
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Simon Drescher | + 3184 word(s) | 3184 | 2020-12-28 06:46:34 | | | |
| 2 | Camila Xu | Meta information modification | 3184 | 2021-02-04 07:55:47 | | |
Video Upload Options
Phospholipids are unique and versatile molecules. They are of natural occurrence and the main components in cellular membranes. Arranged as a lipid bilayer, phospholipids play a significant role in the structure and functionality of biological membranes. They are amphiphilic and consist of a hydrophilic headgroup and a lipophilic/hydrophobic tail.
1. Introduction
In phospholipids, the sn-1 and sn-2 position of the glycerol backbone are esterified with fatty acids of varying length and degree of saturation. The remaining sn-3 position is esterified with phosphoric acid, which, in turn, is esterified with an alcohol [1]. Depending on the structure of this alcohol, different types of phospholipids comprise, for example, phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), or phosphatidylserine (PS) [2]. The specific and non-random distribution of substituents over the positions sn-1, sn-2, and sn-3 of the glycerol introduces chirality. Depending on the structure of the polar headgroup and pH of the surrounding medium, PE and PC are zwitterionic and have a neutral charge at pH 7, whereas PG, PI, and PS are negatively charged at this pH value. As an example, the chemical structure of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and the different types with alternative headgroups are shown in Figure 1.
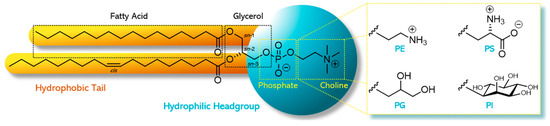
Figure 1. Chemical structure of a phospholipid as exampled by 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and the different alternative headgroups varying in the type of alcohol: phosphatidylethanolamine (PE) with ethanolamine, phosphatidylglycerol (PG) with glycerol, phosphatidylserine (PS) with serine, and phosphatidylinositol (PI) with inositol.
The physiological functions of phospholipids are manifold. For instance, besides their functional role in cell membranes, phospholipids, mainly PC, have digestion/metabolic functions in bile (as monoacyl-phospholipids, i.e., lyso-phospholipids) to solubilize cholesterol and fatty components in food and lipophilic drug substances [3]. Moreover, phospholipids act as lipoprotein components for transport of fat between gut and liver, as source for acetylcholine (in the case of PC), and as source of (essential) fatty acids and energy [4]. In addition, in lung surfactant, a specific phospholipid, namely 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), occurs [4], lowering the surface tension at the air/water interface within the alveoli of the lung. PS is a component of the lipid–calcium–phosphate complex for deposition during bone formation [5], and it is active in regulation of blood coagulation [6] and apoptosis [7].
Due to their physiological roles, phospholipids possess a very low toxicity profile and can be used for any route of administration [8]. The structural diversity of phospholipids and the resulting variability of chemical, biophysical, and technological properties leads to wide use of phospholipids in various pharmaceutical formulations [9][10][11][12][13]. They can be technologically used as an emulsifier, wetting agent, solubilizer, and agent in the formation of liposomes and mixed micelles, to name but a few. From a pharmaceutical perspective, they are used as key excipients for parenteral administration for solubilizing formulations such as liposomes, mixed micelles, and oil-in-water (o/w) emulsions; in liposomes for drug targeting and slow release; and for topical administration to the lung and the skin for slow release and enhanced skin interaction, respectively. After oral administration, phospholipids are used to suppress gastrointestinal (GI) side effects of, for example, non-steroidal anti-inflammatory drugs (NSAIDs) and explored as solubilizers to enhance the oral absorption of poorly water-soluble compounds. Nevertheless, phospholipids are still not widely applied as excipients for the development and manufacture of pharmaceuticals, although they represent, as natural compounds, for instance, very effective alternatives to synthetic (non-natural) emulsifiers such as polysorbates, polyoxyethylene castor oil derivatives, and sucrose esters. To change this, renowned international scientists conducting research on phospholipids founded the Phospholipid Research Center Heidelberg (PRC) in 2006 with the support of Lipoid GmbH (Ludwigshafen, Germany) and PHOSPHOLIPID GmbH (Cologne, Germany) [14]. The main vision of the PRC is to translate the physiological and physicochemical properties and benefits of phospholipids into their optimal pharmaceutical use as excipients.
Since the foundation of the PRC, 90 research projects related to phospholipids have been supported as of 2020. In this comprehensive review, we classify the projects according to the main application route of phospholipid-based formulations in parenteral (51), oral (20), and topical projects (10). In addition, nine projects related to basic phospholipid research are included. The distribution of the projects according to their main application route/basic research as well as the location of the institutes of the principal investigator is provided in Figure 2. Besides the countries mentioned, the term “other” stands for one project each from Croatia, Finland, France, Iran, Israel, Nigeria, Poland, Portugal, Sweden, and the United Kingdom, i.e., 46% of all projects are in Germany, 48% in Europe (without Germany), and 6% in the rest of the world. This review gives an overview of current (drug delivery-related) phospholipid research and highlights possible future research tendencies [15].
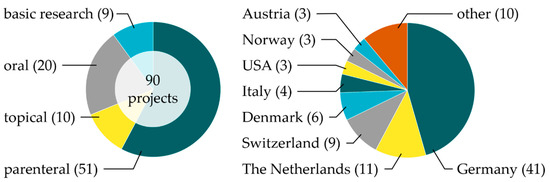
Figure 2. Distribution of the 90 research projects supported so far by the Phospholipids Research Center Heidelberg (PRC) regarding the phospholipids’ administration type/basic research (left) and location of projects across the world (right), with the number of projects in parentheses.
However, before performing research with phospholipids, their quality (sources, purification, and resulting purity) and the intended use should be taken into consideration. Depending on the type of application of the phospholipids, there are different requirements to prove their pharmaceutical quality.
2. Phospholipids—General Aspects
First, one must be aware of the different meanings and uses of the term “phosphatidylcholine” and “lecithin”. According to the United States Pharmacopeia (USP), lecithin is described as “a complex mixture of acetone-insoluble phosphatides (i.e., phospholipids), which consist chiefly of PC, PE, PI, and phosphatic acid (PA), present in conjunction with various amounts of other substances such as triglycerides, fatty acids, and carbohydrates, as separated from the crude vegetable oil source. It contains not less than 50% of acetone-insoluble matter.” Unfortunately, especially in the American literature, lecithin is also used as a synonym of PC, which is the pure compound. Descriptions of the phospholipid component in products as “lecithin” leaves open which lipid is used. We therefore recommend using for natural phospholipids the term “lecithin” only when the product contains less than 80% by weight total phospholipids and the term “phospholipid” when the product contains 80–100% by weight phospholipids (e.g., PC, PE); the specific phospholipid should be mentioned when the product contains more than 90% by weight of that specific phospholipid.
2.1. Natural Phospholipids
In principal, one can distinguish between natural and synthetic phospholipids. Natural phospholipids can be obtained from vegetable sources such as soybeans, sunflower, rape (canola) seed, wheat germ, and flax seed; and animal material such as hen egg yolk, milk, or krill. The phospholipid and fatty acid profiles of the various lecithins depend, of course, on the raw material sources. The composition of de-oiled vegetable lecithin from variable sources and egg lecithin, respectively, are provided as example in Table 1. Besides PC, PE, and PI, other components can also occur in natural lecithins, namely sphingomyelin (SM), PA, and minor amounts of phospholipids containing only one acyl chain in the sn-1 position, namely lyso-phosphatidylcholine (LPC) and lyso-phosphatidylethanolamine (LPE). In all cases, PC is the main phospholipid component. To convert these materials into high-quality excipients, meeting pharmacopeial and regulatory requirements for parenteral and other specific formulations, extraction and chromatography procedures are applied (see Section 2.3).
Table 1. Phospholipid composition of vegetable de-oiled lecithins (derived from product specifications [16]) and egg phospholipids of different PC contents (after extraction and chromatography [17]), respectively. Data taken from [9].
| Phospholipid | Lecithin and Phospholipids (% w/w) | |||||
|---|---|---|---|---|---|---|
| Soybean | Sunflower Seed | Rapeseed | Egg (64–79% PC) |
Egg (80–85% PC) |
Egg (≥98% PC) |
|
| PC | 20–22 | 20–26 | 23–31 | 72 | 81 | 99 |
| PE | 16–22 | 4–10 | 9–15 | 17 | 8.5 | 0.0 |
| PI | 13–16 | 15–19 | 15–18 | - | - | - |
| PA | 5–10 | 2–5 | 5–10 | - | - | - |
| SM | - | - | - | 2.0 | 2.0 | 0.4 |
| LPC | <3 | <3 | <3 | 2.0 | 2.0 | 0.0 |
| LPE | - | - | - | 1.0 | 0.3 | 0.0 |
The fatty acid composition of typical batches of vegetable de-oiled lecithins and egg phospholipid of different PC content, respectively, is given in Table 2 [9]. The corresponding fatty acids are listed in their short notation, with the first digit indicating the number of carbon atoms and the second digit the numbers of cis-double bonds: C14:0, myristic acid (tetradecanoic acid); C16:0, palmitic acid (hexadecenoic acid); C18:0, stearic acid (octadecanoic acid); C18:1, oleic acid (octadecenoic acid); C18:2, linoleic acid (octadecadienoic acid); C18:3, α-linolenic acid (ALA, octadecatrienoic acid); C20:0, arachidic acid (eicosanoic acid); C20:4, arachidonic acid (ARA, eicosatetraenoic acid); C22:0 behenic acid (docosanoic acid); C22:4, docosatetraenoic acid; C22:5, docosapentaenoic acid (DPA); and C22:6, cervonic acid (docosahexaenoic acid, DHA). The presence of the polyunsaturated fatty acids (PUFAs) C20:4 and C22:6 is typical for hen egg yolk phospholipids.
Table 2. Fatty acid composition of vegetable de-oiled lecithins (derived from product specifications [16]) and egg phospholipids of different PC contents (after extraction and chromatography [17]), respectively. Data taken from [9].
| Fatty Acid | Lecithin and Phospholipids (% w/w) | |||||
|---|---|---|---|---|---|---|
| Soybean | Sunflower Seed | Rapeseed | Egg (64–79% PC) |
Egg (80–85% PC) |
Egg (≥98% PC) |
|
| C14:0 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 |
| C16:0 | 21 | 16 | 10 | 31 | 31 | 34 |
| C18:0 | 4.7 | 5.3 | 0.8 | 15 | 14 | 12 |
| C18:1 | 9.9 | 21 | 49 | 24 | 28 | 28 |
| C18:2 | 57 | 54 | 31 | 16 | 15 | 16 |
| C18:3 | 5.0 | 0.2 | 4.4 | - | - | - |
| C20:0 | 0.1 | 0.3 | 0.1 | - | - | - |
| C20:4 | - | - | - | 5.6 | 4.8 | 3.6 |
| C22:0 | 0.4 | 1.5 | 0.1 | - | - | - |
| C22:4 | - | - | - | 0.3 | 0.3 | 0.2 |
| C22:5 | - | - | - | 0.2 | 0.2 | 0.1 |
| C22:6 | - | - | - | 2.2 | 1.8 | 1.8 |
2.2. Synthetic Phospholipids
Due to the presence of unsaturated fatty acids in natural phospholipids, the liquid crystalline to gel phase transition temperature (Tm) is below 0 °C. These phospholipids are at ambient temperature in the liquid crystalline state (Lα phase) and form upon hydration flexible structures/mesophases [2], suitable for specific pharmaceutical technological applications. In some formulations, however, when, for example, more physically stable liposomes with increased stability in blood plasma or phospholipids with more powder-like properties are required, phospholipids with higher Tm are preferred [18]. Phospholipids with saturated fatty acids possess these properties and can be obtained by hydrogenation of natural phospholipids with unsaturated fatty acids [19], resulting in, for example, HSPC (hydrogenated soybean PC). The progress of the hydrogenation can be followed by monitoring the iodine value, which is a measure of the degree of unsaturation of fatty substances.
Nowadays, alternative biochemical synthesis routes via enzyme-catalyzed reactions serve as a viable alternative to organic-chemical synthesis steps. In that respect, the use of enzymes for phospholipid modification has moved quickly in recent years, not only in academic research but also in industry [20]. The fast-growing use of enzymes for polar lipid modification arises from factors such as milder reaction conditions, less environmental pollution, better specificity for improved quality, and higher efficiency of reactions. Specific enzymes are suitable for different modification purposes to modify/synthesize phospholipids. For acyl modifications, natural enzymes such as phospholipase A1 and A2 (PLA1 and PLA2), which selectively cleave the fatty acid in sn-1 and sn-2, respectively, leading to lyso-phospholipids, can be used. Phospholipase B (PLB) is an enzyme with a combination of both PLA1 and PLA2 activities; that is, it can cleave acyl chains from both the sn-1 and sn-2 positions. Phospholipase C (PLC) can hydrolyze the bond between the glycerol oxygen and the phosphate group, leading to the formation of diacylglycerol and phosphocholine. Unfortunately, this enzyme family is only active for the hydrolysis reaction and not for the reformation process. Therefore, PLC and PLB are not used industrially. Finally, phospholipase D (PLD) is the only potential enzyme for polar group modification. This enzyme cleaves the bond between the phosphate and the choline. Examples of enzyme-modified (“semi-synthetic”) natural phospholipids prepared from natural PC are lyso-PC, soybean PE, soybean PG, egg PG, and their saturated analogs.
To study more mechanistic biochemical or biophysical aspects of phospholipids at the molecular level in natural environments or in model membranes, various synthetic approaches to chemically well-defined phospholipids have been developed. These “full-synthetic” phospholipids are then homogeneous with respect to the polar headgroup and fatty acid composition [2][20][21]. This aspect has been reviewed by Hoogevest and Wendel [9], and we refer to this review and the literature cited therein. In addition to the need for synthetic analogs of natural phospholipids, further synthetic phospholipids were designed to, for example, optimize the drug targeting properties of liposomes. Examples are the PEGylated phospholipids, which are phospholipids bearing a polyethylene glycol (PEG) chain of variable length attached to their headgroup [22], and the cationic phospholipid 1,2-diacyl-P-O-ethylphosphatidylcholine [23], to name but a few.
For the preparation of “full-synthetic” phospholipids, several starting compounds are conceivable. The classical approach to synthesize diacyl-PCs is to start from d-mannitol. However, this synthetic method is lengthy, requires toxic chemicals and solvents in large excess, and reaction intermediates may be unstable and subject to partial racemization [24][25]. A simpler method to synthesize symmetrical glycerophospholipids, which are phospholipids bearing two identical fatty acids, is a method starting from (R) or (S) glycidyl tosylates [26]. The enantiomerically pure glycidyl derivative is, however, expensive, making large scale syntheses of glycerophospholipids impractical [2]. Another route to produce phospholipids with defined fatty acids is to start from glycerophosphocholine (GPC), which is also termed sn-glycero-3-phosphocholine, l-α-glyceryl-phosphorylcholine, α-GPC, or choline alfoscerate. GPC can be synthesized in an enantioselective manner using a biotransformation procedure based on the phosphorylation of glycerol by adenosine triphosphate (ATP) catalyzed glycerol kinase [27]or, preferably, produced by means of alkaline hydrolysis from natural PC maintaining the natural stereoisomeric structure. Starting from the latter, symmetrical phospholipids can be synthesized in a one step process using activated acyl derivatives, such as acylimidazolides or anhydrides [21][28], and various coupling reagents such as dicyclohexylcarbodiimide (DCC) in combination with 4-(dimethylamino)pyridine (DMAP) [29] or 2-methyl-6-nitrobenzoic anhydride (MNBA) [30]. For the further synthesis from GPC to asymmetrical, i.e., mixed fatty acid chain phospholipids, organic chemical methods as well as enzymatic procedures can be applied. Which synthesis route will finally be selected is, from an industrial perspective, dependent on the (commercial) availability of key intermediates or starting materials.
2.3. Industrial Production of Phospholipids
To exemplify the production process of natural phospholipid excipients starting from plant oil, the production process of soybean lecithin is given. First, crude soybean lecithin is isolated by degumming of the crude soybean oil as obtained by extraction from soybeans [31] (Figure 3).
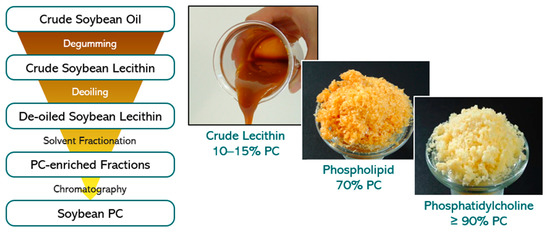
Figure 3. (Left) Flow chart of isolation process steps of soybean PC derived from crude soybean oil. (Right) Visual appearance of soybean lecithin/PC fractions with variable PC content.
The crude soybean lecithin obtained serves as starting material for production at a large scale of soybean lecithin fractions with higher PC content. These fractions are obtained in high yields by extraction methods using the non-toxic solvents acetone and ethanol followed by chromatographic purification procedures and appropriate solvent removal methods. All solvents can be recycled and reused. By selecting appropriate sequential extraction and chromatography methods, several lecithin fractions differing in PC content from 20 to 80% up to pure PC (≥98%) and in ratios of phospholipids to non-polar lipids can be reproducibly achieved. This procedure applies to soybean oil and all vegetable oils used to produce lecithin, and high-purity phospholipids/PC. Furthermore, egg phospholipids are isolated from hen egg yolk with similar extraction and chromatography methods as for soybean lecithin.
2.4. Regulatory and Safety Aspects
Natural phospholipids are in general well known to regulatory authorities. Moreover, their track record as excipients with very high tolerability and biocompatibility is outstanding. The World Health Organization (WHO) places no limit on the oral intake of lecithin. Furthermore, no limit for the value of acceptable daily intake (ADI) for lecithin as a food additive is given [32]. The pediatric oral use of phospholipids (soybean) is in general allowed, of course with precautions for soybean allergy (see below). Alternatively, sunflower phospholipids, with no allergy warnings, can be used. After parenteral administration, egg and soybean lecithin—unsaturated and saturated variations—are well tolerated.
The European Commission declares that lecithin is a food additive (E322) “generally permitted for use in foodstuffs” [33]. Furthermore, no ADI value has been fixed for lecithin in Europe; the material may be used quantum satis [34]. The US Food and Drug Administration (FDA) assigned the generally recognized as safe (GRAS) affirmation for lecithin [35] and enzyme-modified lecithin [36].
Although phospholipids on their own do not have an allergy potential, phospholipids derived from soybean and hen egg yolk must be labelled as potentially allergenic because of the soy and egg origin [37][38]. It is known that these allergies are caused by residues of soy and egg proteins. In purified soybean phospholipids used for pharmaceutical application, the protein residues were found to be below the lower limit of detection (LLOD) of a soybean specific enzyme-linked immunosorbent assay (ELISA), which was 1 ppm [39]. The egg protein content of purified egg lecithin for parenteral administration was tested by Lipoid GmbH [40]. The most sensitive immunological detection method of proteins showed less than the LLOD of 0.5 ppm of egg protein in purified egg phospholipids. Since, however, from an immunological perspective one molecule of soy protein or egg protein represents a theoretical risk, still the origin of such products has, according to regulatory authorities, to be labelled to alert allergic individuals. In a recent study on a propofol emulsion with soybean oil and egg phospholipids as emulsifier and its parenteral use in children with allergies to egg, peanut, soybean, or other legumes, it was concluded that genuine serious allergic reaction to product was rare and is not reliably predicted by a history of food allergy [41].
2.5. Use of Phospholipids in Pharmaceutical Formulations
Phospholipids—natural as well as synthetic—are broadly used in pharmaceutical technology as wetting agents, emulsifiers, and builders or components of different lipid mesophases such as liposomes, micelles, mixed micelles, inverted micelles, cubosomes, etc. [9]. These functional properties are applied in many types of pharmaceutical formulations such as suspensions, different types of emulsions, solid dispersions, lipid nanoparticles, drug/phospholipid complexes, etc. [42][43]. With respect to their physiological role, phospholipids possess a very low toxicity profile and can be used for any route of administration, namely parenteral, oral, and topical. Regarding the versatility, phospholipids are superior excipients compared to synthetic non-biodegradable polymers, which are not suitable to be used for every administration route and which are, by definition, un-physiological. In case natural phospholipids derived from hen egg yolk or soybean are selected, attention should be paid to the minimal quality in phospholipid content, which depends on the administration route. For oral and dermal administration, natural phospholipids with at least 45% PC can be used, whereas for parenteral use, at least 70% PC is common [44]. For specific high-tech parenteral products (for injection), more expensive, synthetic, and chemically well-defined phospholipids of high purity may be the best choice, whereas for topical and oral administration (and of course other parental applications), cost effective natural phospholipids are better.
The categories of pharmaceutical formulations, which are of interest to study the occurrence of natural and synthetic phospholipids, respectively, are products used for parenteral administration [9]. These are mainly liposomes, o/w emulsions, mixed micelles for intravenous (i.v.) use and slow release and vaccine vehicles, and drug suspensions for intramuscular (i.m.) and subcutaneous (s.c.) administration. Liposomal products for i.v. administration are formulated with synthetic as well as natural phospholipids. Liposomes can also be used as vehicles for slow release after local parenteral administration, i.e., at the surgical site, epidural or intrathecal. In this case, mainly synthetic phospholipids are applied. Egg phospholipids are used in o/w emulsions for parenteral nutrition (for example Intralipid) [45] and as carriers for oil-soluble drug substances [46]. In mixed micellar formulations, including phospholipids and different choate salts, exclusively soybean phospholipids are used. Here, the phospholipids act as solubilizer for poorly water-soluble substances or as active principles (soybean PC), through the presence of PUFAs for treatment of liver disorders [47]. Considering finally products comprising phospholipids for pulmonary administration, natural and synthetic phospholipids are applied. Most of these products are used for respiratory distress syndrome in infants [48] or bacterial lung infections [49]. More details regarding the use of phospholipids in pharmaceutical formulations, including a variety of examples and drug products, can be found in the literature [8][9].
References
- IUPAC-IUB. Nomenclature of phosphorus-containing compounds of biochemical importance (Recommendations1976). Proc. Natl. Acad. Sci. USA 1977, 74, 2222–2230.
- Cevc, G. Phospholipids Handbook; Taylor & Francis Inc.: London, 1993; Volume 1, p. 1004.
- Vertzoni, M.; Markopoulos, C.; Symillides, M.; Goumas, C.; Imanidis, G.; Reppas, C. Luminal lipid phases after administration of a triglyceride solution of danazol in the fed state and their contribution to the flux of danazol across Caco-2 cell monolayers. Mol. Pharm. 2012, 9, 1189–1198, doi:10.1021/mp200479f.
- Hanin, I.; Pepeu, G. Phospholipids. Biochemical, Pharmaceutical, and Analytical Considerations; Plenum Press: New York, NY, USA, 1990; Volume 1.
- Merolli, A.; Santin, M. Role of phosphatidyl-serine in bone repair and its technological exploitation. Molecules 2009, 14, 5367–5381, doi:10.3390/molecules14125367.
- Lentz, B.R. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 2003, 42, 423–438, doi:10.1016/s0163-7827(03)00025-0.
- Devitt, A.; Pierce, S.; Oldreive, C.; Shingler, W.H.; Gregory, C.D. CD14-dependent clearance of apoptotic cells by human macrophages: The role of phosphatidylserine. Cell Death Differ. 2003, 10, 371–382, doi:10.1038/sj.cdd.4401168.
- van Hoogevest, P.; Tiemessen, H.; Metselaar, J.M.; Drescher, S.; Fahr, A. The Use of Phospholipids to make Pharmaceutical Form Line Extensions Eur. J. Lipid Sci. Technol. 2021, revision submitted.
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107, doi:10.1002/ejlt.201400219.
- van Hoogevest, P. Review—An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12, doi:10.1016/j.ejps.2017.07.008.
- van Hoogevest, P.; Luciani, P. Recent Advances in the Use of Phospholipid Excipients in Local or Injectable Depot Formulations. Pharm. Ind. 2018, 8, 1104.
- van Hoogevest, P.; Fahr, A. Phospholipids in Cosmetic Carriers (Phospholipids, Liposomes, Emulsions, Lamellar structures, Solubilizers, Biocompatibility). In Nanocosmetics—From Ideas to Products; Cornier, J., Keck, C., Van de Voorde, M., Eds.; Springer: Cham, Switzerland, 2019; Volume 6, pp. 97–140.
- van Hoogevest, P. Non‐Aqueous Phospholipid Concentrates for Increasing the Bioavailability of Poorly Soluble Compounds. Eur. J. Lipid Sci. Technol. 2020, 122, 1900411, doi:10.1002/ejlt.201900411.
- PRC. Phospholipid Research Center (The PRC—Vision and Mission). Available online: https://www.phospholipid-institute.com/about/the-prc/ (accessed on 13 August 2020).
- PRC. Phospholipid Research Center (Funded Projects). Available online: https://www.phospholipid-institute.com/funding/funded-projects/ (accessed on 18 August 2020).
- Analytical data; Lipoid GmbH: Ludwigshafen, Germany. Available on request: https://www.lipoid.com/en/headquarter-and-manufacturing (accessed on 16 December 2020).
- Phospholipid Research Center, Analytics Newsletter, Number 1. Egg Lecithins for Pharmaceutical Application; Heidelberg, Germany, 2008. Available on request: https://www.phospholipid-research-center.com/contact/contact-person/ (accessed on 16 December 2020).
- Senior, J.; Gregoriadis, G. Stability of small unilamellar liposomes in serum and clearance from the circulation: The effect of the phospholipid and cholesterol components. Life Sci. 1982, 30, 2123–2136, doi:10.1016/0024-3205(82)90455-6.
- Pryde, E.H. Chemical Reactions of Phosphatides. In Lecithins; Szuhaj, B.F., List, G.R., Eds.; American Oil Chemists’ Society: Champaign, IL, USA, 1985; Volume 1, pp. 213–246.
- Xu, X.; Vikbjerg, A.F.; Guo, Z.; Zhang, L.; Acharya, A.K. Chapter 3—Enzymatic modification of phospholipids and related polar lipids. In Phospholipid Technology and Applications; Gunstone, F.D., Ed.; Woodhead Publishing: Bridgewater, CT, USA, 2012; pp. 41–82, doi:10.1533/9780857097880.41.
- Paltauf, F.; Hermetter, A. Strategies for the synthesis of glycerophospholipids. Prog. Lipid Res. 1994, 33, 239–328, doi:10.1016/0163-7827(94)90028-0.
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine 2005, 1, 193–212, doi:10.1016/j.nano.2005.06.004.
- MacDonald, R.C.; Rakhmanova, V.A.; Choi, K.L.; Rosenzweig, H.S.; Lahiri, M.K. O-ethylphosphatidylcholine: A metabolizable cationic phospholipid which is a serum-compatible DNA transfection agent. J. Pharm. Sci. 1999, 88, 896–904, doi:10.1021/js990006q.
- Eibl, H. Phospholipide als funktionelle Bausteine biologischer Membranen. Angew. Chem. 1984, 96, 247–262.
- Ohno, M.; Fujita, K.; Nakai, H.; Kobayashi, S.; Inoue, K.; Nojima, S. An enantioselective synthesis of platelet-activating factors, their enantiomers, and their analogues from D- and L-tartaric acids. Chem. Pharm. Bull. 1985, 33, 572–582, doi:10.1248/cpb.33.572.
- Virtanen, J.A.; Brotherus, J.R.; Renkonen, O.; Kates, M. Synthesis of monoacid 2,3-diacyl-sn-glycerols via 1,6-ditrityl-d-mannitol. Chem. Phys. Lipids 1980, 27, 185–190, doi:10.1016/0009-3084(80)90034-1.
- Crans, D.C.; Whitesides, G.M. Glycerol Kinase: Synthesis of Dihydroxyacetone Phosphate, sn-Glycerol-3-Phosphate, and Chiral Analogues. J. Am. Chem. Soc. 1985, 107, 7019–7027.
- Warner, T.G.; Benson, A.A. An improved method for the preparation of unsaturated phosphatidylcholines: Acylation of sn-glycero-3-phosphorylcholine in the presence of sodium methylsulfinylmethide. J. Lipid Res. 1977, 18, 548–552.
- Neises, B.; Steglich, W. Einfaches Verfahren zur Veresterung von Carbonsäuren. Angew. Chem. 1978, 90, 556–557, doi:10.1002/ange.19780900718.
- Yasuda, T.; Kinoshita, M.; Murata, M.; Matsumori, N. Detailed Comparison of Deuterium Quadrupole Profiles between Sphingomyelin and Phosphatidylcholine Bilayers. Biophys. J. 2014, 106, 631–638, doi:10.1016/j.bpj.2013.12.034.
- Wendel, A. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk, R.E., Othmer, D.F., Eds.; John Wiley & Sons: New York, NY, USA; Chichester, UK; Weinheim, Germany; Brisbane, Australia; Singapore; Toronto, ON, Canada, 1995; pp. 191–210.
- WHO. WHO Food Additives Series No. 5. Available online: http://www.inchem.org/documents/jecfa/jecmono/v05je42.htm (accessed on 13 August 2020).
- EMA. COMMISSION REGULATION (EU) No 231/2012 of 9 March 2012 Laying down Specifications for Food Additives Listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council European Commission. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32012R0231 (accessed on 13 August 2020).
- Publications Office of the EU (Report from the Commission on Dietary Food Additive Intake in the European Union). Available online: https://op.europa.eu/en/publication-detail/-/publication/26105dba-6d8f-4515-a641-0e43fe3f5498/language-en (accessed on 20 August 2020).
- FDA. 21CFR184.1400. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1400 (accessed on 20 August 2020).
- FDA. 21CFR184.1063. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1063 (accessed on 20 August 2020).
- Singh, T.P.; Singh, P.; Kumar, P. Egg allergy: An emerging foodborne problem J. Foodborne Zoonotic Dis. 2014, 2, 19–26.
- Warner, J.; Meyer, R. SoyaAllergy: The Facts (PDF File). Available online: https://www.allergywise.org.uk/wp-content/uploads/2017/12/Soya.pdf (accessed on 10 August 2020).
- Analytical Data; Phospholipid GmbH: Cologne, Germany, Available on request: https://www.lipoid.com/en/headquarter-and-manufacturing (accessed on 16 December 2020).
- Study of the Allergenicity and Protein Content in Egg Lecithin; ifp Institut für Produktqualität: Berlin, Germany, Available on request: https://www.produktqualitaet.com/de/ (accessed on 16 December 2020).
- Sommerfield, D.L.; Lucas, M.; Schilling, A.; Drake-Brockman, T.F.E.; Sommerfield, A.; Arnold, A.; von Ungern-Sternberg, B.S. Propofol use in children with allergies to egg, peanut, soybean or other legumes. Anaesthesia 2019, 74, 1252–1259, doi:10.1111/anae.14693.
- Fricker, G.; Kromp, T.; Wendel, A.; Blume, A.; Zirkel, J.; Rebmann, H.; Setzer, C.; Quinkert, R.-O.; Martin, F.; Müller-Goymann, C.C. Phospholipids and Lipid-Based Formulations in Oral Drug Delivery. Pharm. Res. 2010, 27, 1469–1486, doi:10.1007/s11095-010-0130-x.
- van Hoogevest, P.; Liu, X.; Fahr, A.; Leigh, M.L.S. Role of phospholipids in the oral and parenteral delivery of poorly water soluble drugs. J. Drug Delivery Sci. Technol. 2011, 21, 5–16, doi:10.1016/S1773-2247(11)50001-2.
- Lipoid. (Products). Available online: https://www.lipoid.com/en/node/10 (accessed on 10 August 2020).
- Driscoll, D.F. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm. Res. 2006, 23, 1959–1969, doi:10.1007/s11095-006-9092-4.
- Shah, P.; Bhalodia, D.; Shelat, P. Nanoemulsion: A pharmaceutical review. Syst. Rev. Pharm. 2013, 1, 24–32.
- Hammad, M.A.; Müller, B.W. Increasing drug solubility by means of bile salt-phosphatidylcholine-based mixed micelles. Eur. J. Pharm. Biopharm. 1998, 46, 361–367, doi:10.1016/s0939-6411(98)00037-x.
- Gregory, T.J.; Steinberg, K.P.; Spragg, R.; Gadek, J.E.; Hyers, T.M.; Longmore, W.J.; Moxley, M.A.; Cai, G.Z.; Hite, R.D.; Smith, R.M.; et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1997, 155, 1309–1315, doi:10.1164/ajrccm.155.4.9105072.
- Vandevanter, D.R.; Geller, D.E. Tobramycin administered by the TOBI® Podhaler® for persons with cystic fibrosis: A review. Med. Devices (Auckl) 2011, 4, 179–188, doi:10.2147/mder.S16360.




