| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jessica Griffith | + 2552 word(s) | 2552 | 2020-12-15 08:38:55 | | | |
| 2 | Nicole Yin | Meta information modification | 2552 | 2021-01-11 07:27:21 | | |
Video Upload Options
Barriers between the brain and systemic circulation are dynamic and highly specialized to strictly regulate the access of a wide variety of molecules to the brain. These barriers allow for the delivery of nutrients and other molecules necessary for neuronal functioning, but often limit the permeation of xenobiotics, including drugs. In brain tumors, these barrier functions may be disrupted or altered. However, this disruption is often heterogeneous and not reliable to guaranteee the delivery of efficacious concentrations of antineoplastic agents to brain tumors.
1. Introduction
The blood–brain barrier (BBB) remains one of the greatest obstacles to effective pharmaceutical interventions in the treatment of central nervous system (CNS) disease, including brain tumors. While it is true that some loss of neurovascular and barrier integrity may occur in and around brain tumors, the magnitude of this change is not consistent, and new pharmaceutical strategies for the treatment of brain tumors have yet to show significant efficacy in the clinic[1][2][3]. This lack of efficacy is largely attributed to insufficient drug delivery due to the presence of the BBB. The dense vascular network of the brain works to strictly regulate the transport of substances into and out of the brain parenchyma in order to maintain ionic homeostasis, nutrient supply, and removal of waste for optimal neuronal function. In recent decades, research has revealed that the BBB is composed of specialized endothelial cells (ECs), which are surrounded and supported by pericytes and astrocytes and are regulated by neuronal signaling, forming what is referred to as the neurovascular unit (NVU)[4]. A lack of vesicular transport across these specialized ECs and the presence of active efflux proteins help to further restrict the access of drugs to the CNS[5]. Currently, treatment for the majority of brain tumors involves maximal surgical resection, if possible, followed by radiation, and in the case of glioblastoma multiforme (GBM), concomitant temozolomide (TMZ)[3]. However, these treatments often prove to be palliative, and malignant brain tumors are nearly always fatal within five years of initial diagnosis[6][7].
2. Barriers and Boundaries in the Brain
Rational drug delivery to any organ requires a thorough understanding of the structures and properties of the target tissue. This section includes detailed features of the dynamic NVU model that is rapidly supplanting the former static BBB concept. Furthermore, the role that these features serve in guiding the molecular basis for current therapies for CNS tumors is illustrated.
2.1. CNS Blood–Tissue Barriers
Any strategy for blood-borne drug delivery into the CNS must consider several structural obstacles related to blood–tissue interfaces[8][9]. First, the brain is covered by layers of cells collectively described as the dura–arachnoid–pia membranes. The dura separates peripheral vessels within the cranium from the cerebrospinal fluid (CSF) in which the brain resides. Several compact layers of epithelial cells, with tight junctional contacts and relatively low surface areas, prevent materials from traversing this boundary. Within the CSF compartment, the pial layer contains vessels that penetrate the brain parenchyma. These vessels also exhibit very low permeability and surface area. Other major barriers include relatively small regions of CNS circulation and include the choroid plexus, circumventricular organs (CVOs), and ependymal cells. Each of these has specialized epithelial cells with properties that highly restrict water-soluble chemicals from penetrating the mass of parenchymal tissue. The choroid plexus that produces CSF, for example, contains specialized epithelial cells with tight junctions (TJs) encasing a fenestrated vascular endothelium that allows the exchange of blood constituents with the extracellular space. The CVOs are also composed of fenestrated endothelial cells, but their location is confined by surrounding tanycytes, a specialized epithelial cell that also has tight junctional contacts that localize and restrict the interstitial fluid. Direct exchange between the CSF and interstitial fluid is restricted by ependymal cells that line the ventricular surface and form a selectively permeable cellular barrier.
2.2. Neurovascular Unit
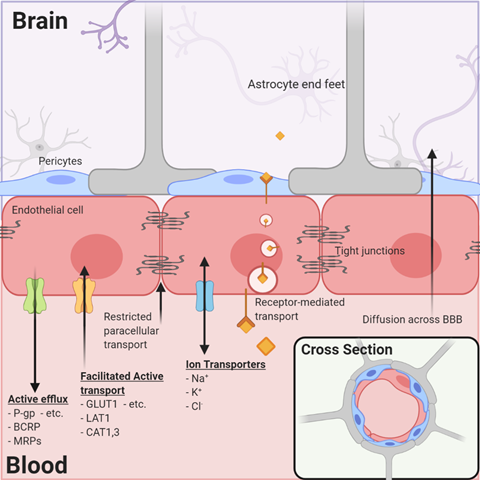
By far, the major blood–tissue interface in the brain is the microvasculature network extending from arterioles to capillaries and to venules. By illustration, in one gram of human brain, the length of vessels, if joined end to end, approximates the length of 4.5 football fields and almost a quarter (~23%) of the surface area of a sheet of photocopy paper. The current model of the brain microvasculature consists of several different cell types working collectively to form a functional NVU[4][10]. Endothelial cells joined by tight junctions that block paracellular diffusion are surrounded by pericytes that form gap junctions with multiple adjacent endothelial cells. Astrocytic endfeet also cover >99% of the endothelial–pericyte cell surface (Figure 1). The astrocytes, in turn, extend processes that monitor synaptic activity and react by signaling endothelial cells and pericytes to respond to increased metabolic demands by increasing nutrient delivery. Microglia, the resident immune cells, are extravascular when dormant but react swiftly to remove cellular debris (by phagocytosis) or respond to inflammatory signals associated with disease or injury. Loss of pericytes and their signaling in the NVU by injury or genetic means leads to reduced expression of endothelial tight junction proteins and dysfunction of their permeability barrier[11][12]. These examples illustrate the remarkable dynamics, plasticity, and interdependence of signaling among the NVU cells to maintain functional stability in the contemporary model of the neurovasculature.
Figure 1. The neurovascular unit/blood–brain barrier (NVU/BBB) is composed of specialized endothelial cells and support cells, including pericytes and astrocytes. The cross-sectional view illustrates that the majority of the abluminal surface of the endothelial cell is covered by pericytes and astrocytic foot processes. Paracellular transport across the BBB/NVU is restricted by tight junction proteins, and even small, lipophilic molecules that might diffuse across the BBB may be subject to active efflux by a variety of proteins. Facilitated active transport, receptor-mediated transport, and ion transporters allow the brain to be supplied with nutrients while maintaining strict homeostasis.
2.3. Blood-to-Brain Permeability and Transport
As the brain depends on external nutrients for growth and development and yet must be protected from the influence of circulating toxins or xenobiotics, the specialized endothelial cells of the brain express critical membrane-imbedded proteins that function as transporters. One group of transporters includes facilitated carriers and secondary active transporters for the delivery of energy substrates and essential nutrients (Table 1). The glucose transporter (GLUT1), monocarboxylic acid transporter (MCT1), and amino acid transporters are examples of the >40 transporters detected by functional and transcriptomic analyses[13].
A second type of transporter that is critical to brain drug delivery is the ABC (ATP-binding cassette) superfamily that uses the energy of ATP hydrolysis to expel endogenous and exogenous xenobiotics from the cell (and from the brain) and return them to the blood for transformation and excretion (Table 2). The most relevant ABC transporters expressed by brain endothelial cells are P-glycoprotein (P-gp, ABCB1), breast-cancer-related protein (BCRP, ABCG2), and the multiple drug-related proteins (MRP1, -4, -5; ABCC1, -4, -5). Many anticancer drugs are substrates of the ABC transporters and, therefore, may influence their effectiveness as brain cancer drugs (Figure 1). The relevance of ABC transporters in anticancer drug delivery in brain tumors with apparently high permeability is illustrated by the fact that the most permeable tumor vasculatures have influx rate constants several-fold less than the rate constants for other organs such as muscle, heart, lung, kidney, and liver[14]. Therefore, efflux mechanisms are likely important even in CNS tumors, in which the permeability of the brain vasculature may be compromised and elevated compared to the surrounding tissue.
Table 1. Endothelial cell membrane transporters: partial list of common carriers.
|
Transport System |
Typical Substrate |
SLC Family |
Common Name |
|
Carbohydrates |
|||
|
Hexose |
Glucose |
SLC2A1 |
Glut1 |
|
Sodium Myo-inositol |
Myo-inositol |
SLC5A3 |
SMIT |
|
Monocarboxylates |
|||
|
Monocarboxylic acid |
Lactic acid ketones |
SLC16A1 |
MCT1 |
|
Amino Acids |
|||
|
Large neutral amino acid |
Phenylalanine |
SLC7A5 |
LAT1 |
|
Small neutral amino acid |
Alanine |
SLC38A2 |
SNAT2, -3, -5 |
|
Cationic amino acid |
Lysine |
SLC7A1 |
Cat1, CAT3 |
|
Beta amino acid |
Taurine |
SLC6A6 |
TauT |
|
Ala-Ser-Cys |
Ala, ser, cys |
SLC1A4 |
ASCT1, -2 |
|
Excitatory amino acid |
Glutamic acid |
SLC1A2 |
EAAT-1, -2, -3 |
|
Glycine |
Glycine |
SLC6A9, A5 |
GT-1 |
|
Others |
|||
|
Fatty acids |
Essential FA LPC-PC (DHA) |
SLC44A1/2 Mfsd2A |
FATP-1, -4 Mfsd2A |
|
Nucleoside |
Adenosine |
SLC29A1 SLC28A1 |
ENT-1, -2; CNT1–3 |
|
Hormones |
Thyroid T3 Thyroid T4 |
SLC16A2 OATP1C1 |
MCT8 OATP1C1 |
|
Biotin, pantothenic acid |
biotin |
SLC5A6 |
SMVT |
|
Folic acid |
Folinic acid |
SLC46A1 |
PCFT |
|
Copper |
Cu+ |
SLC31A1 |
CTR1 |
Table 2. Brain endothelial cell transporters of xenobiotics/drugs. Members of the ABC (ATP-binding cassette) superfamily of transporters demonstrated in brain endothelial cells and non-ABC transporters of organic chemicals potentially present are listed.
|
Transport System |
Common Name |
Typical Substrate |
|
ATP Binding Cassette Transporter (ABC) |
||
|
ABCB1 |
P-gp |
Broad-spectrum, xenobiotics |
|
ABCG2 |
BCRP |
mitoxantrone anthracycline xenobiotics |
|
ABCC1 |
MRP1 |
GSSG, leukotrienes |
|
ABCC5 |
MRP5 |
Thiopurines, cyclic nucleotides |
|
ABCC4 |
MRP4 |
Organic anions |
|
Non-ABC Transporters |
||
|
SLC22A7 SLC22A8 SLC20A2 SLCO1A4 SLCO2B1 |
OAT2-3 OATP1A4 OATP2B1 OCTN2 OCT1-3 |
Organic ions |
3. Heterogeneous Blood–Tumor Barrier Permeability
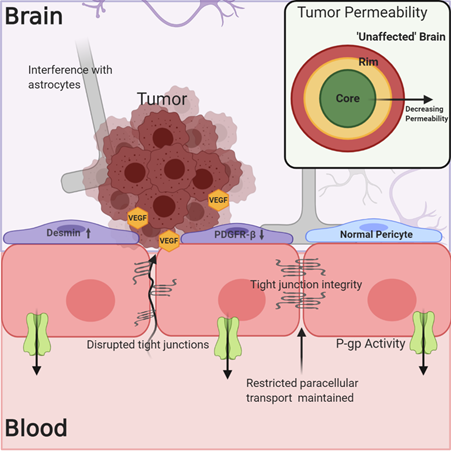
The understanding of the BBB’s physical and biochemical barrier functions, including the expression of tight junction proteins, restricted paracellular transport, and active efflux mechanisms, has been well established. However, determining the integrity of the NVU/BBB in and around tumors and how this affects tumor treatment has been less straightforward. In the case of both primary and metastatic tumors, the NVU/BBB is subject to changes due to tumor growth and signaling, and these alterations in NVU/BBB integrity and physiology result in what will hereafter be referred to as the blood–tumor barrier (BTB). The BTB may be characterized by an inflammatory environment with increased numbers of activated astrocytes, vascular endothelial growth factor (VEGF)-induced reduction in the expression of tight junction proteins like claudin-5, breakdown of the basal lamina, and tumor cell interference in associations between endothelial cells and astrocytic endfeet (Figure 2)[15][16][17]. There is also evidence for a change in the phenotype of BTB-associated pericytes, which may show decreased platelet-derived growth factor receptor-b (PDGFR-b) expression in addition to increased desmin expression[18]. As a result of these changes, the BTB can be, on average, somewhat “leakier” (more permeable) than the normal NVU/BBB in the absence of disease[17][19][20]. The predominant question with regards to BBB breakdown and the treatment of brain tumors has therefore been, is the breakdown of the NVU/BBB in the case of brain tumors significant and uniform enough to allow for the accumulation of efficacious drug concentrations?
Figure 2. The blood–tumor barrier (BTB) is characterized by increased cytokine and VEGF signaling from the tumor, which may lead to decreased expression of tight junction (TJ) proteins like claudin-5. Alterations in pericyte phenotype and disruption of astrocytic associations with endothelial cells may contribute to decreased barrier integrity. However, this is not a uniform phenomenon within or among tumors, and the expression of efflux transporters limits drug permeation into the tumor. Evidence exists showing decreased permeability of the BTB in regions distant to the core of the tumor, which more closely resemble “unaffected” brain.
As this question has been repeatedly investigated, various preclinical tumor models have routinely led to conflicting results. In some cases, tumor vascular permeability, assessed by the accumulation of fluorescent tracers, has been previously correlated with growth patterns, tumor size, or peripheral tumor of origin[21]. In other cases, including a variety of brain-trophic metastatic breast cancer models developed at the National Institutes of Health (NIH), no correlation between tumor size and permeability has been found[14][19]. These studies also found that the variability of BTB permeability among tumors in the same animal and even among regions of the same tumors, as assessed by the accumulation of fluorescent tracers and small molecules like paclitaxel, doxorubicin, and lapatinib, could be as much as 100-fold[14][22][23]. More recent studies in HER2+ brain-trophic breast cancer metastasis models have shown a poor correlation between drug accumulation and tracer accumulation, as well as inconsistent drug uptake and variable efficacy of biologics like trastuzumab and other antibody-based therapies[22][24][25]. Another model of lung cancer brain metastases found two-fold increases in permeability to small molecules like 3H-mannitol but concluded that this small relative increase in addition to functional P-gp was still a significant limitation to systemic drug therapy[26]. In addition, a number of studies utilizing transporter-knockout mice and patient-derived xenograft (PDX) models of GBMs and brain metastases have shown that the efficacy of systemic administration of various small molecules is consistently limited by the presence of the NVU/BBB and BTB, active efflux, and the fact that vascular permeability is widely variable within and around the tumor region[20][27][28][29][30][31][32][33]. This heterogeneity in permeability at the BBB leads to wide variability in drug/tracer accumulation and has also been confirmed by elegant correlated ultramicroscopy and MRI techniques in preclinical tumor models[34]. These studies point to the conclusion that relying on the potential for increased BTB permeability is unlikely to result in efficacious treatment through the systemic administration of novel therapies and their subsequent regulatory approval for such applications.
Although the aforementioned evidence has been largely preclinical, it agrees with clinical observations when considered in the appropriate context. Increased permeability of the BTB, relative to normal brain, is observed clinically, as increased uptake of tracers in magnetic resonance imaging (MRI) and positron emission tomography (PET) imaging allows for definitive diagnosis of brain tumors and informs many aspects of their treatment[35]. However, especially in the case of diffuse and invasive tumors like GBM, it has also been shown that nonenhancing, infiltrating regions of brain tumors often exist outside of the region of T1-weighted contrast enhancement[36][37]. This indicates that some portions of the malignant tumor are protected by a relatively uncompromised NVU/BBB. The patterns of treatment failure are strongly correlated with and attributed to these nonenhancing regions, and maximal resection that includes these regions improves survival[38][39][40]. Increasingly, early-phase studies, in which patients receive drugs prior to tumor resection and biopsy, are being utilized to determine the real extent of antineoplastic drug permeability to the BTB[1][2]. Although fold-increases in drug concentrations relative to normal brain may be observed at the core of the tumor, this may still not be adequate to cause cell death. As has been evidenced in many of the aforementioned preclinical models, it is unlikely that these drug concentrations are representative of concentrations in the entirety of the tumor. In the case of GBM, the infiltrative boundaries of the tumor are likely to have a more competent and intact BTB, closer to that of “unaffected brain”[20][31][41][42]. This heterogeneous drug distribution among different regions of the tumor is also clinically evidenced in drug concentrations from biopsies of non-contrast-enhancing tumor regions[43].
As there has been a great success with novel treatments of peripheral disease, the culmination of decades of brain tumor research has led to the conclusion that it is imperative that molecules and delivery strategies be designed foremost with an intact NVU/BBB in mind. As an example, GNE317, a small molecule that was designed specifically to avoid active efflux, showed significantly higher activity in a model of brain metastases of lung cancer than another counterpart PI3K inhibitor not designed to penetrate the BBB/NVU[44][45]. Other brain-penetrant inhibitors like osimertinib, an EGFR inhibitor, have also shown better preclinical and potential clinical efficacy[46][47]. While designing small lipophilic molecules in an attempt to optimize tumor penetration and minimize active efflux is certainly one potential method towards effective treatments for brain tumors, there are a vast number of other drug delivery strategies and novel molecules in development for this application.
References
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2018, 20, 184–191.
- Pitz, M.W.; Desai, A.; Grossman, S.A.; Blakeley, J.O. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J. Neuro-Oncol. 2011, 104, 629–638.
- Arvold, N.D.; Lee, E.Q.; Mehta, M.P.; Margolin, K.; Alexander, B.M.; Lin, N.U.; Anders, C.K.; Soffietti, R.; Camidge, D.R.; Vogelbaum, M.A.; et al. Updates in the management of brain metastases. Neuro-Oncology 2016, 18, 1043–1065.
- N. Joan Abbott; Adjanie A. K. Patabendige; Diana E. M. Dolman; Siti R. Yusof; David J. Begley; Structure and function of the blood–brain barrier. Neurobiology of Disease 2010, 37, 13-25, 10.1016/j.nbd.2009.07.030.
- Tetsuya Terasaki; Sumio Ohtsuki; Brain-to-blood transporters for endogenous substrates and xenobiotics at the blood-brain barrier: An overview of biology and methodology. Neurotherapeutics 2005, 2, 63-72, 10.1007/bf03206643.
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 1985–1996.
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012.
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Leybaert, L.; Molnár, Z.; O’Donnell, M.E.; Povlishock, J.T.; et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011, 12, 169–182.
- Mastorakos, P.; McGAVERN, D.B. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019, 4, eaav0492.
- Melanie D. Sweeney; Zhen Zhao; Axel Montagne; Amy R. Nelson; Berislav V. Zlokovic; Blood-Brain Barrier: From Physiology to Disease and Back. Physiological Reviews 2019, 99, 21-78, 10.1152/physrev.00050.2017.
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566.
- Liu, Q.; Lin, W.J.; Tang, Y. New Insights into the Dysfunctions of Pericytes and Neurovascular Units in Neurodegenerative Diseases. Neurosci. Bull. 2020.
- Rucha Pandit; Liyu Chen; Jürgen Götz; The blood-brain barrier: Physiology and strategies for drug delivery. Advanced Drug Delivery Reviews 2020, 165-166, 1-14, 10.1016/j.addr.2019.11.009.
- Paul R. Lockman; Rajendar K. Mittapalli; Kunal S. Taskar; Vinay Rudraraju; Brunilde Gril; Kaci A. Bohn; Chris E. Adkins; Amanda Roberts; Helen R. Thorsheim; Julie A. Gaasch; et al.Suyun HuangDiane PalmieriPatricia S. SteegQuentin R. Smith Heterogeneous Blood–Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clinical Cancer Research 2010, 16, 5664-5678, 10.1158/1078-0432.ccr-10-1564.
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982.
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 1–15.
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41.
- L. Tiffany Lyle; Paul R. Lockman; Chris E. Adkins; Afroz Shareef Mohammad; Emily Sechrest; Emily Hua; Diane Palmieri; David J. Liewehr; Seth M. Steinberg; Wojciech Kloc; et al.Ewa Izycka-SwieszewskaRenata DuchnowskaNaema NayyarPriscilla K. BrastianosPatricia S. SteegBrunilde Gril Alterations in Pericyte Subpopulations Are Associated with Elevated Blood–Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clinical Cancer Research 2016, 22, 5287-5299, 10.1158/1078-0432.ccr-15-1836.
- Adkins, C.E.; Mohammad, A.S.; Terrell-Hall, T.B.; Dolan, E.L.; Shah, N.; Sechrest, E.; Griffith, J.; Lockman, P.R. Characterization of passive permeability at the blood-tumor barrier in five preclinical models of brain metastases of breast cancer. Clin. Exp. Metastasis 2016, 33, 373–383.
- Gampa, G.; Kenchappa, R.S.; Mohammad, A.S.; Parrish, K.E.; Kim, M.; Crish, J.F.; Luu, A.; West, R.; Hinojosa, A.Q.; Sarkaria, J.N.; et al. Enhancing Brain Retention of a KIF11 Inhibitor Significantly Improves its Efficacy in a Mouse Model of Glioblastoma. Sci. Rep. 2020, 10, 1–13.
- R. D. Zhang; J. E. Price; T. Fujimaki; C. D. Bucana; I. J. Fidler; Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice.. The American Journal of Pathology 1992, 141, 1115-1124.
- Terrell-Hall, T.B.; Nounou, M.I.; El-Amrawy, F.; Griffith, J.I.G.; Lockman, P.R. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 2017, 8, 83734–83744.
- Taskar, K.S.; Rudraraju, V.; Mittapalli, R.K.; Samala, R.; Thorsheim, H.R.; Lockman, J.; Gril, B.; Hua, E.; Palmieri, D.; Polli, J.W.; et al. Lapatinib Distribution in HER2 Overexpressing Experimental Brain Metastases of Breast Cancer. Pharm. Res. 2012, 29, 770–781.
- Gril, B.; Wei, D.; Zimmer, A.S.; Robinson, C.; Khan, I.; Difilippantonio, S.; Overstreet, M.G.; Steeg, P.S. HER2 antibody-drug conjugate controls growth of breast cancer brain metastases in hematogenous xenograft models, with heterogeneous blood–tumor barrier penetration unlinked to a passive marker. Neuro-Oncology 2020, 22, 1625–1636.
- Askoxylakis, V.; Ferraro, G.B.; Kodack, D.P.; Badeaux, M.; Shankaraiah, R.C.; Seano, G.; Kloepper, J.; Vardam, T.; Martin, J.D.; Naxerova, K.; et al. Preclinical Efficacy of Ado-trastuzumab Emtansine in the Brain Microenvironment. J. Natl. Cancer Inst. 2016.
- Ngoc H. On; Ryan Mitchell; Sanjot D. Savant; Corbin. J. Bachmeier; Grant M. Hatch; Donald W. Miller; Examination of blood–brain barrier (BBB) integrity in a mouse brain tumor model. Journal of Neuro-Oncology 2012, 111, 133-143, 10.1007/s11060-012-1006-1.
- Agarwal, S.; Elmquist, W.F. Insight into the Cooperation of P-glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) at the Blood–Brain Barrier: A Case Study Examining Sorafenib Efflux Clearance. Mol. Pharm. 2012, 9, 678–684.
- Parrish, K.E.; Pokorny, J.; Mittapalli, R.K.; Bakken, K.; Sarkaria, J.N.; Elmquist, W.F. Efflux Transporters at the Blood-Brain Barrier Limit Delivery and Efficacy of Cyclin-Dependent Kinase 4/6 Inhibitor Palbociclib (PD-0332991) in an Orthotopic Brain Tumor Model. J. Pharmacol. Exp. Ther. 2015, 355, 264–271.
- Gampa, G.; Kim, M.; Mohammad, A.S.; Parrish, K.E.; Mladek, A.C.; Sarkaria, J.N.; Elmquist, W.F. Brain Distribution and Active Efflux of Three panRAF Inhibitors: Considerations in the Treatment of Melanoma Brain Metastases. J. Pharmacol. Exp. Ther. 2019, 368, 446–461.
- Mittapalli, R.K.; Chung, A.H.; Parrish, K.E.; Crabtree, D.; Halvorson, K.G.; Hu, G.; Elmquist, W.F.; Becher, O.J. ABCG2 and ABCB1 Limit the Efficacy of Dasatinib in a PDGF-B-Driven Brainstem Glioma Model. Mol. Cancer Ther. 2016, 15, 819–829.
- Pokorny, J.L.; Calligaris, D.; Gupta, S.K.; Iyekegbe, D.O.; Mueller, D.; Bakken, K.K.; Carlson, B.L.; Schroeder, M.A.; Evans, D.L.; Lou, Z.; et al. The efficacy of the wee1 inhibitor MK-1775 combined with temozolomide is limited by heterogeneous distribution across the blood-brain barrier in glioblastoma. Clin. Cancer Res. 2015, 21, 1916–1924.
- Lakoma, A.; Barbieri, E.; Agarwal, S.; Jackson, J.; Chen, Z.; Kim, Y.; McVay, M.; Shohet, J.M.; Kim, E.S. The MDM2 small-molecule inhibitor RG7388 leads to potent tumor inhibition in p53 wild-type neuroblastoma. Cell Death Discov. 2015, 1, 15026.
- Kim, M.; Ma, D.J.; Calligaris, D.; Zhang, S.; Feathers, R.W.; Vaubel, R.A.; Meaux, I.; Mladek, A.C.; Parrish, K.E.; Jin, F.; et al. Efficacy of the MDM2 Inhibitor SAR405838 in Glioblastoma Is Limited by Poor Distribution Across the Blood–Brain Barrier. Mol. Cancer Ther. 2018, 17, 1893–1901.
- Michael O. Breckwoldt; Julia Bode; Felix Sahm; Thomas Krüwel; Gergely Solecki; Artur Hahn; Peter Wirthschaft; Anna S. Berghoff; Maximilian Haas; Varun Venkataramani; et al.Andreas Von DeimlingWolfgang WickChristel Herold-MendeSabine HeilandMichael PlattenMartin BendszusFelix T. KurzFrank WinklerBjörn Tews Correlated MRI and Ultramicroscopy (MR-UM) of Brain Tumors Reveals Vast Heterogeneity of Tumor Infiltration and Neoangiogenesis in Preclinical Models and Human Disease. Frontiers in Neuroscience 2019, 12, 1004, 10.3389/fnins.2018.01004.
- James R. Fink; Mark Muzi; Melinda Peck; Kenneth A. Krohn; Multimodality Brain Tumor Imaging: MR Imaging, PET, and PET/MR Imaging. Journal of Nuclear Medicine 2015, 56, 1554-1561, 10.2967/jnumed.113.131516.
- Watanabe, M.; Tanaka, R.; Takeda, N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 1992, 34, 463–469.
- Kelly, P.J.; Daumas-Duport, C.; Kispert, D.B.; Kall, B.A.; Scheithauer, B.W.; Illig, J.J. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J. Neurosurg. 1987, 66, 865–874.
- Pessina, F.; Navarria, P.; Cozzi, L.; Ascolese, A.M.; Simonelli, M.; Santoro, A.; Clerici, E.; Rossi, M.; Scorsetti, M.; Bello, L. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: Is it useful and safe? A single institution retrospective experience. J. Neuro-Oncol. 2017, 135, 129–139.
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–766.
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma. JAMA Oncol. 2016, 2, 1460–1469.
- Agarwal, S.; Mittapalli, R.K.; Zellmer, D.M.; Gallardo, J.L.; Donelson, R.; Seiler, C.; Decker, S.A.; SantaCruz, K.S.; Pokorny, J.L.; Sarkaria, J.N.; et al. Active efflux of dasatinib from the brain limits efficacy against murine glioblastoma: Broad implications for the clinical use of molecularly targeted agents. Mol. Cancer Ther. 2012, 11, 2183–2192.
- Sanai, N.; Li, J.; Boerner, J.; Stark, K.; Wu, J.; Kim, S.; Derogatis, A.; Mehta, S.; Dhruv, H.D.; Heilbrun, L.K.; et al. Phase 0 trial of azd1775 in first-recurrence glioblastoma patients. Clin. Cancer Res. 2018, 24, 3820–3828.
- Michael T. Milano; Paul Okunieff; Rosemary S. Donatello; Nimish A. Mohile; Joohee Sul; Kevin A. Walter; David N. Korones; Patterns and Timing of Recurrence After Temozolomide-Based Chemoradiation for Glioblastoma. International Journal of Radiation Oncology*Biology*Physics 2010, 78, 1147-1155, 10.1016/j.ijrobp.2009.09.018.
- Osswald, M.; Blaes, J.; Liao, Y.; Solecki, G.; Gömmel, M.; Berghoff, A.S.; Salphati, L.; Wallin, J.J.; Phillips, H.S.; Wick, W.; et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin. Cancer Res. 2016, 22, 6078–6087.
- Salphati, L.; Heffron, T.P.; Alicke, B.; Nishimura, M.; Barck, K.; Carano, R.A.; Cheong, J.; Edgar, K.A.; Greve, J.; Kharbanda, S.; et al. Targeting the PI3K pathway in the brain—Efficacy of a PI3K inhibitor optimized to cross the blood-brain barrier. Clin. Cancer Res. 2012, 18, 6239–6248.
- Ballard, P.; Yates, J.W.T.; Yang, Z.; Kim, D.W.; Yang, J.C.H.; Cantarini, M.; Pickup, K.; Jordan, A.; Hickey, M.; Grist, M.; et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin. Cancer Res. 2016, 22, 5130–5140.
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297.