
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zinnia Shah | + 3114 word(s) | 3114 | 2020-12-31 03:38:49 | | | |
| 2 | Dean Liu | -923 word(s) | 2191 | 2021-01-06 02:56:46 | | |
Video Upload Options
Marine bacterial species contribute to a significant part of the oceanic population, which substantially produces biologically effectual moieties having various medical and industrial applications. The use of marine-derived bacterial pigments displays a snowballing effect in recent times, being natural, environmentally safe, and health beneficial compounds. Although isolating marine bacteria is a strenuous task, these are still a compelling subject for researchers, due to their promising avenues for numerous applications. Marine-derived bacterial pigments serve as valuable products in the food, pharmaceutical, textile, and cosmetic industries due to their beneficial attributes, including anticancer, antimicrobial, antioxidant, and cytotoxic activities. Biodegradability and higher environmental compatibility further strengthen the use of marine bio-pigments over artificially acquired colored molecules. Besides that, hazardous effects associated with the consumption of synthetic colors further substantiated the use of marine dyes as color additives in industries as well.
1. Introduction
1.1. Microbial Pigments
The production of bio-pigments from bacterial species is being conducted globally with soaring interest under the research of microbial autecology. A massive array of these compounds, also referred to as “bioactive pigmented molecules”, can be derived from both Gram-positive and Gram-negative bacterial species. Production of these pigments in the marine environment is mediated through the complex mechanism of “quorum sensing”[1] or also can be induced through exposure to different stress conditions in external environments. Quorum sensing is the mechanism whereby individual bacterial cells can coordinate with others in their colony to carry out constitutive functions especially involving the secretion of numerous specific chemical compounds. These compounds can help them with survival, competence, bioluminescence, biofilm formation, and even sporulation, etc. Bio-pigments can be produced by triggering regulatory quorum sensing mechanisms of these species and can be extensively used in various bio-medical and bio-industrial sectors, including textiles, food, pharmaceutical, and cosmetic industries, owing to their beneficial attributes and biological activities[2][3]. These are moreover convenient to harvest in large volumes through utilizing simple gene manipulating strategies. The rising consumer concerns regarding safety and quality of industrial products holds a significant ground as to why scientists are shifting their focus towards naturally derived, non-toxic, and eco-friendly pigment alternatives[4].
1.2. Bacterial Pigments as Natural Colorants
The use of synthetic pigments goes back to the 1850s when these were put in trend for the first time due to their supercilious coloring properties, lower prices, and easy production strategies[5], the significance of which remains empirically the same to this day. The importance of artificial/synthetic coloring agents is still based on the fact that the appearance of food items influences consumer’s emotions, attitudes, and preferences. Let us say, if a carrot is not red, the consumer is most probably expected to reject it. The same can be applied in regards with the cosmetic industry, where the product apparel decides its fate. Thus, need for “synthetic pigments” cannot be overseen if client orientation is to be fulfilled[6]. The only progress made today is the shift towards naturally derived pigments rather than continuing the use of artificially synthesized ones, which have been denounced for their serious threat to consumer’s well-being[7]. Cancers of skin, liver, and bladder have been found positively related to the use of artificial pigments because of their high azo-dye/heavy metal compositions. Furthermore, the precursors involved and the waste generated through their production process is environmentally hazardous as well[8][9]. The outcry against the use of synthetic colorants in many health-conscious countries has already caused the ban of several artificial colorants, including Blue NO 1, Blue NO 2, Blue FCF, and Yellow NO 6[10].
Bio-pigments, however, are eco-friendly and proved additionally propitious as antitoxic, antitumor, antioxidant, anticancer, and antimicrobial agents[2]. Other advantages include fast and economic extraction techniques, higher yield, and time- and cost-efficient production. Moreover, the production of microbial pigments can also be made more convenient by the optimization capacity of their growth parameters [11]. Keeping the capacity of bio-pigments into consideration, many biotech industries are now developing protocols for efficient extraction of natural pigments as a replacement to synthetic counterparts. For instance, natural pigments such as zeaxanthin, saproxanthin, myxol and many others which illicit antioxidant activities are being instigated against artificial antioxidants such as butylated hydroxyl toluene and butylated hydroxyl acids[12][13].
1.3. Marine Ecosystem as a Source of Pigment Producing Bacterial Species
The study of a likely natural ecosystem serves as the initial-most important research step needed to find an environment that can entertain the diversity of bio-pigment sources. The marine environment is a habitat for almost 80% of all life forms[14]. It serves as a rich source of aquatic microbial species that exhibit comparatively more augmented diversity than their telluric counterparts[15]. The marine environment is presently being considered as an attractive fount for bio-pigment sources[16]. Numerous bacterial isolates from such biotopes have already been tested for pigment production. At the same time, many of them are also being utilized for various industrial purposes as well [15]. The preference of pigments produced by marine microorganisms is based on their ability to persist in extremities such as highly acidic/alkaline environments (pH < 4 and >9), extreme temperatures (−2–15 °C and 60–110 °C), and under limited substrate availability[17][18]. Apart from bacterial isolates, halophilic archaea are extensively disseminated in the marine ecosystem. Pigmented compounds from marine archaea are also prioritized owing to their ability to tolerate hyper saline and basic pH environments, besides their potential to withstand osmolytes (such as 2-sulfotrehalose) or high ionic strength[19][20].
Concerns regarding environmental conservation and consumers’ preferences have stimulated the interests of researchers and stake holders in exploring nontoxic, eco-friendly, and biodegradable commodities. Bacterially produced bio-pigments (bpBPs) have growing importance not only on account of their dyeing potential, but also due to their medicinal properties. Likewise, awareness regarding the carcinogenic and other pernicious effects of synthetic colorants has kindled a fresh enthusiasm towards the utilization of bacterial pigments in the food industry as safer alternatives to use as antioxidants, color intensifiers, flavor enhancers, and food additives.
Extraction of natural pigments from microorganisms populating environments exclusive of soil is a topic of current interest. Marine environment has become a captivating subject matter for microbiologists, pharmacologists, and biochemists in order to extract water based bacterial pigments. With the recent increase in awareness towards the benefits of natural over synthetic products, the bio-pigment industry is likely to increase its global market. The review aims at discussing the therapeutic and industrial significance of marine derived bacterial pigments helping to delineate the consequence of furthering the scope of these studies. It provides a comprehensive overview of potentiality and competence of marine bacteria as a source of bio-pigments by critically summarizing the scientific researches and accumulated data in the literature and the prominence of these bio-pigments in strengthening the overall pigment market by reviewing latest industry market research, reports, and statistics.
2. Biosynthesis of Bacterial Pigments
The potential of marine bacterial isolates as a leading source of bio-pigments demands an extensive understanding of bio-mechanisms responsible for yielding pigmented molecules. Different studies have reported the proposed biosynthetic pathways of pigment production by marine bacterial isolates along with biochemically characterized enzymatic transformations (Figure 1). However, it is still unclear if the proposed pathways are distinct for marine or terrestrial bacterial species, or may be the same in both cases.
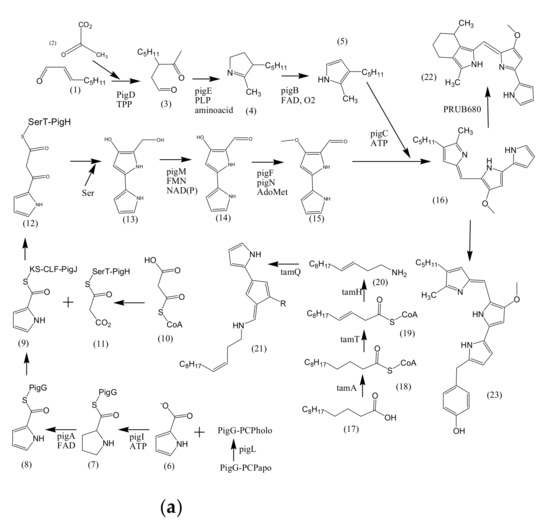
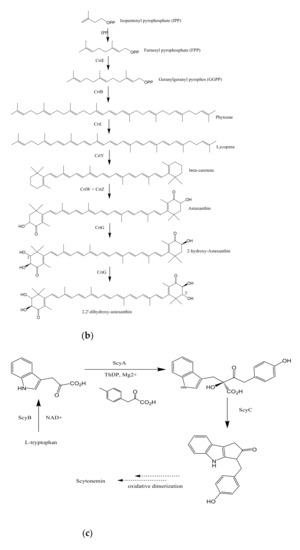
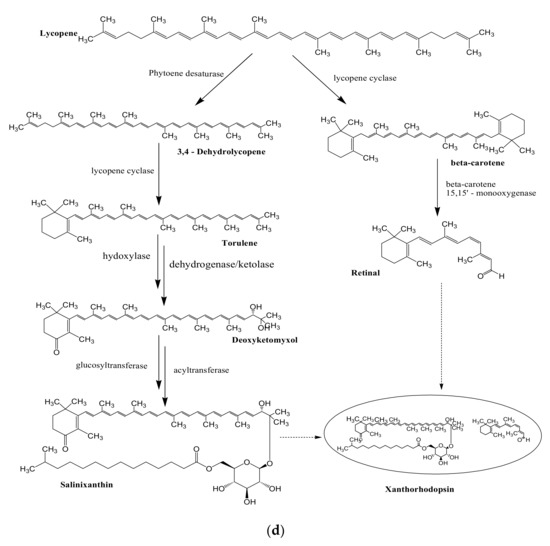
Figure 1. Proposed biosynthetic pathways of few bacterially produced bio-pigments. (a) Biosynthesis of Prodiginine analogs; MAP biosynthesis; MBC biosynthesis; Tambjamine biosynthesis; Cyloprodigiosin biosynthesis; 2-(p-hydroxybenzyl)prodigiosin (HBPG) biosynthesis. (b) Biosynthesis of carotenoids. (c) Biosynthesis of scytonemin. (d) Biosynthesis of salinixanthin and retinal pigments. (a) Biosynthesis of prodigioinine analogs. MAP Biosythesis (Green): (1) 2octenal, (2) Pyruvate, (3) 3-acetyloctanal, (4) H2MAP, (5) MAP. MBC Biosynthesis (Blue), (6) L-proline, (7) L-prolyl-S-PCP intermediate, (8) Pyrrolyl2-carboxyl-S-PCP, (9) Pyrrole-2-carboxyl thioester, (10) Malonyl-CoA, (11) Bound malonyl, (12) pyrrolyl-β-ketothioester on PigH, (13) 4-hydroxy-2,20-bipyrrole-5methanol (HBM), (14) 4-hydroxy-2,20-bipyrrole-5-carbaldehyde (HBC), (15) MBC, (16) Prodigiosin. Tambjamine Biosynthesis, (17) Dodecenoic acid, (18) Activated fatty acid, (19) CoA-ester, (20) Enamine, (21) Tambjamine, (22) Cycloprodigiosin (cPrG) &, (23) 2-(p-hydroxybenzyl)prodigiosin(HBPG) Biosynthesis. (b). Biosynthesis of carotenoids: CrtE: GGPP synthase, IPP: Isopentenyl pyrophosphate, GGPP: Geranylgeranyl pyrophos, CrtB: Phytoene synthase, CrtI: Phytoene desaturase, CrtY: lycopene β-cyclase, CrtW: β-carotene ketolase, CrtZ: β-carotene hydroxylase, CrtG: Astaxanthin 2,2′-β-ionone ring hydroxylase gene. (c). Biosynthesis of scytonemin: Scytonemin biosynthetic enzymes: ScyA, ScyB, ScyC (ScyA: a thiamin-dependent enzyme, ScyC: enzyme annotated as a hypothetical protein), ThDP: Thiamine diphosphate, NAD: Nicotinamide adenine dinucleotide, Mg2+: Magnesium ion.
3.1. Biosynthesis of Prodiginine Analogs
2-methyl-3-n-amyl-pyrrole (MAP) biosynthesis: This pathway involves three genes; pigB, pigD, and pigE. At first, PigD carries out the addition of pyruvate to 2-octenal in the presence of coenzyme thiamine pyrophosphate (TPP). As a result, 3-acetyloctanal formation occurs along with the release of CO2 molecule. PigE catalyzes the transfer of an amino group to the aldehyde, followed by cyclization, resulting in the formation of H2MAP. PigB carries out further oxidation to form MAP (Figure 1a)[21][22].
4-methoxy-2,2′-bipyrrole-5-carbaldehyde (MBC) biosynthesis: This pathway involves seven genes: pigA, pigF-J, pigL, and pigM. 4′-phosphopantetheinyl transferase (PigL) carries out the activation of peptidyl carrier protein (PCP) domain of PigG by introducing 4′-phosphopantetheinyl group. Formation of L-prolyl-S-PCP intermediate occurs by the transfer of L-prolyl group of L-proline to the thiol group of phosphopantetheine, carried out by PigI and ATP. PigA further catalyzes the oxidation of the intermediate to pyrrolyl-2-carboxyl-S-PCP. Pyrrole-2-carboxyl thioester is generated by the transfer of pyrrole-2-carboxyl group of PigG to the cysteine active site at PigJ. Phosphopantetheinylated ACP domains of PigH provide binding sites for malonyl group of malonyl-CoA. Decarboxylation of bound malonyl results in condensation with pyrrole-2-carboxyl thioester and leads to the formation of pyrrolyl-β-ketothioester on PigH. Generation of 4-hydroxy-2,2′-bipyrrole-5-methanol (HBM) occurs by decarboxylation between serine and pyrrolyl-β-ketothioester, catalyzed by PigH[21][23]. 4-hydroxy-2,2′-bipyrrole-5-carbaldehyde (HBC) is formed when PigM oxidizes the alcohol group of HBM. Methyltransferase (PigF) and oxidoreductase (PigN) further carries out the methylation of HBC hydroxyl group to form MBC[22]. After the formation of MAP and MBC, PigC utilizes ATP to perform terminal condensation of these pyrroles, synthesizing prodigiosin.
Cycloprodigiosin (cPrG) biosynthesis: The cyclization of undecylprodiginine in order to form metacycloprodigiosin and butyl-meta-cycloheptylprodiginine is carried out by mcpG and redG, respectively [24]. Studies also revealed that a homologus gene (PRUB680) encodes an alkylglycerol monooxygenase-like protein away from pig biosynthetic gene cluster[25]. The respective enzyme demonstrates regiospecificity through C-H activation, resulting in cyclization of prodigiosin to form cPrG[26].
Tambjamine (tam) biosynthesis: Tambjamines have MBC moiety but lack MAP moiety, rather have an enamine group. Enamine biosynthetic pathway involves three genes; tamT, tamH, and afaA[27]. Acyl CoA synthetase (TamA) activates dodecenoic acid[28]. Dehydrogenase (TamT) carries out the oxidation of activated fatty acid, incorporating a π-bond to the fatty acyl side chain at its C-3 carbon. Further, the reduction of CoA-ester, followed by transamination to dodec-3-en-1-amine is facilitated by reductase/aminotransferase (TamH). MBC and enamine then undergoes condensation in order to form tambjamine, catalyzed by TamQ[27].
3.2. Biosynthesis of Carotenoids
Carotenoids are yellow, orange, and red colored pigmented compounds that are further subdivided into carotenes and xanthophylls. So far, 700 carotenoids have been reported, and among them beta-carotene, lutein, canthaxanthin, astaxanthin, lycopene, and zeaxanthin are the highly valued carotenoids [29]. Universal precursors for C40 and C50 carotenoid biosynthesis are two 5 C subunits: isopentenyl diphosphate (IPP) plus its isomeric form dimethylallyl diphosphate (DMAPP). IPP/DMAPP isomerase (IDI) carries out the isomerization of IPP into DMAPP. Geranylgeranyl diphosphate (GGPP) synthase further catalyzes the addition of one DMAPP molecule with three IPP molecules to generate an immediate precursor, i.e., C20 geranylgeranyl diphosphate (GGPP)[30]. Phytoene synthase carries out the first committed step of carotenoid biosynthesis i.e., condensing two GGPP molecules to form phytoene (C40), which is further desaturated by phytoene desaturase by the incorporation of four double bonds in its structure. This desaturated structure is a red-colored compound unlike its colorless parent molecule, and is called lycopene. Lycopene further undergoes several modifications to produce different carotenoids. Beta-carotene is generated by the cyclization of lycopene, carried out by lycopene beta-cyclase. It is then converted into canthaxanthin and zeaxanthin by catalytic activity of two protein classes: beta-carotene ketolase and beta-carotene hydroxylase, respectively, next to the formation of astaxanthin[31]. Beta-carotene ketolase represented by CrtW and CrtO types adds the ketone group to carbon 4/40 of the b-ionone ring. However, beta-carotene hydroxylase, encompassed by CrtR, CrtZ, and P450 types carries out the hydroxylation of carbon 3/30 of the b-ionone ring[32]. 2,2′-β-ionone ring hydroxylase introduces hydroxyl group to the β-ionone ring of astaxanthin and results in the formation of 2,2′-dihydroxy-astaxanthin (Figure 1b)[33].
3.3. Biosynthesis of Scytonemin
Biosynthesis of scytonemin involves three scytonemin biosynthetic enzymes; ScyA, ScyB, and ScyC. ScyB carries out the conversion of L-tryptophan to 3-indole pyruvic acid. ScyA (thiamin-dependent enzyme) performs the coupling of 3-indole pyruvic acid with p-hydroxyphenylpyruvic acid and results in the formation of b-ketoacid, whose cyclization is further carried out by ScyC (enzyme annotated as hypothetical protein)[34]. The resulting tricyclic ketone resembles half of the skeleton of scytonemin (Figure 1c)[35].
3.4. Biosynthesis of Salinixanthin and Retinal
Retinal: Lycopene cyclase converts lycopene into β-carotene. Breakdown of β-carotene into two retinal molecules is further catalyzed by a gene annotated as β-carotene 15,15′-monooxygenase (orf4) (Figure 1d)[36][37].
Salinixanthin: Xanthorhodopsin (orf2) (a light-driven proton pump) has two chromophores; retinal and salinixanthin [38][39]. Phytoene desaturase (CrtI) converts lycopene to 3,4-dehydrolycopene, which is further converted to torulene by lycopene cyclase[40]. Subsequently, conversion of torulene to salinixanthin is catalyzed by hydroxylase, ketolase or dehydrogenase, glucosyltransferase, and acyltransferase, having reactions involved similar to that of biosynthetic reactions of myxol and canthaxanthin[41][42].
References
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R. Marine Pigmented Bacteria: A Prospective Source of Antibacterial Compounds. J. Nat. Sci. Biol. Med. 2019, 10, 104–113.
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial Pigments and Their Applications. Process Biochem. 2013, 48, 1065–1079.
- Saviola, B. Pigments and Pathogenesis. J. Mycobact. Dis. 2014, 4, 5.
- Shindo, K.; Misawa, N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs 2014, 12, 1690–1698.
- Azman, A.-S.; Mawang, C.-I.; Abubakar, S. Bacterial Pigments: The Bioactivities and as an Alternative for Therapeutic Applications. Nat. Prod. Commun. 2018, 13, 1747–1754.
- Dufossé, L. Pigments, Microbial. In Reference Module in Life Sciences; Elsevier: London, UK, 2016.
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. Curr. Opin. Food Sci. 2016, 7, 20–26.
- Kumar, A.; Vishwakarma, H.S.; Singh, J.; Dwivedi, S.; Kumar, M. Microbial Pigments: Production and Their Applications in Various Industries. Int. J. Pharm. Chem. Biol. Sci. 2015, 5, 203–212.
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.; Khan, A.L.; Shinwari, Z.K.; Al‑Harrasi, A. Therapeutic Applications of Bacterial Pigments: A Review of Current Status and Future Opportunities. 3Biotech. 2018, 8, 207.
- The Menace of Synthetic Non-Food Colours. Business Recorder. Available online: https://fp.brecorder.com/2008/02/20080216695580/ (accessed on 4 June 2020).
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96.
- Petruk, G.; Roxo, M.; Lise, F.D.; Mensitieri, F.; Notomista, F.; Wink, M.; Izzo, V.; Monti, D.M. The Marine Gram-Negative Bacterium Novosphingobium sp. PP1Y as a Potential Source of Novel Metabolites with Antioxidant Activity. Biotechnol. Lett. 2019, 41, 273–281.
- Stolz, P.; Obermayer, B. Manufacturing Microalgae for Skin Care. Cosmet. Toilet. 2015, 120, 99–106.
- Bruckner, A.W. Life-Saving Products from Coral Reefs. Issues Sci. Technol. 2002, 18, 35.
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive Pigments from Marine Bacteria: Applications and Physiological Roles. Evid. Based Complement. Altern. Med. 2011, 1–17.
- Pawar, R.; Mohandass, C.; Rajasabapathy, R.; Meena, R.M. Molecular Diversity of Marine Pigmented Bacteria in the Central Arabian Sea with Special Reference to Antioxidant Properties. Cah. Biol. Mar. 2018, 59, 409–420.
- Baharum, S.N.; Beng, E.K.M.; Mokhtar, M.A.A. Marine Microorganisms: Potential Application and Challenges. J. Biol. Sci. 2010, 10, 555–564.
- Podar, M.; Reysenbach, A.-L. New Opportunities Revealed by Biotechnological Explorations of Extremophiles. Curr. Opin. Biotechnol. 2006, 17, 250–255.
- Giani, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments. Mar. Drugs 2019, 17, 524.
- Oren, A. Halophilic Microbial Communities and Their Environments. Curr. Opin. Biotechnol. 2015, 33, 119–124.
- Harris, A.K.P.; Williamson, N.R.; Slater, H.; Cox, A.; Abbasi, S.; Foulds, I.; Simonsen, H.T.; Leeper, F.J.; Salmond, G.P.C. The Serratia Gene Cluster Encoding Biosynthesis of the Red Antibiotic, Prodigiosin, Shows Species- and Strain-Dependent Genome Context Variation. Microbiology 2004, 150, 3547–3560.
- Williamson, N.R.; Simonsen, H.T.; Ahmed, R.A.A.; Goldet, G.; Slater, H.; Woodley, L.; Leeper, F.J.; Salmond, G.P.C. Biosynthesis of the Red Antibiotic, Prodigiosin, in Serratia: Identification of a Novel 2-methyl-3-n-amyl-Pyrroie (MAP) Assembly Pathway, Definition of the Terminal Condensing Enzyme, and Implications for Undecylprodigiosin Biosynthesis in Streptomyces. Mol. Microbiol. 2005, 56, 971–989.
- Garneau-Tsodikova, S.; Dorrestein, P.C.; Kelleher, N.L.; Walsh, T.C. Protein Assembly Line Components in Prodigiosin Biosynthesis: Characterization of PigA, G, H, I, J. J. Am. Chem. Soc. 2006, 128, 12600–12601.
- Kimata, S.; Izawa, M.; Kawasaki, T.; Hayakawa, Y. Identification of a Prodigiosin Cyclization Gene in the Roseophilin Producer and Production of a New Cyclized Prodigiosin in a Heterologous Host. J. Antibiot. 2017, 70, 196–199.
- De Rond, T.; Stow, P.; Eigl, I.; Johnson, R.E.; Chan, L.J.G.; Goyal, G.; Baidoo, E.E.K.; Hillson, N.J.; Petzold, C.J.; Sarpong, R.; et al. Oxidative Cyclization of Prodigiosin by an Alkylglycerol Monooxygenase-Like Enzyme. Nat. Chem. Biol. 2017, 13, 1155–1159.
- Sakai-Kawada, F.E.; Ip, C.G.; Hagiwara, K.A.; Awaya, J.D. Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review. Front. Microbiol. 2019, 10, 1715.
- Burke, C.; Thomas, T.; Egan, S.; Kjelleberg, S. The Use of Functional Genomics for the Identification of a Gene Cluster Encoding for the Biosynthesis of an Antifungal Tambjamine in the Marine Bacterium Pseudoalteromonas tunicata: Brief Report. Environ. Microbiol. 2007, 9, 814–818.
- Marchetti, P.M.; Kelly, V.; Simpson, J.P.; Ward, M.; Campopiano, D.J. The Carbon chain-Selective Adenylation Enzyme TamA: The Missing Link between Fatty Acid and Pyrrole Natural Product Biosynthesis. Org. Biomol. Chem. 2018, 16, 2735–2740.
- Asker, D.; Awad, T.S.; Beppu, T.; Ueda, K. Rapid and Selective Screening Method for Isolation and Identification of Carotenoid-Producing Bacteria. Methods Mol. Biol. 2018, 1852, 143–170.
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017.
- Huang, Z.; Dong, L.; Lai, Q.; Liu, J. Spartinivicinus ruber gen. nov., sp. nov., a Novel Marine Gamma proteobacterium Producing Heptylprodigiosin and Cycloheptylprodigiosin as Major Red Pigments. Front. Microbiol. 2020, 11, 11.
- Scaife, M.A.; Burja, A.M.; Wright, P.C. Characterization of Cyanobacterial β-Carotene Ketolase and Hydroxylase Genes in Escherichia coli, and Their Application for Astaxanthin Biosynthesis. Biotechnol. Bioeng. 2009, 103, 944–955.
- Liu, H.; Zhang, C.; Zhang, X.; Tana, K.; Zhang, H.; Cheng, D.; Ye, T.; Li, S.; Ma, H.; Zheng, H. A Novel Carotenoids-Producing Marine Bacterium from Noble Scallop Chlamys nobilis and Antioxidant Activities of Its Carotenoid Compositions. Food Chem. 2020, 320, 126629.
- Soule, T.; Stout, V.; Swingley, W.D.; Meeks, J.C.; Garcia-Pichel, F. Molecular Genetics and Genomic Analysis of Scytonemin Biosynthesis in Nostoc punctiforme ATCC 29133. J. Bacteriol. 2007, 189, 4465–4472.
- Balskus, E.P.; Case, R.J.; Walsh, C.T. The Biosynthesis of Cyanobacterial Sunscreen Scytonemin in Intertidal Microbial Mat Communities. FEMS Microbiol. Ecol. 2011, 77, 322–332.
- Estrada, A.F.; Maier, D.; Scherzinger, D.; Avalos, J.; Al-Babili, S. Novel Apo Carotenoid Intermediates in Neurospora crassa Mutants Imply a New Biosynthetic Reaction Sequence Leading to Neurosporaxanthin Formation. Fungal Genet. Biol. 2008, 45, 1497–1505.
- Liao, L.; Su, S.; Zhao, B.; Fan, C.; Zhang, J.; Li, H.; Chen, B. Biosynthetic Potential of a Novel Antarctic Actinobacterium Marisediminicola antarctica ZS314T Revealed by Genomic Data Mining and Pigment Characterization. Mar. Drugs. 2019, 17, 388.
- Balashov, S.P.; Imasheva, E.S.; Boichenko, V.A.; Antón, J.; Wang, J.M.; Lanyi, J.K. Xanthorhodopsin: A Proton Pump with a Light-Harvesting Carotenoid Antenna. Science 2005, 309, 2061–2064.
- Lanyi, J.K.; Balashov, S.P. Xanthorhodopsin: A Bacteriorhodopsin-Like Proton Pump with a Carotenoid Antenna. Biochim. Biophys. Acta 2008, 1777, 684–688.
- Graham, J.E.; Bryant, D.A. The Biosynthetic Pathway for Myxol-2’fucoside (Myxoxanthophyll) in the Cyanobacterium Synechococcus sp. Strain PCC 7002. J. Bacteriol. 2009, 191, 3292–3300.
- Hannibal, L.; Lorquin, J.; Dortoli, N.A.; Garcia, N.; Chaintreuil, C.; Massonboivin, C.; Dreyfus, B.; Giraud, E. Isolation and Characterization of Canthaxanthin Biosynthesis Genes from the Photosynthetic Bacterium Bradyrhizobium sp. Strain ORS278. J. Bacteriol. 2000, 182, 3850–3853.
- Huang, Z.; Dong, L.; Lai, Q.; Liu, J. Spartinivicinus ruber gen. nov., sp. nov., a Novel Marine Gamma proteobacterium Producing Heptylprodigiosin and Cycloheptylprodigiosin as Major Red Pigments. Front. Microbiol. 2020, 11, 11.




