
Video Upload Options
Metastasis of prostate cancer often results in death of the patient. A cluster of fatty acid-binding protein (FABP) genes involved in transportation, accumulation and utilization of fatty acids are co-amplified and preferentially expressed in metastatic prostate cancer compared to localized disease. These genes, namely FABP12, FABP4, FABP9, FABP8 and FABP5, individually and collectively, promote properties associated with prostate cancer metastasis. Levels of these FABP genes may serve as an indicator of prostate cancer aggressiveness, and that inhibiting the action of FABP genes may provide a new approach to prevent and/or treat metastatic prostate cancer.
1. Introduction
Prostate cancer (PCa) is ranked as the second most frequent cancer and the fifth leading cause of cancer deaths in men worldwide. In 2018, there were ~1.3 million new cases of PCa and 359,000 associated deaths [1]. Localized low grade PCa tumors can usually be successfully treated; however, metastatic PCa is resistant to treatment, resulting in relapse and death [2][3][4]. The most common sites of PCa metastasis are the bones and lymph nodes, although metastasis also occurs in lung and liver [5][6]. The exact mechanisms of PCa metastasis are currently unknown, although a number of key players in metastasis have been investigated [7][8]. The first step in metastasis is local invasion whereby the invasive cells reduce their cell–cell and cell–matrix adhesive characteristics and acquire the ability to migrate and break down the extracellular matrix (ECM). Breaching the endothelial barriers allows the cancer cells to enter the vascular or lymphatic circulation. Cells can then extravasate and transmigrate through the endothelial layer to reach the interstitium, where, if the environment is favorable, they proliferate and produce a metastatic tumor [9]. It is critical that biological factors and pathways that drive PCa metastasis be identified and studied, to allow precise clinical intervention.
Three features affect the clinical management of PCa. First, PCa is highly heterogeneous, making it difficult to predict response to treatment. Second, as there is an overall lack of molecular signatures to stratify tumor subtypes, treatment is almost exclusively based on histological architecture (Gleason score) [10][11], prostate-specific antigen (PSA) levels [12] and local disease state (TNM, WHO 2009) [13][14]. Third, unlike other cancers which are characterized by increased glucose consumption and elevated energy production from glycolysis, PCa shows reduced glycolysis and mainly relies on fatty acid oxidation for its energy supply [15][16][17].
FAs are hydrophobic molecules that require fatty acid-binding proteins (FABPs) for their intracellular trafficking [18]. FABPs therefore regulate the cellular accumulation, distribution, utilization and fate of FAs [19]. There are ten FABPs, with each FABP displaying distinct tissue distribution and ligand preference [18][20]. FABPs are receiving increasing attention in oncology because of their emerging roles in the prevention and treatment of cancer [21][22]. In particular, FABPs are implicated in metastatic progression in various cancers [23][24][25][26], including prostate cancer [27][28][29]. They are also recognized as important factors in metabolic diseases [30][31][32], particularly as related to PPAR (peroxisome proliferator-activated receptor) function [33][34][35][36][37][38].
Chromosome 8q21 is the most commonly amplified region in PCa metastases [39]. We previously identified a novel fatty acid-binding protein gene, FABP12, in this region (8q21.13), located within a cluster of four other members of the FABP family (FABP4, FABP5, FABP8/PMP2 and FABP9) [20]. Roles for these FABPs in PCa progression have been reported, especially through the modulation of lipid metabolic pathways and metastatic transformation. This review aims to decipher how FABPs, through unique, synergetic or combinatorial actions, can affect PCa invasion and metastasis.
2. Fatty Acid-Binding Protein (FABP) and Prostate Cancer
Metastasis is a multistep process that involves many molecular and physiological alterations. Studies of the FABP cluster on chromosome 8 support a common role for all five FABPs in promoting PCa aggressiveness and metastasis. However, the biological actions of these FABPs appear to be synergic rather than redundant (Figure 1). FABP4 mainly functions as a secreted protein which mediates cancer cell–microenvironment interactions [24][40][41]. FABP5 mainly affects de novo fatty acid synthesis, lipolysis and angiogenesis pathways [42][43][44][45], whereas FABP12 induces EMT and oxidative phosphorylation [29]. PPARs are important mediators of FABP functions presumably through fatty acid transfer from FABPs to PPARs, although the details remain elusive. As well, further studies are needed to determine the precise role of FABPs in modulating lipid metabolism reprogramming and lipid-derived bioenergetics during metastasis. Future in-depth investigations on the cross-talk between fatty acid-FABP-PPAR and androgen-AR signaling pathways may shed light on the mechanism underlying castration resistance and metastasis in PCa. It will be important to further explore the roles of these FABPs in chemotherapy drug resistance in relation to FABP-induced lipid metabolism alterations. As well, it will be interesting to address the role of FABPs in the homing of prostate cancer cells to metastatic sites. Such a role for FABP4 has previously been described for the homing of ovarian cancer cells to omental adipose tissue [40].
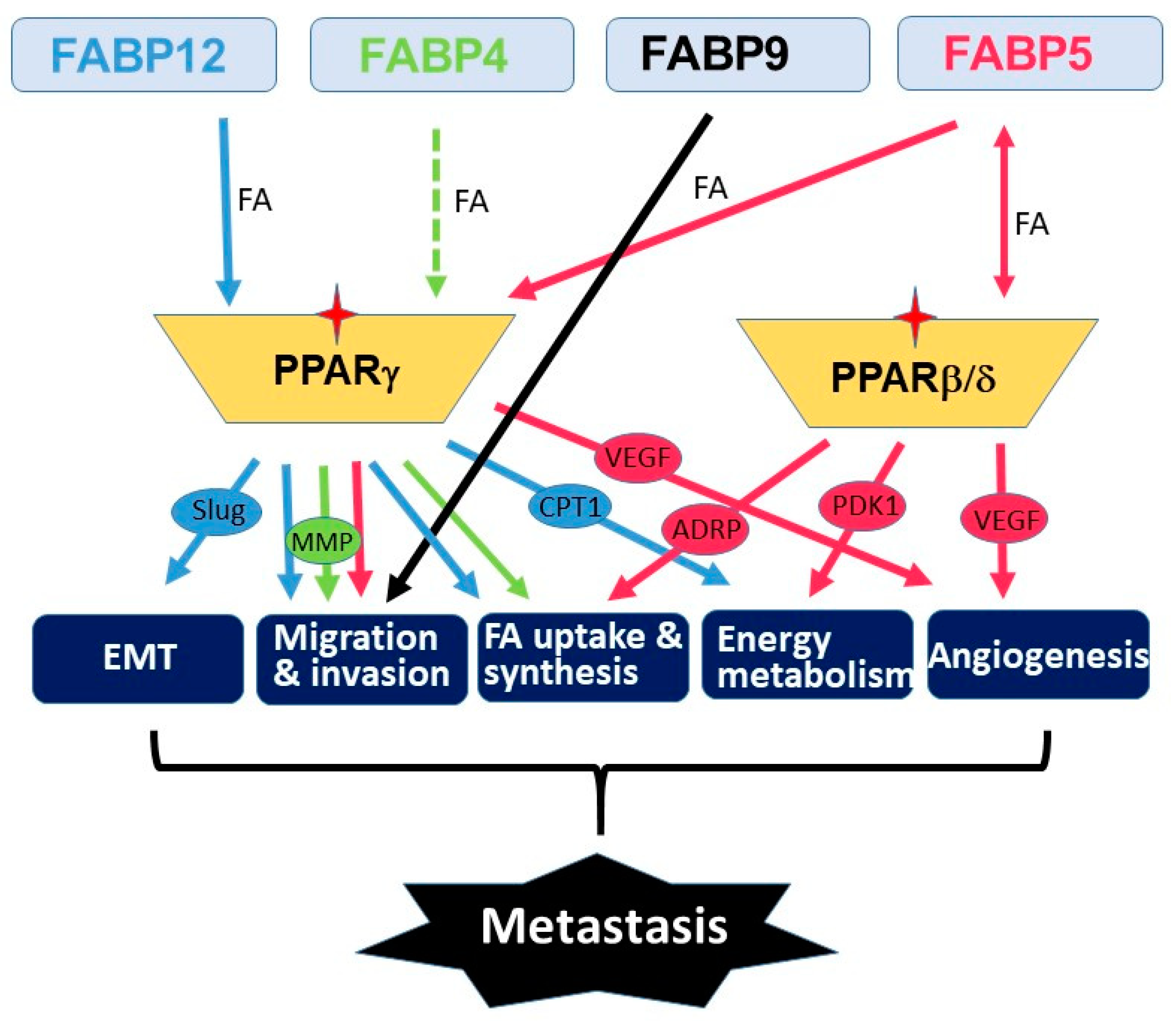
As FABPs induce lipid metabolism reprogramming in cancer cells, a property associated with cancer stemness, FABPs may also affect response and resistance to therapy. In fact, FABP5 inhibitors have been reported to synergize with chemotherapy drugs (docetaxel and cabazitaxel) to inhibit PCa growth in vitro and in vivo [46]. Carefully designed PCa patient cohort analyses will be needed to determine whether FABPs, singly or in combination, can serve as predictive biomarkers for anti-tumor therapies. Importantly, FABPs, including FABP4, FABP5, FABP9 and FABP12, have all been shown to have significant prognostic value in PCa patient populations [29][36][47][48]. Whether any of these FABPs, singly or in combination, could be used as independent prognostic biomarkers remains to be seen. The unique influence of lipid metabolism on PCa progression, the preferential amplification and enrichment of FABPs in metastatic PCa and the recent in vitro and in vivo evidence showing their emerging roles in promoting PCa metastasis and progression all point to FABPs as being valid therapeutic targets for advanced PCa carrying this amplified FABP cluster. Initial studies have shown that either a small molecule inhibitor of FABP5/7 (SBFI-26) or a mutated recombinant FABP5 construct (dmrFABP5) exhibit potent inhibitory effects on tumorigenesis and metastasis in xenograft animal models of PCa [49][50]. These promising results, combined with the documented roles of FABPs in cancer progression, support further development of FABP-targeted inhibitors and therapies for the treatment of PCa.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Massague, J.; Obenauf, A.C. Metastatic colonization by circulating tumour cells. Nature 2016, 529, 298–306.
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669.
- Asmane, I.; Ceraline, J.; Duclos, B.; Rob, L.; Litique, V.; Barthelemy, P.; Bergerat, J.P.; Dufour, P.; Kurtz, J.E. New strategies for medical management of castration-resistant prostate cancer. Oncology (Williston Park) 2011, 80, 1–11.
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.D.; et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014, 74, 210–216.
- Shou, J.; Zhang, Q.; Wang, S.; Zhang, D. The prognosis of different distant metastases pattern in prostate cancer: A population based retrospective study. Prostate 2018, 78, 491–497.
- Clarke, N.W.; Hart, C.A.; Brown, M.D. Molecular mechanisms of metastasis in prostate cancer. Asian J. Androl. 2009, 11, 57–67.
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Ruger, R. Molecular Mechanisms of Bone Metastasis. Cancer Genom. Proteom. 2016, 13, 1–12.
- Chiang, A.C.; Massague, J. Molecular basis of metastasis. N. Engl. J. Med. 2008, 359, 2814–2823.
- Gleason, D.F. Classification of prostatic carcinomas. Cancer Chemother. Rep. 1966, 50, 125–128.
- Gleason, D.F.; Mellinger, G.T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 1974, 111, 58–64.
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.A.; Scardino, P.T.; Flanigan, R.C.; deKernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6630 men. J. Urol. 1994, 151, 1283–1290.
- Draisma, G.; Etzioni, R.; Tsodikov, A.; Mariotto, A.; Wever, E.; Gulati, R.; Feuer, E.; de Koning, H. Lead time and overdiagnosis in prostate-specific antigen screening: Importance of methods and context. J. Natl. Cancer Inst. 2009, 101, 374–383.
- Ramirez, M.L.; Nelson, E.C.; Evans, C.P. Beyond prostate-specific antigen: Alternate serum markers. Prostate Cancer Prostatic Dis. 2008, 11, 216–229.
- Andersen, K.F.; Divilov, V.; Sevak, K.; Koziorowski, J.; Lewis, J.S.; Pillarsetty, N. Influence of free fatty acids on glucose uptake in prostate cancer cells. Nucl. Med. Biol. 2014, 41, 254–258.
- Liu, Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostat. Dis. 2006, 9, 230–234.
- Liu, Y.; Zuckier, L.S.; Ghesani, N.V. Dominant uptake of fatty acid over glucose by prostate cells: A potential new diagnostic and therapeutic approach. Anticancer Res. 2010, 30, 369–374.
- Haunerland, N.H.; Spener, F. Fatty acid-binding proteins-insights from genetic manipulations. Prog. Lipid Res. 2004, 43, 328–349.
- Chmurzynska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48.
- Liu, R.Z.; Li, X.; Godbout, R. A novel fatty acid-binding protein (FABP) gene resulting from tandem gene duplication in mammals: Transcription in rat retina and testis. Genomics 2008, 92, 436–445.
- Elsherbiny, M.E.; Emara, M.; Godbout, R. Interaction of brain fatty acid-binding protein with the polyunsaturated fatty acid environment as a potential determinant of poor prognosis in malignant glioma. Prog. Lipid Res. 2013, 52, 562–570.
- Storch, J.; Corsico, B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 2008, 28, 73–95.
- Amiri, M.; Yousefnia, S.; Seyed Forootan, F.; Peymani, M.; Ghaedi, K.; Nasr Esfahani, M.H. Diverse roles of fatty acid binding proteins (FABPs) in development and pathogenesis of cancers. Gene 2018, 676, 171–183.
- Gharpure, K.M.; Pradeep, S.; Sans, M.; Rupaimoole, R.; Ivan, C.; Wu, S.Y.; Bayraktar, E.; Nagaraja, A.S.; Mangala, L.S.; Zhang, X.; et al. FABP4 as a key determinant of metastatic potential of ovarian cancer. Nat. Commun. 2018, 9, 2923.
- Tian, W.; Zhang, W.; Zhang, Y.; Zhu, T.; Hua, Y.; Li, H.; Zhang, Q.; Xia, M. FABP4 promotes invasion and metastasis of colon cancer by regulating fatty acid transport. Cancer Cell Int. 2020, 20, 512.
- Uehara, H.; Takahashi, T.; Oha, M.; Ogawa, H.; Izumi, K. Exogenous fatty acid binding protein 4 promotes human prostate cancer cell progression. Int. J. Cancer 2014, 135, 2558–2568.
- Adamson, J.; Morgan, E.A.; Beesley, C.; Mei, Y.; Foster, C.S.; Fujii, H.; Rudland, P.S.; Smith, P.H.; Ke, Y. High-level expression of cutaneous fatty acid-binding protein in prostatic carcinomas and its effect on tumorigenicity. Oncogene 2003, 22, 2739–2749.
- Forootan, S.S.; Bao, Z.Z.; Forootan, F.S.; Kamalian, L.; Zhang, Y.; Bee, A.; Foster, C.S.; Ke, Y. Atelocollagen-delivered siRNA targeting the FABP5 gene as an experimental therapy for prostate cancer in mouse xenografts. Int. J. Oncol. 2010, 36, 69–76.
- Liu, R.Z.; Choi, W.S.; Jain, S.; Dinakaran, D.; Xu, X.; Han, W.H.; Yang, X.H.; Glubrecht, D.D.; Moore, R.B.; Lemieux, H.; et al. The FABP12/PPARgamma pathway promotes metastatic transformation by inducing epithelial-to-mesenchymal transition and lipid-derived energy production in prostate cancer cells. Mol. Oncol. 2020, 14, 3100–3120.
- Thumser, A.E.; Moore, J.B.; Plant, N.J. Fatty acid binding proteins: Tissue-specific functions in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 124–129.
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503.
- Hotamisligil, G.S.; Bernlohr, D.A. Metabolic functions of FABPs--mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015, 11, 592–605.
- Schug, T.T.; Berry, D.C.; Shaw, N.S.; Travis, S.N.; Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 2007, 129, 723–733.
- Schug, T.T.; Berry, D.C.; Toshkov, I.A.; Cheng, L.; Nikitin, A.Y.; Noy, N. Overcoming retinoic acid-resistance of mammary carcinomas by diverting retinoic acid from PPARbeta/delta to RAR. Proc. Natl. Acad. Sci. USA 2008, 105, 7546–7551.
- Bao, Z.; Malki, M.I.; Forootan, S.S.; Adamson, J.; Forootan, F.S.; Chen, D.; Foster, C.S.; Rudland, P.S.; Ke, Y. A novel cutaneous Fatty Acid-binding protein-related signaling pathway leading to malignant progression in prostate cancer cells. Genes Cancer 2013, 4, 297–314.
- Forootan, F.S.; Forootan, S.S.; Malki, M.I.; Chen, D.; Li, G.; Lin, K.; Rudland, P.S.; Foster, C.S.; Ke, Y. The expression of C-FABP and PPARgamma and their prognostic significance in prostate cancer. Int. J. Oncol. 2014, 44, 265–275.
- Morgan, E.; Kannan-Thulasiraman, P.; Noy, N. Involvement of Fatty Acid Binding Protein 5 and PPARbeta/delta in Prostate Cancer Cell Growth. PPAR Res. 2010, 2010.
- Schroeder, F.; Petrescu, A.D.; Huang, H.; Atshaves, B.P.; McIntosh, A.L.; Martin, G.G.; Hostetler, H.A.; Vespa, A.; Landrock, D.; Landrock, K.K.; et al. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 2008, 43, 1–17.
- Cher, M.L.; Bova, G.S.; Moore, D.H.; Small, E.J.; Carroll, P.R.; Pin, S.S.; Epstein, J.I.; Isaacs, W.B.; Jensen, R.H. Genetic alterations in untreated metastases and androgen-independent prostate cancer detected by comparative genomic hybridization and allelotyping. Cancer Res. 1996, 56, 3091–3102.
- Mukherjee, A.; Chiang, C.Y.; Daifotis, H.A.; Nieman, K.M.; Fahrmann, J.F.; Lastra, R.R.; Romero, I.L.; Fiehn, O.; Lengyel, E. Adipocyte-Induced FABP4 Expression in Ovarian Cancer Cells Promotes Metastasis and Mediates Carboplatin Resistance. Cancer Res. 2020, 80, 1748–1761.
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503.
- Carbonetti, G.; Wilpshaar, T.; Kroonen, J.; Studholme, K.; Converso, C.; d’Oelsnitz, S.; Kaczocha, M. FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Sci. Rep. 2019, 9, 18944.
- Senga, S.; Kobayashi, N.; Kawaguchi, K.; Ando, A.; Fujii, H. Fatty acid-binding protein 5 (FABP5) promotes lipolysis of lipid droplets, de novo fatty acid (FA) synthesis and activation of nuclear factor-kappa B (NF-kappaB) signaling in cancer cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1057–1067.
- Senga, S.; Kawaguchi, K.; Kobayashi, N.; Ando, A.; Fujii, H. A novel fatty acid-binding protein 5-estrogen-related receptor alpha signaling pathway promotes cell growth and energy metabolism in prostate cancer cells. Oncotarget 2018, 9, 31753–31770.
- Forootan, F.S.; Forootan, S.S.; Gou, X.; Yang, J.; Liu, B.; Chen, D.; Al Fayi, M.S.; Al-Jameel, W.; Rudland, P.S.; Hussain, S.A.; et al. Fatty acid activated PPARgamma promotes tumorigenicity of prostate cancer cells by up regulating VEGF via PPAR responsive elements of the promoter. Oncotarget 2016, 7, 9322–9339.
- Carbonetti, G.; Converso, C.; Clement, T.; Wang, C.; Trotman, L.C.; Ojima, I.; Kaczocha, M. Docetaxel/cabazitaxel and fatty acid binding protein 5 inhibitors produce synergistic inhibition of prostate cancer growth. Prostate 2020, 80, 88–98.
- Al Fayi, M.S.; Gou, X.; Forootan, S.S.; Al-Jameel, W.; Bao, Z.; Rudland, P.R.; Cornford, P.A.; Hussain, S.A.; Ke, Y. The increased expression of fatty acid-binding protein 9 in prostate cancer and its prognostic significance. Oncotarget 2016, 7, 82783–82797.
- Harraz, A.M.; Atia, N.; Ismail, A.; Shady, A.; Farg, H.; Gabr, H.; Fouda, M.; Abol-Enein, H.; Abdel-Aziz, A.F. Evaluation of serum fatty acid binding protein-4 (FABP-4) as a novel biomarker to predict biopsy outcomes in prostate biopsy naive patients. Int. Urol. Nephrol. 2020, 52, 1483–1490.
- Al-Jameel, W.; Gou, X.; Forootan, S.S.; Al Fayi, M.S.; Rudland, P.S.; Forootan, F.S.; Zhang, J.; Cornford, P.A.; Hussain, S.A.; Ke, Y. Inhibitor SBFI26 suppresses the malignant progression of castration-resistant PC3-M cells by competitively binding to oncogenic FABP5. Oncotarget 2017, 8, 31041–31056.
- Al-Jameel, W.; Gou, X.; Jin, X.; Zhang, J.; Wei, Q.; Ai, J.; Li, H.; Al-Bayati, A.; Platt-Higgins, A.; Pettitt, A.; et al. Inactivated FABP5 suppresses malignant progression of prostate cancer cells by inhibiting the activation of nuclear fatty acid receptor PPARgamma. Genes Cancer 2019, 10, 80–96.




