
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | María Begoña Coco Martín | + 4182 word(s) | 4182 | 2020-12-17 07:27:00 | | | |
| 2 | Lily Guo | -68 word(s) | 4114 | 2020-12-30 01:47:59 | | | | |
| 3 | Lily Guo | Meta information modification | 4114 | 2021-01-04 09:05:14 | | |
Video Upload Options
Active vision therapy using perceptual learning and/or dichoptic or binocular environments has shown its potential effectiveness in amblyopia, but some doubts remain about the type of stimuli and the mode and sequence of presentation that should be used.
1. Introduction
Amblyopia is a visual developmental disorder consisting of a reduced best-corrected visual acuity in one or, rarely, both eyes without the presence of any ocular pathology. Etiology is often a high refractive error, anisometropia, strabismus, or a combination of these factors. Less common is deprivation amblyopia, which is caused by a pathology that avoids the eye stimulation during childhood and causes a severe visual deficit[1][2]. The prevalence of amblyopia in childhood is approximately between 1 and 3%, although these values differ among authors[3][4]. In amblyopia, the loss of visual acuity and the presence of many other monocular and binocular visual deficits are the consequence of the anomalies in the visual pathway of the amblyopes, mainly in the striate and extra-striate cortex[5]. Amblyopic eyes showed worse neural adaptation in V1, V2, V3, V3a, Vp and V4, that is, a reduced cortical automation after a repeated visual task compared with fellow eyes, and a decreased neural response assessed by functional magnetic resonance imaging (fMRI)[6] as well as a worse effective connectivity between the implied brain regions, which is correlated with the reduced visual acuity[7].
Over the last few years, vision therapy has been suggested to be an effective option to promote visual rehabilitation and even to accelerate recovery when combined with patching[8]. New trends in computer-based active vision therapy have been developed for amblyopia treatment, such as the use of perceptual learning environments[9][10][11], dichoptic stimulation[12][13][14] and binocular training[15][16]. These video games use visual and perceptual tasks, such as orientation discrimination or letter recognition, among others, to cause a response in neuro-modulatory pathways and the enhancement of attentional skills, according to neurophysiological studies[17][18]. These options may support and optimize treatments with spectacles, patching and penalization[19][20].
One of the critical issues when active vision therapy is programmed using perceptual learning and/or a dichoptic or binocular environment is the type of stimuli and the mode and sequence of presentation that should be used. Additionally, depending on the treatment phase, stimuli and environments are specifically selected for promoting visual deficit recovery in a monocular phase, or improving interocular fusion and stereopsis in the binocular phase. Different approaches are commercially available in the form of adaptative software to perform the training at home with online control of the compliance by the practitioner. However, more research is needed on the adequacy of the visual stimuli used for visual training and which psychophysical method would be the most appropriate for the presentation of such stimuli. The aim of the current review article is to gather worthy information on all the main characteristics of the stimulus and the psychophysical method that should be used in amblyopia, depending on the baseline characteristics of the patient, to provide researchers and clinicians a useful guideline for optimizing active vision therapy programs in amblyopia.
2. Related Studies in Stimuli Characteristics and Psychophysical Requirements for Visual Training in Amblyopia
One hundred and forty-three articles were obtained in the first search for documentary support, but only those which met the inclusion criteria were selected. Then, a manual search was performed based on the bibliography of the articles selected. The results were organized in structured sections for a better understanding of the concepts described and an appropriate follow-up of the reader.
2.1. Visual Deficits in Amblyopia
For an appropriate treatment, the stimuli used for visual training should be scientific-based and adapted to the specific characteristics of the amblyopic visual system. Thus, a good comprehension of the neural mechanisms and visual deficits of amblyopia is needed.
2.1.1. Crowding, Flankers and Masking
Crowding is one of the most important visual deficits in amblyopia and is described by Levi as a “deleterious influence of nearby contours of visual discrimination, is a form of inhibitory interaction which is ubiquitous in spatial vision” [21]. Crowding is also present in the normal population when the target is surrounded by other stimuli called flankers, with the consequent impairment of the recognition of an object in a clutter[22]. In the peripheral vision of non-amblyope subjects, a letter becomes unrecognizable when there are other stimuli around it, despite both target and flankers being clearly separated in the retinal point spread function (PSF), this being dependent on target eccentricity[23][24]. In addition, in the foveal vision of the non-amblyope subject, crowding can occur when the target–flankers distance is less than 4–6 min of arc[22][25][26]. In amblyopes, crowding occurs with larger distances between targets and flankers, and is related to spatial frequency and target size[27].
Crowding affects many visual tasks such as letter recognition[22][23][24], orientation discrimination[28][29], vernier visual acuity[30] and stereoacuity tasks[31]. Furthermore, crowding seems to be the result of a global visual deficit in amblyopia[21][32][33], although the values of visual acuity (VA), contrast sensitivity and stereoacuity may not have a large impact on it[27].
Flankers are the objects located around the target that impair the target perception due to crowding. In strabismic and mixed amblyopia, which present both anisometropia and strabismus, flankers reduce VA[34]. According to previous authors, when the flankers are complex stimuli, such as letters, the correct term is crowding, but when flankers are sidebars, the term contour interaction should be used[22].
Finally, masking is the impairment of target perception due to an overlapped element called a mask. For people with amblyopia, obtaining the information of the target from an array of stimuli is harder than it is for normal subjects. Repeated practice of a masking task leads to improvements in target detection time in non-amblyopic subjects[35], as well as in crowded and uncrowded visual acuity in amblyopes[26]. Therefore, adding masking tasks to vision therapy might be interesting for future research.
From a neuronal perspective, cortical neuron insufficiency, elevated cortical noise, and abnormal lateral interactions in V1 seem to be related with spatial processing deficits in amblyopic eyes[33].
2.1.2. Contrast Sensitivity Reduction
Contrast sensitivity is the ability to detect differences in contrast luminance at different spatial frequencies of a grating. Amblyopic eyes can present decreased contrast sensitivity at high spatial frequencies or even at all the spatial frequencies, which is the consequence of alterations in the lateral geniculate nucleus and visual striate cortex[36]. Specifically, there are some differences according to its etiology. In anisometropic amblyopia, a significant decrease in global contrast sensitivity compared with normal subjects is commonly reported, while strabismic amblyopes can show normal or increased low frequency contrast sensitivity[37]. It is interesting that a loss in contrast sensitivity could persist despite the recovery of the visual acuity after amblyopia treatment. However, several authors reported improvements in contrast sensitivity after vision training with perceptual learning in amblyopic subjects [11][38][39]. Thus, amblyopia management should involve contrast sensitivity tasks for a global approach[38], with the aim of obtaining similar values in both eyes and facilitating binocular vision.
2.1.3. Stereopsis Impairment
Stereopsis or stereoacuity is the perception of three-dimensionality because of the cortical combination between the images from each eye. For obtaining correct stereoscopic perception, both eyes should have an adequate and similar visual acuity and contrast sensitivity in the presence of ocular alignment [40]. Therefore, stereopsis is one of the main features that should be assessed in the clinical management of amblyopia, since it usually is decreased or absent due to the differences in the perceived images between the amblyopic and the fellow eye. In anisometropic amblyopia, stereopsis is reduced, although it depends on the degree of anisometropia and the loss of visual acuity in the amblyopic eye. In strabismic or mixed amblyopia with constant deviation greater than 12 prism diopters, subjects are stereoblind due to the lack of bifoveal fixation[41]. Stereopsis is important for appropriate performance in activities such as driving, sports or hand-to-eye coordination tasks, although further research is needed to understand more about how different values of stereopsis interfere with daily activities[16]. In accordance with the aim of this review, it is relevant to note that during the treatment of amblyopia, stereopsis should be assessed and trained, since some authors suggested that vision therapy can also improve stereoacuity[16][42][43][44]. Thus, designing exercises with specific stimuli for stereopsis training assumes great significance as part of the treatment in amblyopic subjects.
Recent evidence from functional magnetic resonance imaging (fMRI) studies on the cortical processing of vision in amblyopia shows that, during amblyopic eye stimulation, not only do primary and secondary visual areas present reduced activation when compared to fellow-fixing eye stimulation, but so too do higher-level visual areas, such as the parieto-occipital and temporal cortex. These findings could explain binocular vision deficits in amblyopic patients [45].
2.2. Visual Stimuli Used for Vision Training in Amblyopia
There are many types of stimuli which are used in vision training for amblyopia. For example, video games or films are usually used in published studies, commonly in dichoptic training. Despite the fact that they seem to be effective, they also have some features which could be a limitation. Some of these treatments are based on dichoptic training or monocular stimulation, and use eye–hand coordination games [12,14,46] and popular films [47] which are not specifically designed for treating amblyopia, or do not adjust the difficulty of the training to the patient’s progress. However, there are four types of stimuli which have shown to be effective in clinical research that can be easily added to the amblyopia training software, with the possibility of modification according to the patient’s evolution, and these are letter optotypes [48,49], Gabor’s patches [9,48,49,50,51,52], Vernier’s stimuli [53] and random-dot stereograms [16,54] (Table 1).
| Authors | Design | N | Type of Amblyopia | Enviroment | Stimulus | Population | Main Outcome |
|---|---|---|---|---|---|---|---|
| Jia et al., 2018 | NRSI | 19 | Aniso | Perceptual learning | Gabor’s patch | Young adult | AUCSF improved from 8.41 ± 1.09 to 15.48 ± 1.61 VA increased about 2 lines |
| Liu et al., 2018 | NRSI | 13 | 9 aniso, 1 strab, 3 mixed | DT | Gabor’s patch | Young adults | Stereopsis improved 26.5% ± 6.9% |
| Avram et al., 2013 | Case series | 5 | Aniso | PL | Letter optotypes | Children | Significant improvement of VA and CS after training |
| Chen et al., 2016 | NRSI | 13 | Aniso | PL | Gabor’s patch | Teenagers and young adults | VA increased 1.64 ± 0.06 lines CSF improved significantly |
| Gambacorta et al., 2018 | NRSI | 29 | Aniso | PL and DT | Gabor’s patch | Teenagers | VA improved 0.1 ± 0.03 LogMAR after 10 h of training |
| Zhang et al., 2013 | Retrospective | 341 | Aniso, strab, ametropic and mixed | PL | Gabor’s patch Letter optotype |
Children | Improvement in VA with PL was similar to with patching |
| Levi et al., 1997 | NRSI | 11 | 4 aniso, 4 strab, 3 mixed | PL | Vernier’s stimulus | Adults | Improvement in Vernier acuity |
| Portela-Camino et al., 2018 | RCT | 32 | 2 aniso, 18 strab, 10 mixed, 2 isoametropic | PL | RDS | Children | Stereopsis increased about 50% with RPST and 46.42% with Wirt circles |
| Martín-González et al., 2020 | NRSI | 16 | 2 aniso, 8 strab, 4 mix, 2 isoametropic | PL | RDS | Children | Stereopsis experienced a significant improvement |
2.2.1. Letter Optotypes
As aforementioned, letter recognition is affected by crowding and interaction contours in amblyopia [55], but its use in visual training using perceptual learning approaches can be useful, since many of the parameters of these optotypes can be modified, such as size, contrast sensitivity, presence or absence of flankers, orientation, or motion (Figure 1). For this reason, visual training based on letter optotypes has been used in several scientific articles [56,57].

2.2.2. Gabor’s Patches
These are sinusoidal gratings with a Gaussian envelope, which also are commonly used in amblyopia studies, since experimental research demonstrated that gratings with a neutral background can cause selective cortical responses for orientation and contrast, which additionally correlates with fMRI findings (Figure 2) [58,59]. Amblyopia affects high spatial frequency, contrast sensitivity and orientation discrimination, among others visual deficits. Therefore, Gabor´s patches also can be used in perceptual learning, since they allow clinicians to apply many visual tasks, adapting contrast, spatial frequency, size, and other parameters according to each individual case [9,48,49,50,51,52,60].

2.2.3. Random-Dot Stereograms
This stimulus consists of an array of dots randomly presented to the subjects for specific stereoscopic perception stimulation (Figure 3). Usually, random-dot stimuli are used for stereoacuity assessment in clinical practice, but recent published works showed their application also in improving stereopsis values in amblyopia [16,54]. The use of a random-dot stereogram entails a cortical activation in the early visual cortex, particularly with mixed-polarity stimulus [61], and a small but significant activation of the pupil and accommodation responses [62]. Additionally, the optimal size of the dots should be considered for well-designed random-dot stimuli, since the literature suggests that larger dots implicate better stereo-perception [63].
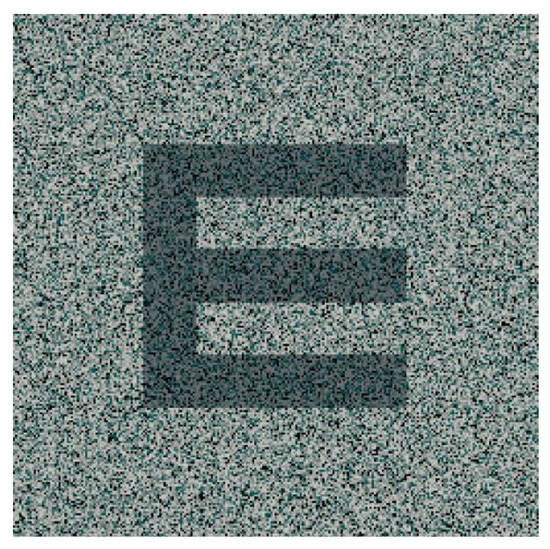
Figure 3. Example of a random-dot stimulus. The dark “E” represents the section of the image that is perceived in depth when seen by subjects with an appropriate binocular vision.
2.2.4. Vernier’s Stimulus
3.3. Parameters That Can Be Modified in the Visual Stimuli
3.3.1. Spatial–Temporal Frequency
3.3.2. Contrast Sensitivity and Luminance
3.3.3. First- and Second-Order Stimulus
2.3.4. Color and Achromatic Stimuli
2.3.5. Figure–Ground Segregation
3.3.6. Signal–Noise
2.3.7. Steady and Dynamic Stimulus
References
- Barrett, B.T.; Bradley, A.; McGraw, P.V. Understanding the neural basis of amblyopia. Neuroscientist 2004, 10, 106–117.
- Webber, A.L.; Wood, J. Amblyopia: Prevalence, natural history, functional effects and treatment. Clin. Exp. Optom. 2005, 88, 365–375.
- Carlton, J.; Karnon, J.; Czoski-Murray, C.; Smith, K.J.; Marr, J. The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: A systematic review and economic evaluation. Health Technol. Assess. 2008, 12, 194.
- Powell, C.; Hatt, S.R. Vision screening for amblyopia in childhood. Cochrane Database Syst. Rev. 2009, 3, CD005020.
- Körtvélyes, J.; Bankó, E.M.; Andics, A.; Rudas, G.; Németh, J.; Hermann, P.; Vidnyánszky, Z. Visual cortical responses to the input from the amblyopic eye are suppressed during binocular viewing. Acta Biol. Hung. 2012, 63, 65–79.
- Li, X.; Coyle, D.; Maguire, L.; McGinnity, T.M.; Hess, R.F. Long timescale fMRI neuronal adaptation effects in human amblyopic cortex. PLoS ONE 2011, 6, e26562.
- Li, X.; Mullen, K.T.; Thompson, B.; Hess, R.F. Effective connectivity anomalies in human amblyopia. Neuroimage 2011, 54, 505–516.
- Hernández-Rodríguez, C.J.; Piñero, D.P. Active vision therapy for anisometropic amblyopia in children: A systematic review. J. Ophthalmol. 2020, 2020, 9.
- Gambacorta, C.; Nahum, M.; Vedamurthy, I.; Bayliss, J.; Jordan, J.; Bavelier, D.; Levi, D.M. An action video game for the treatment of amblyopia in children : A feasibility study. Vis. Res. 2018, 148, 1–14.
- Deshpande, P.G.; Bhalchandra, P.C.; Nalgirkar, A.R.; Tathe, S.R. Improvement of visual acuity in residual meridional amblyopia by astigmatic axis video games. Indian J. Ophthalmol. 2018, 66, 1156–1160.
- Barollo, M.; Contemori, G.; Battaglini, L.; Pavan, A.; Casco, C. Perceptual learning improves contrast sensitivity, visual acuity, and foveal crowding in amblyopia. Restor. Neurol. Neurosci. 2017, 35, 483–496.
- Iwata, Y.; Handa, T.; Ishikawa, H.; Goseki, T.; Shoji, N. Evaluation of the effects of the Occlu-Pad for the management of anisometropic amblyopia in children. Curr. Eye Res. 2018, 43, 785–787.
- Totsuka, S.; Handa, T.; Ishikawa, H.; Shoji, N. Improvement of Adherence with Occlu-Pad Therapy for Pediatric Patients with Amblyopia. Biomed. Res. Int. 2018, 2018, 2394562.
- Iwata, Y.; Handa, T.; Ishikawa, H.; Goseki, T.; Shoji, N. Comparison between amblyopia treatment with glasses only and combination of glasses and open-type Binocular “Occlu-Pad” device. Biomed. Res. Int. 2018, 2018, 2459696.
- Birch, E.E.; Li, S.L.; Jost, R.M.; Morale, S.E.; De La Cruz, A.; Stager, D.J.; Dao, L.; Stager, D.R.S. Binocular iPad treatment for amblyopia in preschool children. J. AAPOS 2015, 19, 6–11.
- Portela-Camino, J.A.; Martín-González, S.; Ruiz-Alcocer, J.; Illarramendi-Mendicute, I.; Garrido-Mercado, R. A random dot computer video game improves stereopsis. Optom. Vis. Sci. 2018, 95, 523–535.
- Coco-Martin, M.B.; Valenzuela, P.L.; Maldonado-López, M.J.; Santos-Lozano, A.; Molina-Martín, A.; Piñero, D.P. Potential of video games for the promotion of neuroadaptation to multifocal intraocular lenses: A narrative review. Int. J. Ophthalmol. 2019, 12, 1782–1787.
- Dye, M.W.G.; Green, C.S.; Bavelier, D. The development of attention skills in action video game players. Neuropsychologia 2009, 47, 1780–1789.
- Kraus, C.L.; Culican, S.M. New advances in amblyopia therapy I : Binocular therapies and pharmacologic augmentation. Br. J. Ophthalmol. 2018, 102, 1492–1496.
- Foss, A.J.E. Use of video games for the treatment of amblyopia. Curr. Opin. Ophthalmol. 2017, 28, 276–281.
- Levi, D.M. Crowding-An essential bottleneck for object recognition: A mini-review. Vis. Res. 2008, 48, 635–654.
- Flom, M.C.; Weymouth, F.W.; Kahneman, D. Visual Resolution and Contour Interaction. J. Opt. Soc. Am. 1963, 53, 1026–1032.
- Bouma, H. Interaction Effects in Parafoveal Letter Recognition. Nature 1970, 226, 177–178.
- Kooi, F.L.; Toet, A.; Tripathy, S.P.; Levi, D.M. The effect of similarity and duration on spatial interaction in peripheral vision. Spat. Vis. 1994, 8, 255–279.
- Liu, L.; Arditi, A. Apparent string shortening concomitant with letter crowding. Vis. Res. 2000, 40, 1059–1067.
- Yehezkel, O.; Sterkin, A.; Lev, M.; Polat, U. Training on spatiotemporal masking improves crowded and uncrowded visual acuity. J. Vis. 2015, 15, 12.
- Levi, D.M.; Yu, C.; Kuai, S.G.; Rislove, E. Global contour processing in amblyopia. Vis. Res. 2007, 47, 512–524.
- Andriessen, J.J.; Bouma, H. Eccentric vision: Adverse interactions between line segments. Vis. Res. 1976, 16, 71–78.
- Westheimer, G.; Shimamura, K.; McKee, S.P. Interference with line-orientation sensitivity. J. Opt. Soc. Am. 1976, 66, 332–338.
- Westheimer, G.; Hauske, G. Temporal and spatial interference with vernier acuity. Vis. Res. 1975, 15, 1137–1141.
- Butler, T.W.; Westheimer, G. Interference with stereoscopic acuity: Spatial, temporal, and disparity tuning. Vis. Res. 1978, 18, 1387–1392.
- Webber, A. The functional impact of amblyopia. Clin. Exp. Optom. 2018, 101, 1–8.
- Hamm, L.M.; Black, J.; Dai, S.; Thompson, B. Global processing in amblyopia: A review. Front. Psychol. 2014, 5, 583.
- Norgett, Y.; Siderov, J. Effect of stimulus configuration on crowding in strabismic amblyopia. J. Vis. 2017, 17, 5.
- Sterkin, A.; Yehezkel, O. Learning to be fast: Gain accuracy with speed. Vis. Res. 2012, 61, 115–124.
- Meier, K.; Giaschi, D. Unilateral amblyopia affects two eyes: Fellow eye deficits in amblyopia. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1779–1800.
- McKee, S.P.; Levi, D.M.; Movshon, J.A. The pattern of visual deficits in amblyopia. J. Vis. 2003, 3, 380–405.
- Wang, G.; Zhao, C.; Ding, Q.; Wang, P. An assessment of the contrast sensitivity in patients with ametropic and anisometropic amblyopia in achieving the corrected visual acuity of 1.0. Sci. Rep. 2017, 7, 42043.
- Zhou, Y.; Huang, C.; Xu, P.; Tao, L.; Qiu, Z.; Li, X.; Lu, Z.L. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vis. Res. 2006, 46, 739–750.
- Legge, G.E.; Gu, Y.C. Stereopsis and contrast. Vis. Res. 1989, 29, 989–1004.
- Levi, D.; Knill, D.C.; Bavelier, D. Stereopsis and amblyopia: A mini-review. Vis. Res. 2015, 114, 17–30.
- Liu, X.Y.; Zhang, J.Y. Dichoptic De-Masking Learning in Adults With Amblyopia and Its Mechanisms. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2968–2977.
- Kelly, K.R.; Jost, R.M.; Wang, Y.Z.; Dao, L.; Beauchamp, C.L.; Leffler, J.N.; Birch, E.E. Improved binocular outcomes following binocular treatment for childhood amblyopia. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1221–1228.
- Ziak, P.; Holm, A.; Halicka, J.; Mojzis, P.; Piñero, D.P. Amblyopia treatment of adults with dichoptic training using the virtual reality oculus rift head mounted display: Preliminary results. BMC Ophthalmol. 2017, 17, 105.




