
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giustino Varrassi | -- | 10454 | 2025-09-25 10:17:45 | | | |
| 2 | Catherine Yang | -3696 word(s) | 6758 | 2025-09-25 10:57:10 | | |
Video Upload Options
Chronic pain is a complex and persistent condition involving sustained nociceptive input, maladaptive neuroplastic changes, and neuroimmune interactions. Central to its pathophysiology is the dysregulation of neuromodulatory signaling pathways, including neurotransmitters (e.g., dopamine, serotonin, norepinephrine), neuropeptides (e.g., substance P, CGRP), and neurotrophic factors (e.g., BDNF), which modulate both central and peripheral sensitization mechanisms. In disorders such as fibromyalgia, altered monoaminergic transmission has been implicated in the attenuation of descending inhibitory control, thereby enhancing pain perception and reducing responsiveness to conventional therapies. Concurrently, neuroinflammation, driven by glial cell activation and cytokine release, further exacerbates neuronal excitability and reinforces maladaptive signaling loops. Recent technological advances, including transcriptomic profiling, functional neuroimaging, and single-cell RNA sequencing, have provided new insights into patient-specific patterns of neuromodulatory dysfunction, highlighting potential biomarkers for disease stratification and therapeutic targeting. These developments support the hypothesis that dysregulated neuromodulatory circuits not only underlie diverse chronic pain phenotypes but may also serve as intervention points for precision medicine. This narrative review synthesizes current evidence on the roles of neuromodulatory systems in chronic pain, focusing on synaptic plasticity, nociceptor sensitization, and neuroimmune crosstalk. By integrating preclinical findings with clinical observations, we propose a mechanistic framework for understanding pain chronification and guiding future therapeutic strategies. Harnessing neuromodulatory targets, whether pharmacologically or via neuromodulation technologies, could offer more personalized and effective approaches to chronic pain management.
1. Introduction
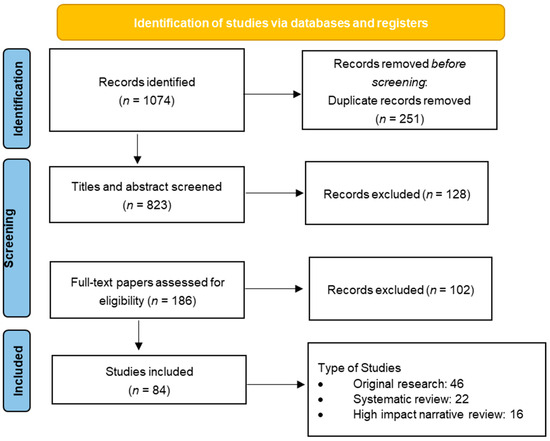
2. Materials and Methods
3. Results
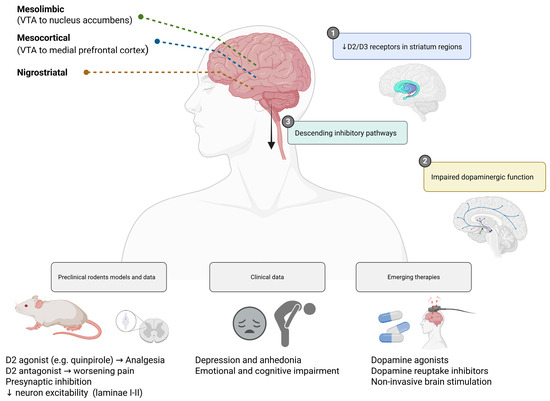
3.1. Dopaminergic Signaling and Pain Regulation
3.2. Serotonergic and Noradrenergic Pathways
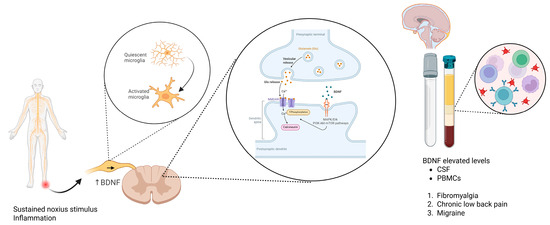
3.3. Brain-Derived Neurotrophic Factor (BDNF)
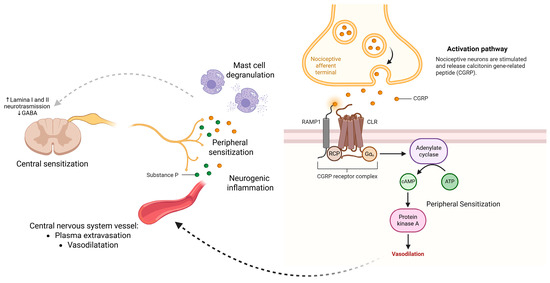
3.4. Neuropeptides: Substance P and CGRP
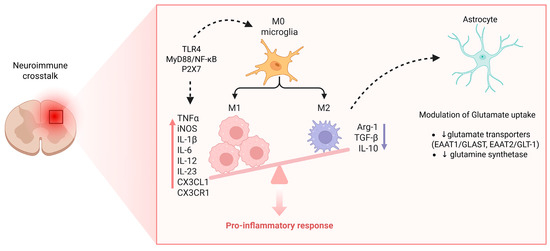
3.5. Glial Cells and Neuroimmune Crosstalk
3.6. Transcriptomics and Functional Neuroimaging
3.7. Translational Challenges and Clinical Perspectives
4. Discussion
Limitations
5. Conclusions
Abbreviations
| ACC | Anterior Cingulate Cortex |
| BDNF | Brain-Derived Neurotrophic Factor |
| CALCA | Calcitonin Gene-Related Peptide Alpha |
| cAMP | Cyclic Adenosine Monophosphate |
| CCL2 | C-C Motif Chemokine Ligand 2 |
| CGRP | Calcitonin Gene-Related Peptide |
| CLR | Calcitonin Receptor-Like Receptor |
| CNS | Central Nervous System |
| CPM | Conditioned Pain Modulation |
| CRPS | Complex Regional Pain Syndrome |
| CSF | Cerebrospinal Fluid |
| CXCL1 | C-X-C Motif Chemokine Ligand 1 |
| CX3CL1 | C-X3-C Motif Chemokine Ligand 1 |
| CX3CR1 | C-X3-C Motif Chemokine Receptor 1 |
| DNIC | Diffuse Noxious Inhibitory Control |
| DMN | Default Mode Network |
| DRG | Dorsal Root Ganglion |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| fMRI | Functional Magnetic Resonance Imaging |
| GABA | Gamma-Aminobutyric Acid |
| 5-HT | 5-Hydroxytryptamine (Serotonin) |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-23 | Interleukin-23 |
| iNOS | Inducible Nitric Oxide Synthase |
| KCC2 | Potassium-Chloride Co-transporter 2 |
| LTP | Long-Term Potentiation |
| mPFC | Medial Prefrontal Cortex |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NAc | Nucleus Accumbens |
| NE | Norepinephrine |
| NF-κB | Nuclear Factor Kappa B |
| NK1 | Neurokinin-1 |
| NLRP3 | NLR Family Pyrin Domain Containing 3 |
| NMDA | N-methyl-D-aspartate |
| NNT | Number Needed to Treat |
| P2X7 | P2X Purinoceptor 7 |
| PAG | Periaqueductal Gray |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PET | Positron Emission Tomography |
| PNS | Peripheral Nervous System |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| QST | Quantitative Sensory Testing |
| RAMP1 | Receptor Activity-Modifying Protein 1 |
| RCP | Receptor Component Protein |
| RCTs | Randomized Controlled Trials |
| RNA | Ribonucleic Acid |
| RVM | Rostral Ventromedial Medulla |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| SCN9A | Sodium Voltage-Gated Channel Alpha Subunit 9 |
| scRNA-seq | Single-Cell RNA Sequencing |
| SNRIs | Serotonin-Norepinephrine Reuptake Inhibitors |
| TAC1 | Tachykinin Precursor 1 |
| TGF-β | Transforming Growth Factor Beta |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor Alpha |
| TrkB | Tropomyosin Receptor Kinase B |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| TSPO | Translocator Protein |
| VTA | Ventral Tegmental Area |
| WoS | Web of Science |
References
- Goldberg, D.S.; McGee, S.J. Pain as a global public health priority. BMC Public Health 2011, 11, 770.
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006.
- Tracey, I.; Bushnell, M.C. How neuroimaging studies have challenged us to rethink: Is chronic pain a disease? J. Pain 2009, 10, 1113–1120.
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152 (Suppl. S3), S2–S15.
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2017, 18, 20–30.
- Denk, F.; McMahon, S.B.; Tracey, I. Pain vulnerability: A neurobiological perspective. Nat. Neurosci. 2014, 17, 192–200.
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231.
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284.
- Yarnitsky, D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 2015, 156 (Suppl. S1), S24–S31.
- Lewis, G.N.; Rice, D.A.; McNair, P.J. Conditioned Pain Modulation in Populations With Chronic Pain: A Systematic Review and Meta-Analysis. J. Pain 2012, 13, 936–944.
- Loggia, M.L.; Berna, C.; Kim, J.; Cahalan, C.M.; Gollub, R.L.; Wasan, A.D.; Harris, R.E.; Edwards, R.R.; Napadow, V. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014, 66, 203–212.
- Taylor, A.M.W.; Becker, S.; Schweinhardt, P.; Cahill, C. Mesolimbic dopamine signaling in acute and chronic pain: Implications for motivation, analgesia, and addiction. Pain 2016, 157, 1194–1198.
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021.
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018, 129, 343–366.
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5.
- Vincent, K.F.; Solt, K. Modulating anesthetic emergence with pathway-selective dopamine signaling. Curr. Opin. Anaesthesiol. 2023, 36, 468–475.
- Bannister, K.; Dickenson, A.H. What do monoamines do in pain modulation? Curr. Opin. Support. Palliat. Care 2016, 10, 143–148.
- Bravo, L.; Llorca-Torralba, M.; Berrocoso, E.; Micó, J.A. Monoamines as Drug Targets in Chronic Pain: Focusing on Neuropathic Pain. Front. Neurosci. 2019, 13, 1268.
- Argoff, C. Mechanisms of pain transmission and pharmacologic management. Curr. Med. Res. Opin. 2011, 27, 2019–2031.
- Ashida, M.; Murayama, N.; Kamio, Y.; Yozaki, M.; Kuwatsuka, Y.; Nakahara, T.; Murota, H. Blood levels of neurotransmitters in Yusho patients: An approach via the descending pain inhibitory pathway for persistent sensory disturbance. J. Dermatol. 2025, 52, 934–938.
- Meseguer-Beltrán, M.; Sánchez-Sarasúa, S.; Landry, M.; Kerekes, N.; Sánchez-Pérez, A.M. Targeting Neuroinflammation with Abscisic Acid Reduces Pain Sensitivity in Females and Hyperactivity in Males of an ADHD Mice Model. Cells 2023, 12, 465.
- Lançon, K.; Tian, J.; Bach, H.; Drapeau, P.; Poulin, J.F.; Séguéla, P. Synergistic deficits in parvalbumin interneurons and dopamine signaling drive ACC dysfunction in chronic pain. Proc. Natl. Acad. Sci. USA 2025, 122, e2502558122.
- Li, C.; Sugam, J.A.; Lowery-Gionta, E.G.; McElligott, Z.A.; McCall, N.M.; Lopez, A.J.; McKlveen, J.M.; Pleil, K.E.; Kash, T.L. Mu Opioid Receptor Modulation of Dopamine Neurons in the Periaqueductal Gray/Dorsal Raphe: A Role in Regulation of Pain. Neuropsychopharmacology 2016, 41, 2122–2132.
- Sheng, H.Y.; Qu, C.L.; Huo, F.Q.; Du, J.Q.; Tang, J.S. D2-like but not D1-like dopamine receptors are involved in the ventrolateral orbital cortex-induced antinociception: A GABAergic modulation mechanism. Exp. Neurol. 2009, 215, 128–134.
- Leknes, S.; Tracey, I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008, 9, 314–320.
- Navratilova, E.; Porreca, F. Reward and motivation in pain and pain relief. Nat. Neurosci. 2014, 17, 1304–1312.
- Porreca, F.; Navratilova, E. Reward, motivation, and emotion of pain and its relief. Pain 2017, 158 (Suppl. S1), S43–S49.
- Arora, V.; Morado-Urbina, C.E.; Aschenbrenner, C.A.; Hayashida, K.; Wang, F.; Martin, T.J.; Eisenach, J.C.; Peters, C.M. Disruption of Spinal Noradrenergic Activation Delays Recovery of Acute Incision-Induced Hypersensitivity and Increases Spinal Glial Activation in the Rat. J. Pain 2016, 17, 190–202.
- Wood, P.B.; Schweinhardt, P.; Jaeger, E.; Dagher, A.; Hakyemez, H.; Rabiner, E.A.; Bushnell, M.C.; Chizh, B.A. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci. 2007, 25, 3576–3582.
- Garcia Guerra, S.; Spadoni, A.; Mitchell, J.; Strigo, I.A. Pain-related opioidergic and dopaminergic neurotransmission: Dual meta-Analyses of PET radioligand studies. Brain Res. 2023, 1805, 148268.
- Martikainen, I.K.; Nuechterlein, E.B.; Peciña, M.; Love, T.M.; Cummiford, C.M.; Green, C.R.; Stohler, C.S.; Zubieta, J.K. Chronic Back Pain Is Associated with Alterations in Dopamine Neurotransmission in the Ventral Striatum. J. Neurosci. 2015, 35, 9957–9965.
- Liu, Y.Y.; Wang, T.X.; Zhou, J.C.; Qu, W.M.; Huang, Z.L. Dopamine D1 and D2 receptors mediate analgesic and hypnotic effects of l-tetrahydropalmatine in a mouse neuropathic pain model. Psychopharmacology 2019, 236, 3169–3182.
- Taylor, B.K.; Joshi, C.; Uppal, H. Stimulation of dopamine D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain Res. 2003, 987, 135–143.
- Fernandes, E.C.; Pechincha, C.; Luz, L.L.; Kokai, E.; Szucs, P.; Safronov, B.V. Primary afferent-driven presynaptic inhibition of C-fiber inputs to spinal lamina I neurons. Prog. Neurobiol. 2020, 188, 101786.
- Borsook, D.; Linnman, C.; Faria, V.; Strassman, A.M.; Becerra, L.; Elman, I. Reward deficiency and anti-reward in pain chronification. Neurosci. Biobehav. Rev. 2016, 68, 282–297.
- Waisman, A.; Katz, J. The autobiographical memory system and chronic pain: A neurocognitive framework for the initiation and maintenance of chronic pain. Neurosci. Biobehav. Rev. 2024, 162, 105736.
- Moisset, X.; Lanteri-Minet, M.; Fontaine, D. Neurostimulation methods in the treatment of chronic pain. J. Neural Transm. 2020, 127, 673–686.
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53.
- Manda, O.; Hadjivassiliou, M.; Varrassi, G.; Zavridis, P.; Zis, P. Exploring the Role of the Cerebellum in Pain Perception: A Narrative Review. Pain Ther. 2025, 14, 803–816.
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151.
- Heinricher, M.M.; Tavares, I.; Leith, J.L.; Lumb, B.M. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009, 60, 214–225.
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474.
- Bannister, K.; Dickenson, A.H. The plasticity of descending controls in pain: Translational probing. J. Physiol. 2017, 595, 4159–4166.
- Kim, W.; Angulo, M.C. Unraveling the role of oligodendrocytes and myelin in pain. J. Neurochem. 2025, 169, e16206.
- Häuser, W.; Wolfe, F.; Tölle, T.; Uçeyler, N.; Sommer, C. The role of antidepressants in the management of fibromyalgia syndrome: A systematic review and meta-analysis. CNS Drugs 2012, 26, 297–307.
- Lunn, M.P.; Hughes, R.A.; Wiffen, P.J. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst. Rev. 2014, 2014, CD007115.
- Welsch, P.; Üçeyler, N.; Klose, P.; Walitt, B.; Häuser, W. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia. Cochrane Database Syst. Rev. 2018, 2, CD010292.
- Yarnitsky, D.; Granot, M.; Nahman-Averbuch, H.; Khamaisi, M.; Granovsky, Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012, 153, 1193–1198.
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of pain and its implications for therapeutic interventions. Signal Transduct. Target. Ther. 2024, 9, 155.
- Asimakopoulos, T.; Tsaroucha, A.; Kouri, M.; Pasqualucci, A.; Varrassi, G.; Leoni, M.L.G.; Rekatsina, M. The Role of Biomarkers in Acute Pain: A Narrative Review. Pain Ther. 2025, 14, 775–789.
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23.
- Pellesi, L.; Yangjeh, A.; Hajjaj, I.; Lababidi, M.; Sarwar, F.; Wang, W.; Martelletti, P. Neurotransmitter Imbalance in Tension-Type Headache: A Systematic Review of Mechanisms and Therapeutic Targets. Pain Ther. 2025, 14, 1279–1291.
- Stefani, L.C.; Leite, F.M.; da Graça L Tarragô, M.; Zanette, S.A.; de Souza, A.; Castro, S.M.; Caumo, W. BDNF and serum S100B levels according the spectrum of structural pathology in chronic pain patients. Neurosci. Lett. 2019, 706, 105–109.
- Ranzolin, A.; Duarte, A.L.; Bredemeier, M.; da Costa Neto, C.A.; Ascoli, B.M.; Wollenhaupt-Aguiar, B.; Kapczinski, F.; Xavier, R.M. Evaluation of cytokines, oxidative stress markers and brain-derived neurotrophic factor in patients with fibromyalgia—A controlled cross-sectional study. Cytokine 2016, 84, 25–28.
- Khan, N.; Smith, M.T. Neurotrophins and Neuropathic Pain: Role in Pathobiology. Molecules 2015, 20, 10657–10688.
- Merighi, A.; Salio, C.; Ghirri, A.; Lossi, L.; Ferrini, F.; Betelli, C.; Bardoni, R. BDNF as a pain modulator. Prog. Neurobiol. 2008, 85, 297–317.
- Merighi, A. Brain-Derived Neurotrophic Factor, Nociception, and Pain. Biomolecules 2024, 14, 539.
- Ma, J.J.; Zhang, T.Y.; Diao, X.T.; Yao, L.; Li, Y.X.; Suo, Z.W.; Yang, X.; Hu, X.D.; Liu, Y.N. BDNF modulated KCC2 ubiquitylation in spinal cord dorsal horn of mice. Eur. J. Pharmacol. 2021, 906, 174205.
- McDonough, K.E.; Hammond, R.; Wang, J.; Tierney, J.; Hankerd, K.; Chung, J.M.; La, J.H. Spinal GABAergic disinhibition allows microglial activation mediating the development of nociplastic pain in male mice. Brain Behav. Immun. 2023, 107, 215–224.
- Constandil, L.; Goich, M.; Hernández, A.; Bourgeais, L.; Cazorla, M.; Hamon, M.; Villanueva, L.; Pelissier, T. Cyclotraxin-B, a new TrkB antagonist, and glial blockade by propentofylline, equally prevent and reverse cold allodynia induced by BDNF or partial infraorbital nerve constriction in mice. J. Pain 2012, 13, 579–589.
- Mazzitelli, M.; Kiritoshi, T.; Presto, P.; Hurtado, Z.; Antenucci, N.; Ji, G.; Neugebauer, V. BDNF Signaling and Pain Modulation. Cells 2025, 14, 476.
- Jaffal, S.M. Neuroplasticity in chronic pain: Insights into diagnosis and treatment. Korean J. Pain 2025, 38, 89–102.
- Casey, C.S.; Pölkki, M.; Suvanen, E.K.; Iso-Mustajärvi, I.; Purmonen, T.; Peltonen, E.J.; Appel, C.K.; Patel, N.J.; Von Arx, L.B. A National Cross-Sectional Survey on Real-World Experiences of Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibody Use in Adults with Migraine in Finland. Pain Ther. 2025, 14, 1045–1061.
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142.
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19.
- Cai, W.; Khoutorsky, A. Revisiting the role of Substance P and CGRPα. eLife 2025, 14, e106766.
- Zieglgänsberger, W. Substance P and pain chronicity. Cell Tissue Res. 2019, 375, 227–241.
- Boyer, N.; Dallel, R.; Artola, A.; Monconduit, L. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain 2014, 155, 1196–1205.
- Lorincz, D.; Drury, H.R.; Lim, R.; Brichta, A.M. Immunohistochemical Identification of Sensory Neuropeptides Calcitonin Gene-Related Peptide, Substance P, and Pituitary Adenylate Cyclase-Activating Polypeptide in Efferent Vestibular Nucleus Neurons. Neuroendocrinology 2025, 115, 269–282.
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350.
- Iyengar, S.; Ossipov, M.H.; Johnson, K.W. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 2017, 158, 543–559.
- Marshall, A.; Elshafei, M.; Preston, F.G.; Burgess, J.; Goodson, N.; Fallon, N.; Frank, B.; Zhao, S.S.; Alam, U. Small Fibre Pathology in Fibromyalgia: A review. Pain Ther. 2025, 14, 461–478.
- Seng, E.; Lampl, C.; Viktrup, L.; Lenderking, W.R.; Karn, H.; Hoyt, M.; Kim, G.; Ruff, D.; Ossipov, M.H.; Vincent, M. Patients’ Experiences During the Long Journey Before Initiating Migraine Prevention with a Calcitonin Gene-Related Peptide (CGRP) Monoclonal Antibody (mAb). Pain Ther. 2024, 13, 1589–1615.
- Taylor, S.S.; Noor, N.; Urits, I.; Paladini, A.; Sadhu, M.S.; Gibb, C.; Carlson, T.; Myrcik, D.; Varrassi, G.; Viswanath, O. Complex Regional Pain Syndrome: A Comprehensive Review. Pain Ther. 2021, 10, 875–892.
- Christiansen, I.M.; Reducha, P.V.; Edvinsson, L.; Holm, A.; Haanes, K.A. Ex vivo stimulation of the trigeminal nucleus caudalis induces peripheral CGRP release in the trigeminal ganglion and reveals a distinct dopamine-endocannabinoid mechanism relevant to migraine. J. Headache Pain 2025, 26, 141.
- Goadsby, P.J.; Reuter, U.; Hallström, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R.A. A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 2017, 377, 2123–2132.
- Silberstein, S.D.; Dodick, D.W.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 2017, 377, 2113–2122.
- Dodick, D.W.; Ashina, M.; Brandes, J.L.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; Lenz, R.A. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018, 38, 1026–1037.
- Yamamoto, S.; Fang, J.; Eter, A.; Liu, G.; Nguyen, A.; Chung, J.M.; La, J.H. The Role of NMDA and NK1 Receptor Signaling in Spine Surgery-induced Central Sensitization. Spine 2025, 50, 1120–1126.
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiologic Approach to Pain Therapy for Complex Pain Entities: A Narrative Review. Pain Ther. 2020, 9, 7–21.
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152.
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311.
- Wang, Y.; Leak, R.K.; Cao, G. Microglia-mediated neuroinflammation and neuroplasticity after stroke. Front. Cell Neurosci. 2022, 16, 980722.
- Yuan, Y.; Liu, H.; Dai, Z.; He, C.; Qin, S.; Su, Z. From Physiology to Pathology of Astrocytes: Highlighting Their Potential as Therapeutic Targets for CNS Injury. Neurosci. Bull. 2025, 41, 131–154.
- Liu, Y.; Cai, X.; Shi, B.; Mo, Y.; Zhang, J.; Luo, W.; Yu, B.; Li, X. Mechanisms and Therapeutic Prospects of Microglia-Astrocyte Interactions in Neuropathic Pain Following Spinal Cord Injury. Mol. Neurobiol. 2025, 62, 4654–4676.
- Kumar, S.; Sharma, V.; Yadav, S. TLR4 Targeting: A Promising Therapeutic Approach Across Multiple Human Diseases. Curr. Protein Pept. Sci. 2025, 26, 241–258.
- Tewari, M.; Michalski, S.; Egan, T.M. Modulation of Microglial Function by ATP-Gated P2X7 Receptors: Studies in Rat, Mice and Human. Cells 2024, 13, 161.
- Haidar, M.A.; Ibeh, S.; Shakkour, Z.; Reslan, M.A.; Nwaiwu, J.; Moqidem, Y.A.; Sader, G.; Nickles, R.G.; Babale, I.; Jaffa, A.A.; et al. Crosstalk between Microglia and Neurons in Neurotrauma: An Overview of the Underlying Mechanisms. Curr. Neuropharmacol. 2022, 20, 2050–2065.
- Mogil, J.S.; Parisien, M.; Esfahani, S.J.; Diatchenko, L. Sex differences in mechanisms of pain hypersensitivity. Neurosci. Biobehav. Rev. 2024, 163, 105749.
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083.
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58.
- Lisse, T.S.; Thiele, F.; Fuchs, H.; Hans, W.; Przemeck, G.K.H.; Abe, K.; Rathkolb, B.; Quintanilla-Martinez, L.; Hoelzlwimmer, G.; Helfrich, M.; et al. ER stress-mediated apoptosis in a new mouse model for osteogenesis imperfecta. PLoS Genet. 2008, 4, e7.
- Greenspan, J.D.; Craft, R.M.; LeResche, L.; Arendt-Nielsen, L.; Berkley, K.J.; Fillingim, R.B.; Gold, M.S.; Holdcroft, A.; Lautenbacher, S.; Mayer, E.A.; et al. Studying sex and gender differences in pain and analgesia: A consensus report. Pain 2007, 132 (Suppl. S1), S26–S45.
- Martin, V.T.; Behbehani, M. Ovarian hormones and migraine headache: Understanding mechanisms and pathogenesis. Headache 2006, 46, 365–386.
- Krsek, A.; Ostojic, L.; Zivalj, D.; Baticic, L. Navigating the Neuroimmunomodulation Frontier: Pioneering Approaches and Promising Horizons-A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 9695.
- Stolfi, F.; Abreu, H.; Sinella, R.; Nembrini, S.; Centonze, S.; Landra, V.; Brasso, C.; Cappellano, G.; Rocca, P.; Chiocchetti, A. Omics approaches open new horizons in major depressive disorder: From biomarkers to precision medicine. Front. Psychiatry 2024, 15, 1422939.
- Xiong, W.; Liu, Y.; Ge, X.; Wang, J.; Wang, Z. Transcriptome Analysis of Non-coding RNAs and mRNAs in the Dorsal Root Ganglion of Peripheral Nerve Injury-Induced Neuropathic Pain. Biochem. Genet. 2025, 1–21.
- Sannes, A.C.; Ghani, U.; Niazi, I.K.; Moberget, T.; Jonassen, R.; Haavik, H.; Gjerstad, J. Investigating Whether a Combination of Electro-Encephalography and Gene Expression Profiling Can Predict the Risk of Chronic Pain: A Protocol for an Observational Prospective Cohort Study. Brain Sci. 2024, 14, 641.
- Jiang, B.C.; Liu, T.; Gao, Y.J. Chemokines in chronic pain: Cellular and molecular mechanisms and therapeutic potential. Pharmacol. Ther. 2020, 212, 107581.
- Xie, K.; Cheng, X.; Zhu, T.; Zhang, D. Single-cell transcriptomic profiling of dorsal root ganglion: An overview. Front. Neuroanat. 2023, 17, 1162049.
- Usoskin, D.; Furlan, A.; Islam, S.; Abdo, H.; Lönnerberg, P.; Lou, D.; Hjerling-Leffler, J.; Haeggström, J.; Kharchenko, O.; Kharchenko, P.V.; et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015, 18, 145–153.
- Renthal, W.; Tochitsky, I.; Yang, L.; Cheng, Y.C.; Li, E.; Kawaguchi, R.; Geschwind, D.H.; Woolf, C.J. Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron 2020, 108, 128–144.e9.
- Denk, F.; McMahon, S.B. Chronic pain: Emerging evidence for the involvement of epigenetics. Neuron 2012, 73, 435–444.
- Kim, D.; Chae, Y.; Park, H.J.; Lee, I.S. Effects of Chronic Pain Treatment on Altered Functional and Metabolic Activities in the Brain: A Systematic Review and Meta-Analysis of Functional Neuroimaging Studies. Front. Neurosci. 2021, 15, 684926.
- Napadow, V.; LaCount, L.; Park, K.; As-Sanie, S.; Clauw, D.J.; Harris, R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheumatol. 2010, 62, 2545–2555.
- Ceko, M.; Bushnell, M.C.; Gracely, R.H. Neurobiology underlying fibromyalgia symptoms. Pain Res. Treat. 2012, 2012, 585419.
- Albrecht, D.S.; Forsberg, A.; Sandström, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Höglund, C.O.; Catana, C.; et al. Brain glial activation in fibromyalgia—A multi-site positron emission tomography investigation. Brain Behav. Immun. 2019, 75, 72–83.
- Alshelh, Z.; Brusaferri, L.; Morrissey, E.J.; Torrado-Carvajal, A.; Kim, M.; Akeju, O.; Grmek, G.; Chane, C.; Murphy, J.; Schrepf, A.; et al. Brain inflammation and its predictive value for post-operative pain in total knee arthroplasty patients. Brain Behav. Immun. 2025, 128, 703–712.
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294.
- Hill, R. NK1 (substance P) receptor antagonists--why are they not analgesic in humans? Trends Pharmacol. Sci. 2000, 21, 244–246.
- Rolan, P.E. The contribution of clinical pharmacology to the development of analgesic drugs. Br. J. Clin. Pharmacol. 2018, 84, 1394–1413.
- Sorge, R.E.; Totsch, S.K. Sex differences in pain. J. Neurosci. Res. 2017, 95, 1271–1281.
- Skolnick, P. The challenges of animal models in conscious drug design. Nat. Rev. Drug Discov. 2018, 17, 467–468.
- Rice, A.S.; Cimino-Brown, D.; Eisenach, J.C.; Kontinen, V.K.; Lacroix-Fralish, M.L.; Machin IMogil, J.S.; Stöhr, T.; on behalf of the Preclinical Pain Consortium. Animal models and the prediction of efficacy in clinical trials of analgesic drugs. Pain 2008, 139, 243–247.
- Edvinsson, L.; Warfvinge, K. Recognizing the role of CGRP and CGRP receptors in migraine and its treatment. Cephalalgia 2019, 39, 366–373.
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics. Nat. Rev. Drug Discov. 2020, 19, 753–769.
- LaCroix-Fralish, M.L.; Austin, J.S.; Zheng, F.Y.; Levitin, D.J.; Mogil, J.S. Patterns of pain: Meta-analysis of microarray studies of pain. Pain 2011, 152, 1888–1898.
- Giglio, M.; Corriero, A.; Preziosa, A.; Varrassi, G.; Puntillo, F. The putative role of immune-inflammatory mechanisms in nociplastic pain pathways: A narrative review. Explor. Immunol. 2025, 5, 1003178.




