
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Massimiliano Lo Faro | + 2861 word(s) | 2861 | 2020-08-06 11:02:35 | | | |
| 2 | Catherine Yang | -3 word(s) | 2858 | 2020-12-31 05:06:38 | | |
Video Upload Options
Two-phase material consisting of a Ruddlesden–Popper-type structure and a solid oxide solution (e.g., α-Fe100-y-zCoyNizOx oxide) was syntheised and demonstrated as an anode for fuel flexible SOFC and as a functinal layer to enhance the fuel flexibility of commercial SOFCs..
1. Introduction
Achieving fuel flexibility in fuel cells has been a focus of high-temperature fuel cells since their discovery [1][2]. As Solid Oxide Fuel Cells (SOFCs) operate at temperatures close or higher than 800 °C, their thermodynamics allow the direct use of dry organic fuels or organic fuels in combination with water [3]. However, practical operation requires a pre-treatment of organic fuels to convert hydrocarbon and remove sulfur traces [4]. In parallel, the discovery of new materials and novel cell designs has allowed reducing the operating temperature to 600–800 °C [5][6]. Several studies have been concerned with the development of novel materials [7][8] and in particular novel anodes for advanced SOFCs operating in fuel-flexible mode [9][10][11][12][13].
Fuel flexibility is treated in this review article in relation to a specific category of electrocatalysts.
Although novel SOFCs designs and several new materials have been investigated, Ni in combination with Yttria-stabilized Zirconia (YSZ) still represents today the selected approach for the anode [14]. This is because the Ni-YSZ cermet provides a proper trade-off in terms of electrochemical, thermal, and mechanical properties [15][16][17][18][19][20][21][22]. In particular, the mechanical and thermal properties of Ni-YSZ are rather flexible and adequate to the production chain of anode-supported SOFC cells [23][24][25]. However, the degradation of Ni-YSZ anodes during SOFC operation is frequently reported. This occurs due to the coarsening of Ni particles [16] and to the carbon deposition [20]. Moreover, another drawback is related to the risk of Ni poisoning due to sulfur contaminants and pore blocking associated with the deposition of carbon tar [26][27][28]. Accordingly, large SOFC systems include a fuel pre-reformer and a desulfurizer [15][29][30][31]. An alternative strategy for SOFC systems’ simplification, which has been quite recently adopted, consists of the use of a functional layer coated on the external side of Ni-YSZ anodes [32]. Due to the favorable electronic properties, Ni alloys in combination with doped ceria electrolyte have been widely investigated to replace bare Ni [33][34][35]. The specific advantage relies on a breaking effect of the crystallographic arrangement of Ni atoms, which is responsible for the cracking mechanism of hydrocarbons [36][37]. This effect has been achieved by the inclusion of a different transition metal, not adsorbing carbon, into the reticular scaffold of Ni. The metals selected for this inclusion were somehow inert toward the cracking reaction (e.g., Cu) [33] or capable of modifying the surface electronic density and the lattice distances of the regular Ni–Ni crystallographic network (e.g., Co) [34], or a combination of all these effects (e.g., Fe) [35]. Then, a further optimization has been achieved by adopting different synthesis methods with the aim of improving the homogeneity of the solid solution and consequently reducing the probability of Ni–Ni bonds’ occurrence on the surface [37].
Moreover, specific catalyst functionalization has been made by increasing its oxygen storage capability [38], e.g., by adding oxygen storage additives such as ceria. This is particularly desired to promote the oxidation of a fuel and to extend the “triple phase boundary”[39][40]. These are the sites where the electrochemical reactions take place. Thus, the alloy is generally mixed with doped ceria, which is a well-known oxygen storage material as a consequence of its peculiar redox properties [10]. Although this approach has been revealed as promising for the direct use of dry organic molecules in SOFCs [41][42], to date, there has been limited evidence about the resistance of these Ni alloy–ceria composite catalysts toward sulfur poisoning.
An approach combining both sulfur resistance and organic fuel oxidation capabilities consists of the use of exsolved perovskites at the anode [43]. ABX3 is the general chemical formula of these materials. A and B are the sites occupied by cations with different sizes (e.g., La and Sr for the A site and Fe and Co for the B site) and different valence states. X is the site for the oxygen anions [44]. These materials may be tuned in both A and B sites by using different cations and doping in order to achieve the desired amount of holes and vacancies that are required for high ionic and electronic conductivity [45][46][47]. The idea of using perovskites at the anode is more recent. It raised from the large amount of evidence about their activity toward the cleavage of C–H, C–C, and C=C bonds [46][48][49] with a relatively good stability in the presence of sulfur-based products [50][51]. These systems are also characterized by mixed electronic and ionic conductivity [52], even if the electronic conductivity is not comparable to that of a metal also at the high operating temperatures of an SOFC. On the other hand, the perovskites may present the drawbacks of a limited structural stability under the reducing environment of an SOFC anode [53][54] To address this issue, the modification of perovskites ex ante with the aim of functionalizing their surface and tailoring the activity for the anodic reactions, while stabilizing the structure and improving the electronic conductivity, has been carried out [43][55]. A further step forward in tailoring these perovskite materials for the use as an anode in SOFCs is relying on the addition of ceria to increase the oxygen storage capacity and the electronic/ionic percolation.
The authors of this mini-review have intensively worked on modified and exsolved perovskite for application as an anode in SOFCs for several years demonstrating an effective oxidation of various organic fuels directly fed to the cell [56][57][58][59][60][61][62][63]. The proper modification of commercial type ferrite-based perovskites, which are commonly used as an SOFC cathode, with small amounts of Ni has been demonstrated. The process has been enhanced by tailoring the thermal treatments to consolidate the modified perovskite structure. In this mini-review, we have summarized the properties of these novel anode materials, their performance in SOFCs, and the specific reaction mechanisms investigated through a combination of electrochemical experiments and the chromatographic analyses of effluents.
2. Surface Exsolution and Physicochemical Studies
The exsolution of fine particles from the perovskite surface is an approach recently used for the functionalization of perovskite materials for anodic applications [64][65][66]. Two main approaches have been developed: (1) synthesis of a non-stoichiometric perovskite host [67] and (2) surface impregnation of a raw perovskite with a foreign transition element [68][69]. The final modification is thermal driven by a process under reducing condition at high temperature in order to achieve a proper thermodynamic stability for the fine exsolved nanoparticles embedded in the perovskite substrate. The latter remains deficient of cations in A and B sites. Both approaches have been addressed to promote a similar distribution of fine exsolved particles on the surface. It was reported that the first method should be preferred in case of high risks for their coarsening [70].
Many exsolved perovskites have been synthesized using these approaches for various applications and with the aim of functionalizing the surface of these materials. One of the most used applications has regarded their use as a novel anode for SOFCs. The subject of this mini-review is specifically addressing the modification of ferrite-based perovskites commonly used as cathodes in commercial-type SOFCs operating in the temperature range between 700 and 800 °C. This type of perovskite generally has the formula La0.6Sr0.4Fe0.8Co0.2O3 (LSFC). The raw LSFC that we have largely used in our previous works had a surface of 5.20 m2 g−1 and was purchased from Praxair. By using a wet impregnation method, 20 cm3 of an aqueous solution containing 3 wt % of Ni as Ni nitrate (Sigma Aldrich) has been deposited drop-by-drop on the perovskite, and this was maintained under stirring condition at 80 °C. After drying at 150 °C for 8 h, the powder has been treated at 500 °C in static air for 2 h and then reduced with 5% of H2 in N2 at 800 °C for 2 h and then re-calcined at 500 °C in static air for 2 h to stabilize the nanoparticles on the surface. The amount of Ni added to the perovskite was evaluated on the basis of a compromise between the need of avoiding large occurrence of metallic nickel on the surface with the consequent risk of carbon deposition during the cracking of organic fuels and to favor just a partial exsolution of Fe and Co from the perovskite. The subsequent step consisted in the grinding for 12 h of this powder together with gadolinia-doped ceria (Gd0.1Ce0.9O2-GDC, Praxair) with a surface area of 38.92 m2 g−1. The doping of perovskite with Ni and its subsequent thermal treatments caused a modification of the initial perovskite phase originated from the depletion of Co and Fe with the consequent formation of a new phase named as n = 1 Ruddlesden–Popper (An+1BnO3n+1) structure [71][72]. Figure 1 shows the XRD spectra of the raw and modified perovskites. Fe and Co are located in the blue octahedrals, and the green spheres are related to the sites of La and Sr, whereas the red spheres are the sites for oxygen ions. As a consequence of perovskite distortion, the ratio La/Sr (0.6/0.4) remained the same during the perovskite modification, whereas the Fe/Co ratio was altered as a consequence of the Co and Fe depletion from the bulk and their migration to the perovskite surface (the Fe/Co ratio was originally 4 in the raw perovskite). The overall amount of Ruddlesden–Popper n = 1 phase was approximately 60 wt % as determined through a least-square fitting profile quantitative analysis procedure. As evidenced by previous High Resolution—Transmission Electronic Microscopy (HR-TEM) and Energy-Dispersive X-ray (EDX) analyses [62], the exsolution process involves the occurrence of a secondary phase related to fine exsolved particles encapsulated on the surface. These are composed of a tri-metallic alloy (e.g., Ni-Fe-Co, about 25 nm) in the core and a shell of mixed oxides (e.g., α-Fe100-y-zCoyNizOx, about 2 nm). Smith et al. [73][74] have reported that this mixed oxide has shown reversible electrochemical behavior starting from low temperatures. Accordingly, it has been also suggested as a substitution of noble metals for the water electrolysis in zero-gap cells [73][74] .
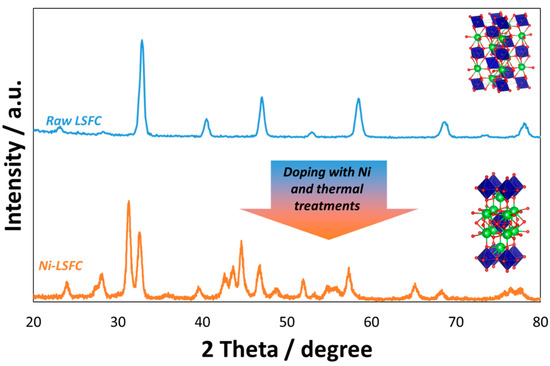
Figure 1. Structural analysis of raw (top) and modified (bottom) perovskites. The figure also reports the related unit cells. As shown, the addition of Ni and subsequent thermal treatments (calcination at 500 °C and reduction at 800 °C) caused the intercalation of one rocksalt-type phase into the perovskite (n = 1 Ruddlesden–Popper phase).
The redox properties of these modified perovskites and their exsoluted fine particles have been the object of specific studies [57]. Figure 2 shows the temperature-programmed reduction (TPR) profile of Ni-LSFC/CGO (i.e., Ce0.8Gd0.2O2−d), the HR-TEM image of a fine particle encapsulated in the surface of the catalyst and its EDX analysis. As discussed, Co and Fe were alloyed with Ni and migrated to the surface of perovskite as proved by the EDX analysis carried out during high-angle annular dark-field (HAADF) imaging, whereas an oxide shell was confirmed by the combination of EDX and HR-TEM analysis. This peculiar structure imparts to the material outstanding catalytic properties [73][74]. The TPR profile showed three signals in the temperature range of 300–650 °C related to the chemisorption of H2 on Co (α1 [75]), Fe (α2 [76]), and Ni (α3 [77]). This indicates that the oxide surface of these nanoparticles turns into a trimetallic system under operation in reducing conditions at high temperature. The TPR profile showed also a broad peak around 650 °C, which is due to the reduction of Ce (Ce4+ → Ce3+, α4 [78][79]). Based on the XPS studies [62], the most probable oxidation states of metals for the fine embedded particles was 3+ for both the cobalt and the iron and 2+ for the nickel. By integrating the three TPR peaks observed in the temperature range between 300 and 650 °C, the following composition α-Fe23Co15Ni12Ox is derived, suggesting an excess of iron in line with the evidence of the EDX profile.
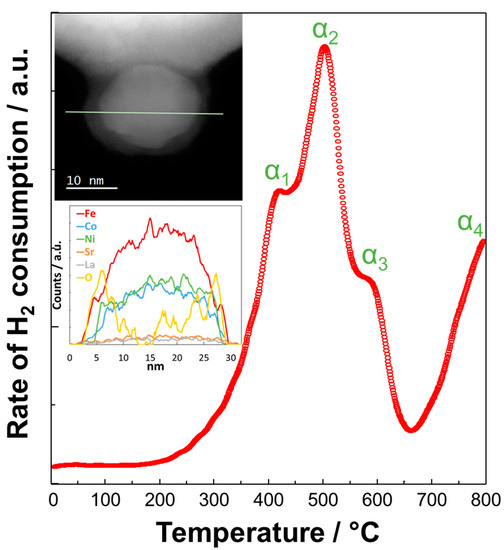
Generally, the surface area of these exsolved perovskite materials is 5.51 m2 g−1 as evaluated by Brunauer–Emmett–Teller (BET) analysis with mesoporous morphology [58]. Thus, the formation of fine embedded particles does not promote a significant increase of the overall surface roughness for the modified perovskite catalyst; instead, the initial surface area is quite similar (e.g., 5.2 m2 g−1).
3. Key Insights on Challenges and Perspectives
The ferrite-based perovskite used as a substrate for a pre-catalytic layer is currently a benchmark for the oxygen electrode in Solid Oxide Fuel Cells (SOFCs) and Solid Oxide Electrolysis Cells (SOECs) operating at intermediate temperature. Specific strengths are the lower cost and the lower constraints compared to the manganite-based perovskites [80][81][82]. Therefore, its use as a fuel electrode or as a promoter for an in situ fuel processor is highly desired [83]. However, this material is not sufficiently stable in a reducing environment [54][84]. It may become chemically stable upon reduction at high temperature and utilization at intermediate temperature. Moreover, as for the most perovskite, the rates for the electroxidation of H2 and organic fuels are low. A possible solution to this issue is the decoration with metallic-based particles as discussed above. Based on the experiments reported in the literature, the modified Ni-LSFC is a promising electrocatalyst for organic fuels that may be easily dehydrogenated at the very early stage of their oxidation. On the other hands, Ni-LSFC has shown strong limitations for the conversion of CH4. Several papers have discussed the use of Ni alloys as a possible electrocatalyst for the oxidation of light organic fuels such as methane [85][86][87]. This allows addressing the low reactivity of exsolved perovskites toward methane, making the pre-catalytic layer more suitable for a fuel-flexible SOFC. We have recently started the investigation of a novel exsolved LSFC by adding 30 wt % of a NiCu alloy (1:1 at.). This electrocatalyst was prepared with the same procedure as that mentioned above. A conventional anode-supported cell (Elcogen) modified with such composite catalyst has shown very promising performance and durability. The cell was fed with dry biogas (30 vol.% CO2 and 70 vol.% CH4), and it showed > 0.2 W cm−2 with biogas and a low decay during a lifetime test carried out at 650 °C for about 100 h.
Figure 3a shows the polarization curves achieved with this novel composite electrocatalyst. The cell has shown an OCV close to 1 V in the presence of biogas. The achieved cell performance approached 200 mW cm−2 at 0.66 V with biogas. The curves seem to be affected only by ohmic constraints due to the intermediate temperature operation (i.e., 650 °C). Figure 3b shows a durability time test at a current density of 150 mA cm−2 with a high fuel flow rate (i.e., 5 cc min−1 cm2). The cell appears very stable under dry biogas feed and no carbon deposition is envisaged under these experimental conditions.
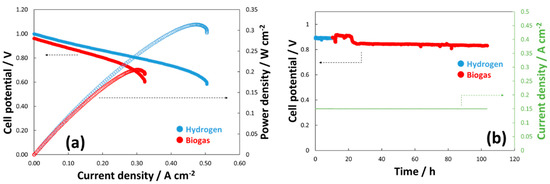
Figure 3. Polarization curves (a) and lifetime test (b) carried out with a novel NiCu-exsolved La0.6Sr0.4Fe0.8Co0.2O3 (LSFC) perovskite composite catalyst. These tests have been carried out at 650 °C with NiCu-LSFC coated on an Elcogen cell fed with H2 and biogas (30 vol.% of CO2). (Standard deviation was < 5 mV for each data point of the overall polarization dataset).
This novel approach should be evaluated in relation to the limited performance achieved for the Ni-LSFC fed with dry methane and reported. As we discussed above, Ni-LSFC showed a low reactivity toward the oxidation of methane, and this is ascribed to the rate-determining step due to the dehydrogenation of this fuel. The present composite catalyst (NiCu-LSFC) registered an OCV close to 1 V, which proves a high reactivity toward CH4. This is in line with the reactivity we have already demonstrated for various Ni-based alloys in several recent papers [11][12][33][34][37][88]. However, some mass transfer limitations at a cell voltage of about 0.6 V have been observed. This effect has a negative impact on the “shuttle mechanism”, causing such voltage decay at sustained currents in the polarization curves.
4. Conclusions
The analysis presented in this mini-review deals with a number of studies carried out over more than a decade on a novel modified perovskite used as an anode electrocatalyst for application in fuel-flexible SOFCs. The exsolved perovskite shows the presence of fine embedded particles made of transition elements such as Fe, Ni, and Co over a depleted perovskite substrate. This structure is characterized by outstanding catalytic activity and proper electronic conductivity under reducing conditions. Moreover, a limited coarsening effect is generally observed for the embedded nanoparticles even upon prolonged operation. Catalytic and electrochemical studies have proved an effective capability of this material to convert organic fuels and good chemical stability under reducing conditions. These evidences make this electrocatalyst a promising anode for electrolyte-supported cells or as a coating layer for anode-supported cells. The exsolved perovskite catalyst can also be modified by the addition of an Ni alloy to form a composite layer. A specific “shuttle mechanism” is envisaged for the catalyst operation in an SOFC. This includes iterative adsorption and desorption steps involving the organic fuel and the reaction intermediates, the reaction at the interface of the formed hydrogen and carbon monoxide, with the final formation of water and CO2. This approach appears very promising for multifuel-fed SOFCs, and it is expected to attract large interest in the coming years.
References
- Dong, Y.; Steinberg, M. Hynol—An economical process for methanol production from biomass and natural gas with reduced CO2 emission. Int. J. Hydrog. Energy 1997, 22, 971–977.
- Gibbs, C.E.; Steel, M.C.F. European opportunities for fuel cell commercialisation. J. Power Sources 1992, 37, 35–43.
- Lee, A.L.; Zabransky, R.F.; Huber, W.J. Internal reforming development for Solid Oxide Fuel Cells. Ind. Eng. Chem. Res. 1990, 29, 766–773.
- Bove, R.; Sammes, N.M. Thermodynamic analysis of SOFC systems using different fuel processors. In Proceedings of the ASME 2004 2nd International Conference on Fuel Cell Science, Engineering and Technology, Rochester, NY, USA, 14–16 June 2004; pp. 461–466.
- Steele, B.C.H. Materials for IT-SOFC stacks—35 years R&D: The inevitability of gradualness? Solid State Ion. 2000, 134, 3–20.
- La Rosa, D.; Lo Faro, M.; Monforte, G.; Antonucci, V.; Arico, A.S. Comparison of the electrochemical properties of intermediate temperature solid oxide fuel cells based on protonic and anionic electrolytes. J. Appl. Electrochem. 2009, 39, 477–483.
- Sauvert, A.L.; Fouletier, J. Research trends: Electrochemical properties of new type of IT-SOFC anode material. Fuel Cells Bull. 2002, 2002, 12.
- Lo Faro, M.; La Rosa, D.; Antonucci, V.; Arico, A.S. Intermediate temperature solid oxide fuel cell electrolytes. J. Indian Inst. Sci. 2009, 89, 363–380.
- Kikuchi, R.; Koashi, N.; Matsui, T.; Eguchi, K.; Norby, T. Novel anode materials for multi-fuel applicable solid oxide fuel cells. J. Alloy. Compd. 2006, 408, 622–627.
- La Rosa, D.; Lo Faro, M.; Monforte, G.; Antonucci, V.; Arico, A.S.; Antonucci, P. Propane conversion over a Ru/CGO catalyst and its application in intermediate temperature solid oxide fuel cells. J. Appl. Electrochem. 2007, 37, 203–208.
- La Rosa, D.; Sin, A.; Lo Faro, M.; Monforte, G.; Antonucci, V.; Arico, A.S. Mitigation of carbon deposits formation in intermediate temperature solid oxide fuel cells fed with dry methane by anode doping with barium. J. Power Sources 2009, 193, 160–164.
- Lo Faro, M.; La Rosa, D.; Frontera, P.; Antonucci, P.; Antonucci, V.; Arico, A.S. Propane-fed Solid Oxide Fuel Cell based on a composite Ni-La-CGO anode catalyst. Catal. Lett. 2010, 136, 57–64.
- De Marco, V.; Iannaci, A.; Lo Faro, M.; Sglavo, V.M. Influence of Copper-based anode composition on intermediate temperature Solid Oxide Fuel Cells performance. Fuel Cells 2017, 17, 708–715.
- 14. Escudero, M.J.; Yeste, M.P.; Cauqui, M.A.; Muñoz, M.A. Performance of a direct methane solid oxide fuel cell using nickel-ceria-yttria stabilized zirconia as the anode. Materials 2020, 13, 599, doi:10.3390/ma13030599.
- Gandiglio, M.; Lanzini, A.; Santarelli, M.; Acri, M.; Hakala, T.; Rautanen, M. Results from an industrial size biogas-fed SOFC plant (the DEMOSOFC project). Int. J. Hydrog. Energy 2020, 45, 5449–5464, doi:10.1016/j.ijhydene.2019.08.022.
- Yokokawa, H.; Suzuki, M.; Yoda, M.; Suto, T.; Tomida, K.; Hiwatashi, K.; Shimazu, M.; Kawakami, A.; Sumi, H.; Ohmori, M.; et al. Achievements of NEDO durability projects on SOFC stacks in the light of physicochemical mechanisms. Fuel Cells 2019, 19, 311–339, doi:10.1002/fuce.201800187.
- Santhanam, S.; Ullmer, D.; Wuillemin, Z.; Varkaraki, E.; Beetschen, C.; Antonetti, Y.; Ansar, A. Experimental analysis of a 25 kWe solid oxide fuel cell module for co-generation of hydrogen and power. Ecs Trans. 2019, 91, 159–166.
- McPhail, S.J.; Pumiglia, D.; Laurencin, J.; Hagen, A.; Leon, A.; Van Herle, J.; Vladikova, D.; Montinaro, D.; Piccardo, P.; Polverino, P.; et al. Developing accelerated stress test protocols for solid oxide fuel cells and electrolysers: The European project AD ASTRA. Ecs Trans. 2019, 91, 563–570.
- Wang, Q.; Wei, H.H.; Xu, Q. A solid oxide fuel cell (SOFC)-based biogas-from-waste generation system for residential buildings in China: A feasibility study. Sustainability 2018, 10, 2395, doi:10.3390/su10072395.
- Stoeckl, B.; Subotić, V.; Preininger, M.; Schroettner, H.; Hochenauer, C. SOFC operation with carbon oxides: Experimental analysis of performance and degradation. Electrochim. Acta 2018, 275, 256–264, doi:10.1016/j.electacta.2018.04.036.
- Maraver, D.; Tondi, G.; Goodchild, R. Overview and current status of eu funded actions on bio-fuelled heating and combined heating & power within the energy challenge of Horizon 2020. In Proceedings of the 26th European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–18 May 2018; pp. 1289–1298.
- Montinaro, D.; Sglavo, V.M.; Bertoldi, M.; Zandonella, T.; Aricò, A.; Lo Faro, M.; Antonucci, V. Tape casting fabrication and co-sintering of solid oxide “half cells” with a cathode–electrolyte porous interface. Solid State Ion. 2006, 177, 2093–2097.
- Murata, K.; Shimotsu, M. Fabrication and evaluation of electrode-supported planar SOFC. Denki Kagaku 1997, 65, 38–43.
- Wincewicz, K.C.; Cooper, J.S. Taxonomies of SOFC material and manufacturing alternatives. J. Power Sources 2005, 140, 280–296, doi:10.1016/j.jpowsour.2004.08.032.
- Lee, H.W.; Park, M.; Hong, J.; Kim, H.; Yoon, K.J.; Son, J.W.; Lee, J.H.; Kim, B.K. Constrained sintering in fabrication of solid oxide fuel cells. Materials 2016, 9, 675, doi:10.3390/ma9080675.
- Singh, D.; Hernández-Pacheco, E.; Hutton, P.N.; Patel, N.; Mann, M.D. Carbon deposition in an SOFC fueled by tar-laden biomass gas: A thermodynamic analysis. J. Power Sources 2005, 142, 194–199, doi:10.1016/j.jpowsour.2004.10.024.
- Grgicak, C.M.; Green, R.G.; Giorgi, J.B. SOFC anodes for direct oxidation of hydrogen and methane fuels containing H2S. J. Power Sources 2008, 179, 317–328, doi:10.1016/j.jpowsour.2007.12.082.
- Schluckner, C.; Subotić, V.; Lawlor, V.; Hochenauer, C. Carbon deposition simulation in porous SOFC anodes: A detailed numerical analysis of major carbon precursors. J. Fuel Cell Sci. Technol. 2015, 12, 051007, doi:10.1115/1.4031862.
- Fernandes, M.D.; Bistritzki, V.; Domingues, R.Z.; Matencio, T.; Rapini, M.; Sinisterra, R.D. Solid oxide fuel cell technology paths: National innovation system contributions from Japan and the United States. Renew. Sustain. Energy Rev. 2020, 127, 109879, doi:10.1016/j.rser.2020.109879.
- Lo Faro, M.; Trocino, S.; Zignani, S.C.; Aricò, A.S.; Maggio, G.; Italiano, C.; Fabiano, C.; Pino, L.; Vita, A. Study of a Solid Oxide Fuel Cell fed with n-dodecane reformate. Part I: Endurance test. Int. J. Hydrog. Energy 2016, 41, 5741–5747, doi:10.1016/j.ijhydene.2016.02.119.
- Lo Faro, M.; Trocino, S.; Zignani, S.C.; Italiano, C.; Vita, A.; Aricò, A.S. Study of a solid oxide fuel cell fed with n-dodecane reformate. Part II: Effect of the reformate composition. Int. J. Hydrog. Energy 2017, 42, 1751–1757, doi:10.1016/j.ijhydene.2016.06.048.
- Zhan, Z.; Lin, Y.; Bamett, S. Anode catalyst layers for direct hydrocarbon and internal reforming SOFCs. Electrochem. Soc. 2005, 9, 1321–1330.
- Lo Faro, M.; Reis, R.M.; Saglietti, G.G.A.; Sato, A.G.; Ticianelli, E.A.; Zignani, S.C.; Aricò, A.S. Nickel-Copper/Gadolinium-doped Ceria (CGO) composite electrocatalyst as a protective layer for a Solid-Oxide Fuel Cell anode fed with ethanol. ChemElectroChem 2014, 1, 1395–1402, doi:10.1002/celc.201402017.
- Lo Faro, M.; Reis, R.M.; Saglietti, G.G.A.; Zignani, S.C.; Trocino, S.; Frontera, P.; Antonucci, P.L.; Ticianelli, E.A.; Aricò, A.S. Investigation of Ni-based alloy/CGO electro-catalysts as protective layer for a solid oxide fuel cell anode fed with ethanol. J. Appl. Electrochem. 2015, 45, 647–656, doi:10.1007/s10800-015-0849-5.
- Lo Faro, M.; Trocino, S.; Zignani, S.C.; Italiano, C.; Reis, R.M.; Ticianelli, E.A.; Aricò, A.S. Nickel–Iron/Gadolinium-doped Ceria (CGO) composite electrocatalyst as a protective layer for a Solid-Oxide Fuel Cell anode fed with biofuels. ChemCatChem 2016, 8, 648–655, doi:10.1002/cctc.201501090.
- Li, J.; Croiset, E.; Ricardez-Sandoval, L. Theoretical investigation of the methane cracking reaction pathways on Ni (1 1 1) surface. Chem. Phys. Lett. 2015, 639, 205–210, doi:10.1016/j.cplett.2015.09.030.
- Lo Faro, M.; Frontera, P.; Antonucci, P.; Aricò, A.S. Ni-Cu based catalysts prepared by two different methods and their catalytic activity toward the ATR of methane. Chem. Eng. Res. Des. 2015, 93, 269–277, doi:10.1016/j.cherd.2014.05.014.
- Laosiripojana, N.; Assabumrungrat, S. The effect of specific surface area on the activity of nano-scale ceria catalysts for methanol decomposition with and without steam at SOFC operating temperatures. Chem. Eng. Sci. 2006, 61, 2540–2549, doi:10.1016/j.ces.2005.11.024.
- Ioselevich, A.; Kornyshev, A.A.; Lehnert, W. Statistical geometry of reaction space in porous cermet anodes based on ion-conducting electrolytes patterns of degradation. Solid State Ion. 1999, 124, 221–237, doi:10.1016/S0167-2738(99)00218-0.
- Pecho, O.M.; Mai, A.; Münch, B.; Hocker, T.; Flatt, R.J.; Holzer, L. 3D microstructure effects in Ni-YSZ anodes: Influence of TPB lengths on the electrochemical performance. Materials 2015, 8, 7129–7144, doi:10.3390/ma8105370.
- Crosbie, G.M.; Murray, E.P.; Bauer, D.R.; Kim, H.; Park, S.; Vohs, J.M.; Gorte, R.J. Solid oxide fuel cells for direct oxidation of liquid hydrocarbon fuels in automotive auxiliary power units: Sulfur tolerance and operation on gasoline. SAE Trans. 2002, 111, 832–839, doi:10.4271/2002-01-0410.
- Mogensen, M.B. Direct conversion of hydrocarbons in Solid Oxide Fuel Cells: A review. In ACS Division of Fuel Chemistry, Preprints, Proceedings of the 224th ACS National Meeting, Boston, MA, USA, 18–22 August 2002; p. 498.
- Sun, Y.F.; Zhou, X.W.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.N.; Zhang, L.Z.; Hua, B.; Li, J.; Li, J.H.; Luo, J.L. An ingenious Ni/Ce co-doped titanate based perovskite as a coking-tolerant anode material for direct hydrocarbon solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 22830–22838, doi:10.1039/c5ta06200d.
- Moure, C.; Peña, O. Recent advances in perovskites: Processing and properties. Prog. Solid State Chem. 2015, 43, 123–148, doi:10.1016/j.progsolidstchem.2015.09.001.
- Perry, N.H.; Ishihara, T. Roles of bulk and surface chemistry in the oxygen exchange kinetics and related properties of mixed conducting perovskite oxide electrodes. Materials 2016, 9, 858, doi:10.3390/ma9100858.
- Cascos, V.; Alonso, J.A.; Fernández-Díaz, M.T. Novel Mg-doped SrMoO3 Perovskites designed as anode materials for solid oxide fuel cells. Materials 2016, 9, 588, doi:10.3390/MA9070588.
- Bernuy-Lopez, C.; Høydalsvik, K.; Einarsrud, M.A.; Grande, T. Effect of A-site cation ordering on chemical stability, oxygen stoichiometry and electrical conductivity in layered LaBaCo2O5+δ double perovskite. Materials 2016, 9, doi:10.3390/ma9030154.
- Yao, Y.-F.Y. The oxidation of hydrocarbons and CO over metal oxides. IV. Perovskite-type oxides. J. Catal. 1975, 36, 266–275, doi:10.1016/0021-9517(75)90036-6.
- Shimizu, T. Partial oxidation of hydrocarbons and oxygenated compounds on perovskite oxides. Catal. Rev. 1992, 34, 355–371, doi:10.1080/01614949208016317.
- Niu, B.; Jin, F.; Yang, X.; Feng, T.; He, T. Resisting coking and sulfur poisoning of double perovskite Sr2TiFe0.5Mo0.5O6–Δ anode material for solid oxide fuel cells. Int. J. Hydrog. Energy 2018, 43, 3280–3290, doi:10.1016/j.ijhydene.2017.12.134.
- Swartz, S.L. Sulfur tolerant fuel processing catalysts. In ACS National Meeting Book of Abstracts, Proceedings of the 230th ACS National Meeting, Washington, DC, USA, 28 August–1 September 2005; p. 1.
- Wang, S.; Jiang, Y.; Zhang, Y.; Li, W.; Yan, J.; Lu, Z. Electrochemical performance of mixed ionic-electronic conducting oxides as anodes for solid oxide fuel cell. Solid State Ion. 1999, 120, 75–84, doi:10.1016/S0167-2738(98)00558-X.
- Sammes, N.M.; Ratnaraj, R. High-temperature mechanical properties of La0.7Sr0.3Cr1-yCoyO3 in reducing environments. J. Mater. Sci. 1997, 32, 687–692, doi:10.1023/A:1018591803417.
- Xu, S.J.; Thomson, W.J. Stability of La0.6Sr0.4Co0.2Fe0.8O3-δ perovskite membranes in reducing and nonreducing environments. Ind. Eng. Chem. Res. 1998, 37, 1290–1299.
- Zhang, Y.; Sun, Y.F.; Luo, J.L. Ce/Ni decorated titanate based perovskite for solid oxide fuel cells. Ecs Trans. 2017, 75, 91–97.
- Lo Faro, M.; La Rosa, D.; Nicotera, I.; Antonucci, V.; Arico, A.S. Electrochemical investigation of a propane-fed solid oxide fuel cell based on a composite Ni-perovskite anode catalyst. Appl. Catal. B-Environ. 2009, 89, 49–57, doi:10.1016/j.apcatb.2008.11.019.
- Lo Faro, M.; Minutoli, M.; Monforte, G.; Antonucci, V.; Arico, A.S. Glycerol oxidation in solid oxide fuel cells based on a Ni-perovskite electrocatalyst. Biomass Bioenergy 2011, 35, 1075–1084, doi:10.1016/j.biombioe.2010.11.018.
- Lo Faro, M.; Stassi, A.; Antonucci, V.; Modafferi, V.; Frontera, P.; Antonucci, P.; Arico, A.S. Direct utilization of methanol in solid oxide fuel cells: An electrochemical and catalytic study. Int. J. Hydrog. Energy 2011, 36, 9977–9986, doi:10.1016/j.ijhydene.2011.05.053.
- Lo Faro, M.; Antonucci, V.; Antonucci, P.L.; Aricò, A.S. Fuel flexibility: A key challenge for SOFC technology. Fuel 2012, 102, 554–559.
- Lo Faro, M.; Modafferi, V.; Frontera, P.; Antonucci, P.; Aricò, A.S. Catalytic behavior of Ni-modified perovskite and doped ceria composite catalyst for the conversion of odorized propane to syngas. Fuel Process. Technol. 2013, 113, 28–33.
- Lo Faro, M.; Arico, A.S. Electrochemical behaviour of an all-perovskite-based intermediate temperature solid oxide fuel cell. Int. J. Hydrog. Energy 2013, 38, 14773–14778, doi:10.1016/j.ijhydene.2013.08.122.
- Lo Faro, M.; Reis, R.M.; Saglietti, G.G.A.; Oliveira, V.L.; Zignani, S.C.; Trocino, S.; Maisano, S.; Ticianelli, E.A.; Hodnik, N.; Ruiz-Zepeda, F.; et al. Solid oxide fuel cells fed with dry ethanol: The effect of a perovskite protective anodic layer containing dispersed Ni-alloy @ FeOx core-shell nanoparticles. Appl. Catal. B Environ. 2018, 220, 98–110, doi:https://doi.org/10.1016/j.apcatb.2017.08.010.
- Lo Faro, M.; Oliveira, V.L.; Reis, R.M.; Saglietti, G.G.A.; Zignani, S.C.; Trocino, S.; Ticianelli, E.A.; Aricò, A.S. Solid Oxide Fuel Cell fed directly with dry glycerol. Energy Technol. 2019, 7, 45–47, doi:doi:10.1002/ente.201700744.
- Jardiel, T.; Caldes, M.T.; Moser, F.; Hamon, J.; Gauthier, G.; Joubert, O. New SOFC electrode materials: The Ni-substituted LSCM-based compounds (La0.75Sr0.25) (Cr0.5Mn0.5-xNix) O3-δ and (La0.75Sr0.25) (Cr0.5-xNixMn0.5)O3-δ. Solid State Ion. 2010, 181, 894–901, doi:10.1016/j.ssi.2010.05.012.
- Van Den Bossche, M.; McIntosh, S. Pulse reactor studies to assess the potential of La0.75Sr 0.25Cr0.5Mn0.4X0.1O 3-δ (X = Co, Fe, Mn, Ni, V) as direct hydrocarbon solid oxide fuel cell anodes. Chem. Mater. 2010, 22, 5856–5865, doi:10.1021/cm101567v.
- Lay, E.; Gauthier, G.; Dessemond, L. Preliminary studies of the new Ce-doped La/Sr chromo-manganite series as potential SOFC anode or SOEC cathode materials. Solid State Ion. 2011, 189, 91–99, doi:10.1016/j.ssi.2011.02.004.
- Li, X.; Dai, L.; He, Z.; Meng, W.; Li, Y.; Wang, L. In situ exsolution of PdO nanoparticles from non-stoichiometric LaFePd0.05O3+δ electrode for impedancemetric NO2 sensor. Sens. Actuators B Chem. 2019, 298, 126827, doi:https://doi.org/10.1016/j.snb.2019.126827.
- Hou, N.; Yao, T.; Li, P.; Yao, X.; Gan, T.; Fan, L.; Wang, J.; Zhi, X.; Zhao, Y.; Li, Y. A-site ordered double perovskite with in situ exsolved core-shell nanoparticles as anode for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2019, 11, 6995–7005, doi:10.1021/acsami.8b19928.
- Lo Faro, M.; La Rosa, D.; Nicotera, I.; Antonucci, V.; Aricò, A.S. Electrochemical behaviour of propane-fed solid oxide fuel cells based on low Ni content anode catalysts. Electrochim. Acta 2009, 54, 5280–5285.
- Vecino-Mantilla, S.; Quintero, E.; Fonseca, C.; Gauthier, G.H.; Gauthier-Maradei, P. Catalytic steam reforming of natural gas over a new Ni exsolved Ruddlesden-Popper manganite in SOFC anode conditions. ChemCatChem 2020, 12, 1453–1466, doi:10.1002/cctc.201902306.
- Kim, J.S.; Lee, J.Y.; Swinnea, J.S.; Steinfink, H.; Reiff, W.M.; Lightfoot, P.; Pei, S.; Jorgensen, J.D. Ruddlesden-Popper phases An+1MnO3n+1. Structures and properties. In Proceedings of the International Conference on the Chemistry of Electronic Ceramic Materials, Jackson, WY, USA, 17–22 August 1990; pp. 301–306.
- Lee, D.; Lee, H.N. Controlling oxygen mobility in ruddlesden-popper oxides. Materials 2017, 10, 368, doi:10.3390/ma10040368.
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Zhang, Z.; Sedach, P.A.; Siu, M.K.J.; Trudel, S.; Berlinguette, C.P. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 2013, 340, 60–63, doi:10.1126/science.1233638.
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Water oxidation catalysis: Electrocatalytic response to metal stoichiometry in amorphous metal oxide films containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 2013, 135, 11580–11586, doi:10.1021/ja403102j.
- Tang, C.-W.; Wang, C.-B.; Chien, S.-H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73, doi:https://doi.org/10.1016/j.tca.2008.04.015.
- Tiernan, M.J.; Barnes, P.A.; Parkes, G.M.B. Reduction of Iron oxide catalysts: The Investigation of kinetic parameters using rate perturbation and linear heating thermoanalytical techniques. J. Phys. Chem. B 2001, 105, 220–228, doi:10.1021/jp003189+.
- Li, C.; Chen, Y.W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465, doi:10.1016/0040-6031(94)02177-P.
- Marrero-Jerez, J.; Larrondo, S.; Rodríguez-Castellón, E.; Núñez, P. TPR, XRD and XPS characterisation of ceria-based materials synthesized by freeze-drying precursor method. Ceram. Int. 2014, 40, 6807–6814, doi:10.1016/j.ceramint.2013.11.143.
- Steele, B.C.H. Oxygen transport and exchange in oxide ceramics. J. Power Sources 1994, 49, 1–14, doi:10.1016/0378-7753(93)01789-k.
- Mai, A.; Haanappel, V.A.C.; Uhlenbruck, S.; Tietz, F.; Stöver, D. Ferrite-based perovskites as cathode materials for anode-supported solid oxide fuel cells: Part I. Variation of composition. Solid State Ion. 2005, 176, 1341–1350, doi:10.1016/j.ssi.2005.03.009.
- Liu, J.; Co, A.C.; Paulson, S.; Birss, V.I. Oxygen reduction at sol-gel derived La0.8Sr0.2Co0.8Fe0.2O3 cathodes. Solid State Ion. 2006, 177, 377–387, doi:10.1016/j.mcm.2006.01.023.
- Dias, J.A.; Andrade, M.A.S.J.; Santos, H.L.S.; Morelli, M.R.; Mascaro, L.H. Lanthanum-Based Perovskites for Catalytic Oxygen Evolution Reaction. ChemElectroChem 2020, 10.1002/celc.202000451, doi:10.1002/celc.202000451.
- Zhu, L.; Wei, B.; Zhang, Y.; Lü, Z.; Wang, Z.; Huang, X.; Cao, Z.; Jiang, W.; Li, Y. Investigation on a novel composite solid oxide fuel cell anode with La0.6Sr0.4Co0.2Fe0.8O3-δ derived phases. Electrochim. Acta 2015, 160, 89–93, doi:10.1016/j.electacta.2015.02.024.
- Benson, S.J.; Waller, D.; Kilner, J.A. Degradation of La0.6Sr0.4Fe0.8Co0.2O3-δ in carbon dioxide and water atmospheres. J. Electrochem. Soc. 1999, 146, 1305, doi:10.1149/1.1391762.
- Kim, H.; Lu, C.; Worrell, W.L.; Vohs, J.M.; Gorte, R.J. Cu-Ni cermet anodes for direct oxidation of methane in solid-oxide fuel cells. J. Electrochem. Soc. 2002, 149, A247, doi:10.1149/1.1445170.
- An, W.; Gatewood, D.; Dunlap, B.; Turner, C.H. Catalytic activity of bimetallic nickel alloys for solid-oxide fuel cell anode reactions from density-functional theory. J. Power Sources 2011, 196, 4724–4728, doi:10.1016/j.jpowsour.2011.01.007.
- Nabae, Y.; Yamanaka, I.; Hatano, M.; Otsuka, K. Catalytic behavior of Pd-Ni/composite anode for direct oxidation of methane in SOFCs. J. Electrochem. Soc. 2006, 153, A140, doi:10.1149/1.2136079.
- Lo Faro, M.; Trocino, S.; Zignani, S.C.; Reis, R.M.; Monforte, G.; Ticianelli, E.A.; Aricò, A.S. Ni-based Alloys as Protective Layer for a Conventional Solid Oxide Fuel Cell Fed with Biofuels. ECS Trans 2015, 68, 2653–2658, doi:10.1149/06801.2653ecst.




