iranian Journal of Basic Medical Sciences, 2015. 18: 635-643.
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. Journal of Ethnopharmacology, 2012. 143: 383-396.
- Ying, H.-Z., Liu, Y.-H., Yu, B., Wang, Z.-Y., Zang, J.-N., Yu, C.-H. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food and Chemical Toxicology, 2013. 52: 53-60.
- Choi, H.J., Song, J.H., Park, K.S., Kwon, D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. European Journal of Pharmaceutical Sciences, 2009. 37: 329-333.
- Mehrbod, P., Ebrahimi, S.N., Fotouhi, F., Eskandari, F., Eloff, J.N., McGaw, L.J., Fasina, F.O. Experimental validation and computational modeling of anti-influenza effects of quercetin-3-O-α-L-rhamnopyranoside from indigenous south African medicinal plant Rapanea melanophloeos. BMC Complementary Medicine and Therapies, 2019. 19: 1-11.
- Murota, K., Terao, J. Antioxidative flavonoid quercetin: implications of its intenstinal absorption and metabolism. . Archives of Biochemistry and Biophysics, 2003. 417: 12-17.
- Abdal Dayem, A., Choi, H.Y., Kim, Y.B., Cho, S.-G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE, 2015. 10:
- Pawlikowska-Pawlega, B., Gruszecki, W., Misiak, L., Paduch, R., Piersiak, T., B., Z., Pawelec, J., Gawron, A. Modification of membranes by quercetin, a naturally occurring flavonoid, via its incorporation in the polar head group. Biochimica et Biophysica Acta-Biomembranes 2007. 1768: 2195-2204.
- Ding, F., Liu, J., Du, R., Yu, Q., Gong, L., Jiang, H., Rong, R. Qualitative and quantitative analysis for the chemical constituents of Tetrastigma hemsleyanum Diels et Gilg using Ultra-High Performance Liquid Chromatography/Hybrid Quadrupole-Orbitrap Mass Spectrometry and preliminary screening for anti-influenza virus components. Evidence-Based Complementary and Alternative Medicine, 2019. 2019.
- Kim, Y., Narayanan, S., Chang, K.-O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Research, 2010. 88: 227-235.
- Ibrahim, A.K., Youssef, A.I., Arafa, A.S., Ahmed, S.A. Anti-H5N1 virus flavonoids from Capparis sinaica Veill. Natural Product Research, 2013. 27: 2149-2153.
- Yang, Z.-F., Bai, L.-P., Huang, W.-b., Li, X.-Z., Zhao, S.-S., Zhong, N.-S., Jiang, Z.-H. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure–activity relationship analysis. Fitoterapia, 2014. 93: 47-53.
- Thapa, M., Kim, Y., Desper, J., Chang, K.-O., Hua, D.H. Synthesis and antiviral activity of substituted quercetins. Bioorganic & Medicinal Chemistry Letters, 2012. 22: 353-356.
- Liu, A.-L., Wang, H.-D., Lee, S.M., Wang, Y.-T., Du, G.-H. Structure–activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorganic & Medicinal Chemistry, 2008. 16: 7141-7147.
- Lall, N., Hussein, A.A., Meyer, J.J.M. Antiviral and antituberculous activity of Helichrysum melanacme constituents. Fitoterapia, 2006. 77: 230-232.
- El-Kashak, W.A., Elshamy, A.I., Mohamed, T.A., El Gendy, A.E.-N.G., Saleh, I.A., Umeyama, A. Rumpictuside A: Unusual 9,10-anthraquinone glucoside from Rumex pictus Forssk. Carbohydrate Research, 2017. 448: 74-78.
- Eropkin, M., Gudkova, T., Konovalova, N., Shchekanova, S., Iaglovskaia, I., Eropkina, E., Kiselev, O. Antiviral action of some antioxidants/antihypoxants and their combinations with remantadine against human influenza A(H3N2) virus studied in in vitro models. Eksperimental'naia i klinicheskaia farmakologiia, 2007. 70: 33-37.
- Bertelli, A.A.E., Mannari, C., Santi, S., Filippi, C., Migliori, M., Giovannini, L. Immunomodulatory activity of shikimic acid and quercitin in comparison with oseltamivir (Tamiflu) in an “in vitro” model. Journal of Medical Virology, 2008. 80: 741-745.
- Zhang, L., Cheng, Y.-X., Liu, A.-L., Wang, H.-D., Wang, Y.-L., Du, G.-H. Antioxidant, anti-inflammatory and anti-influenza properties of components from Chaenomeles speciosa. Molecules, 2010. 15: 8507-8517.
- Cho, W.-K., Weeratunga, P., Lee, B.-H., Park, J.-S., Kim, C.-J., Ma, J.Y., Lee, J.-S. Epimedium koreanum Nakai displays broad spectrum of antiviral activity in vitro and in vivo by inducing cellular antiviral state. Viruses, 2015. 7: 352-377.
- Choi, J.-G., Lee, H., Hwang, Y.-H., Lee, J.-S., Cho, W.-K., Ma, J.Y. Eupatorium fortunei and Its Components Increase Antiviral Immune Responses against RNA Viruses. Frontiers in Pharmacology, 2017. 8: 1-12.
- Mehrbod, P., Abdalla, M.A., Njoya, E.M., Ahmed, A.S., Fotouhi, F., Farahmand, B., Gado, D.A., Tabatabaian, M., Fasanmi, O.G., Eloff, J.N., et al. South African medicinal plant extracts active against influenza A virus. BMC Complementary Medicine and Therapies, 2018a. 18: 1-10.
- Chen, C., Jiang, Z.Y., Yu, B., Wu, X.L., Dai, C.Q., Zhao, C.L., Ju, D.H., Chen, X.Y. Study on the anti-H1N1 virus effects of quercetin and oseltamivir and their mechanism related to TLR7 pathway. Journal of Asian Natural Products Research, 2012. 14: 877-885.
- Wan, Q., Wang, H., Lin, Y., Gu, L., Han, M., Yang, Z., Zhang, Y., Ma, R., Wang, L., Wang, Z. Effects of quercetin on CDK4 mRNA and protein expression in A549 cells infected by H1N1. Biomedical Reports, 2013. 1: 766-770.
- Gannagé, M., Dormann, D., Albrecht, R., Dengjel, J., Torossi, T., Rämer, P.C., Lee, M., Strowig, T., Arrey, F., Conenello, G., et al. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host & Microbe, 2009. 6: 367-380.
- Rossman, J.S., Lamb, R.A. Autophagy, apoptosis, and the influenza virus M2 protein. Cell Host & Microbe, 2009. 6: 367-380.
- Jang, K.S., Kim, M.J., Oh, Y.A., Kim, I.D., No, H.K., Kim, S.D. Effects of various sub-ingredients on sensory quality of Korean cabbage kimchi. Korean Journal of Food and Nutrition, 1991. 20: 233−240.
- Vaidya, B., Cho, S.-Y., Oh, K.-S., Kim, S.H., Kim, Y.O., Jeong, E.-H., Nguyen, T.T., Kim, S.H., Kim, I.S., Kwon, J., et al. Effectiveness of periodic treatment of quercetin against influenza A virus H1N1 through modulation of protein expression. Journal of Agricultural and Food Chemistry, 2016. 64: 4416-4425.
- Choi, J.-G., Lee, H., Kim, Y.S., Hwang, Y.-H., Oh, Y.-C., Lee, B., Moon, K.M., Cho, W.-K., Ma, J.Y. Aloe vera and its components inhibit influenza A virus-induced autophagy and replication. American Journal of Chinese Medicine, 2019. 47: 1307-1324.
- Li, S.-W., Yang, T.-C., Lai, C.-C., Huang, S.-H., Liao, J.-M., Wan, L., Lin, Y.-J., Lin, C.-W. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. European Journal of Pharmacology, 2014. 738: 125-132.
- López-Expósito, I., Castillo, A., Yang, N., Liang, B., Li, X.-M. Chinese herbal extracts of Rubia cordifolia and Dianthus superbus suppress IgE production and prevent peanut-induced anaphylaxis. Chinese Medicine, 2011. 6: 1-10.
- Nile, S.H., Kim, D.H., Nile, A., Park, G.S., Gansukh, E., Kai, G. Probing the effect of quercetin 3-glucoside from Dianthus superbus L against influenza virus infection- In vitro and in silico biochemical and toxicological screening. Food and Chemical Toxicology, 2020. 135: 1-10.
- Rakers, C., Schwerdtfeger, S.-M., Mortier, J., Duwe, S., Wolff, T., Wolber, G., Melzig, M.F. Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorganic & Medicinal Chemistry Letters, 2014. 24: 4312-4317.
- Duan, J., Lou, J., Zhang, Q., Ke, J., Qi, Y., Shen, N., Zhu, B., Zhong, R., Wang, Z., Liu, L., et al. A genetic variant rs1801274 in FCGR2A as a potential risk marker for Kawasaki disease: a case-control study and meta-analysis. PLoS ONE, 2014. 9:
- Lee, I.K., Hwang, B.S., Kim, D.W., Kim, J.Y., Woo, E.E., Lee, Y.J., Choi, H.J., Yun, B.S. Characterization of neuraminidase inhibitors in Korean Papaver rhoeas bee pollen contributing to anti-influenza activities in vitro. Planta Medica, 2016. 82: 524-529.
- Kim, D.H., Park, G.S., Nile, A.S., Kwon, Y.D., Enkhtaivan, G., Nile, S.H. Utilization of Dianthus superbus L and its bioactive compounds for antioxidant, anti-influenza and toxicological effects. Food and Chemical Toxicology, 2019. 125: 313-321.
- Liu, Z., Zhao, J., Li, W., Wang, X., Xu, J., Xie, J., Tao, K., Shen, L., Zhang, R. Molecular docking of potential inhibitors for influenza H7N9. Computational and Mathematical Methods in Medicine, 2015. 2015: 1-8.
- Liu, Z., Zhao, J., Li, W., Shen, L., Huang, S., Tang, J., Duan, J., Fang, F., Huang, Y., Chang, H., et al. Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Scientific Reports, 2016. 6:
- Yin, J., Ma, L., Wang, H., Yan, H., Hu, J., Jiang, W., Li, Y. Chinese herbal medicine compound Yi-Zhi-Hao pellet inhibits replication of influenza virus infection through activation of heme oxygenase-1. Acta Pharmaceutica Sinica B, 2017. 7: 630–637.
- Sadati, S.M., Gheibi, N., Ranjbar, S., Hashemzadeh, M.S. Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuraminidase. Biomedical Reports, 2019. 10: 33-38.
- Xu, L., Jiang, W., Jia, H., Zheng, L., Xing, J., Liu, A., Du, G. Discovery of multitarget-directed ligands against influenza A virus from compound Yizhihao through a predictive system for compound-protein interactions. Frontiers in Cellular and Infection Microbiology, 2020. 10: 1-14.
- Wu, W., Li, R., Li, X., He, J., Jiang, S., Liu, S., Yang, J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses, 2015. 8: 1-6.
- Eşanu, V., Prahoveanu, E., Crişan, I., Cioca, A. The effect of an aqueous propolis extract, of rutin and of a rutin-quercetin mixture on experimental influenza virus infection in mice. Virologie, 1981. 32: 213-215.
- Raju, T.A.N., Lakshmi, A.N.V., Anand, T., Rao, L.V., Sharma, G. Protective effects of quercetin during influenza virus-induced oxidative stress. Asia Pac J Clin Nutr, 2000. 9: 314-317.
- Kumar, P., Khanna, M., Srivastava, V., Tyagi, Y.K., Raj, H.G., Ravi, K. Effect of quercetin supplementation on lung antioxidants after experimental influenza virus infection. Exp Lung Res, 2005. 31: 449-459.
- Savov, V.M., Galabov, A.S., Tantcheva, L.P., Mileva, M.M., Pavlova, E.L., Stoeva, E.S., Braykova, A.A. Effects of rutin and quercetin on monooxygenase activities in experimental influenza virus infection. Exp Toxicol Pathol, 2006. 58: 59-64.
- Friel, H., Lederman, H. A nutritional supplement formula for influenza A (H5N1) infection in humans. Med Hypotheses, 2006. 67: 578-587.
- Davis, J.M., Murphy, E.A., McClellan, J.L., Carmichael, M.D., Gangemi, J.D. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am J Physiol-Reg I, 2008. 295: R505-R509.
- Kim, Y., Narayanan, S., Chang, K.-O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antivir Res, 2010. 88: 227-235.
- Fan, D., Zhou, X., ., Zhao, C., Chen, H., Zhao, Y., Gong, X. Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-D-glucuronide in Polygonum perfoliatum L. Fitoterapia, 2011. 82: 805-810.
- Choi, H.J., Song, J.H., Kwon, D.H. Quercetin 3-rhamnoside exerts antiinfluenza A virus activity in mice. Phytother Res, 2012. 26: 462-464.
- Liu, Z., Zhao, J., Li, W., Shen, L., Huang, S., Tang, J., Duan, J., Fang, F., Huang, Y., Chang, H., et al. Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Sci Rep, 2016. 6:
- Smith, M., Smith, J.C. Repurposing therapeutics for COVID-19: Supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface. ChemRxiv, 2020.
- Omar, S., Bouziane, I., Bouslama, Z., Djemel, A. In-silico identification of potent inhibitors of COVID-19 main protease (Mpro) and angiotensin converting enzyme 2 (ACE2) from natural products: Quercetin, hispidulin, and cirsimaritin exhibited better potential inhibition than hydroxy-chloroquine against COVID-19 main protease active site and ACE2. ChemRxiv, 2020.
- Adem, S., Eyupoglu, V., Sarfraz, I., Rasul, A., Ali, M. Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: An in silico strategy unveils a hope against Corona. Preprint, 2020.
- Das, S., Sarmah, S., Lyndem, S., Singha Roy, A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. Journal of Biomolecular Structure and Dynamics, 2020. just-accepted: 1-18.
- Mullen, W., Boitier, A., Stewart, A.J., Crozier, A. Flavonoid metabolites in human plasma and urine after the consumption of red onions: analysis by liquid chromatography with photodiode array and full scan tandem mass spectrometric detection. Journal of Chromatography A, 2004. 1058: 163-168.
- Zhao, J., Yang, J., Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: an overview. International Jof Pharmaceutics, 2019. 570:
- Williamson, G., Kerimi, A. Testing of natural products in clinical trials targeting the SARS-CoV-2 (Covid-19) viral spike protein-angiotensin converting enzyme-2 (ACE2) interaction. Biochemical Pharmacology, 2020. 178: