
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Phaedra Eleftheriou | -- | 2361 | 2025-09-07 21:15:05 | | | |
| 2 | Phaedra Eleftheriou | + 143 word(s) | 2504 | 2025-09-08 01:46:15 | | | | |
| 3 | Catherine Yang | -1346 word(s) | 1158 | 2025-09-08 03:51:20 | | |
Video Upload Options
A brief review on the Diseases related to increased Antibodies against Food-Derived antigens. (Derived from the article "Increased Concentration of Anti-Egg Albumin Antibodies in Cerebrospinal Fluid and Serum of Patients with Alzheimer’s Disease—Discussion on Human Serpins’ Similarity and Probable Involvement in the Disease Mechanism" ).
Numerous studies have suggested the involvement of antibodies against food derived antigens in the development and progression of several diseases among which, bowel [1][2], auto-immune [3] and degenerative diseases [4]. Migraine [5], other neurological diseases [6][7][8], and mental disorders [9][10][11] have also been corelated with food-derived antigens.
Normally, IgA and IgM antibodies against food antigens may be detected in healthy people because of oral tolerance, a process related to the mucosal immune system’s ability to differentiate between pathogenic and non-pathogenic antigens, mostly described as immunological ignorance. However, dysregulation of this process may lead to increased concentrations of IgG as well as IgM food antigen-specific antibodies in the serum of certain individuals [12][13]. Cross-reaction of these antibodies with human proteins or peptides with increased sequence/structural identity or similarity with the food antigens (molecular mimicry) may be involved in the development or progression of the related diseases [6][14][15].
In general, when conditions like leaky intestine exist, food antigens can cause disorders through at least three different ways, all based on similarity of the molecule with human proteins: a) Production of antibodies which may cross-react with the human proteins usually diminishing the concentration of these proteins, disrupting the mechanism served by them and triggering inflammatory responses [16], b) Direct involvement in a mechanism, since molecular mimicry may enable appropriate interactions [17] and c) Interaction with the target molecule of the homologue protein in an unsuccessful manner preventing the successful interaction of the homologue protein.
Although humans consume a great variety of foods with thousands of proteins and other ingredients, a small number of foods and dietary components have been correlated with diseases. Among the most common food ingredients which are related to diseases (Figure 1) is gliadin, a component of gluten proteins of wheat products, mostly related to intestinal disorders [1][2]. Gluten implication in other diseases such as multiple sclerosis (MS) [7][8], autism [18] and schizophrenia [19] has also been mentioned. However, it is not clear if there is a straight correlation, or the effect concerns the leaking of other factors to the serum, because of the increased intestinal permeability caused by the reaction to gluten. Casein, the main milk protein, has also been related to MS, depression, and bipolar disorder, connecting neurological and mental disorders with dairy food consumption [8][10][12]. Mixed antibodies against egg and bovine antigens have displayed specificity toward human antigens, among which proteins related to Alzheimer’s Disease [12]. Investigating cross-reaction of antibodies to specific food antigens with various purified human tissue antigens indicated a positive reaction between antibodies against egg, milk, wheat and corn with myelin basic protein (MBP) or Aβ42 peptide, both related to amyloid plaque formation [12]. Neu5Gc, a sialic acid present in animals but not in humans, represents another kind of molecules related to immunologic response. It can be incorporated into human glycoproteins triggering immunologic response in some individuals and has been correlated with autoimmune diseases, such as Hashimoto’s Thyroiditis and cancer [3][20].
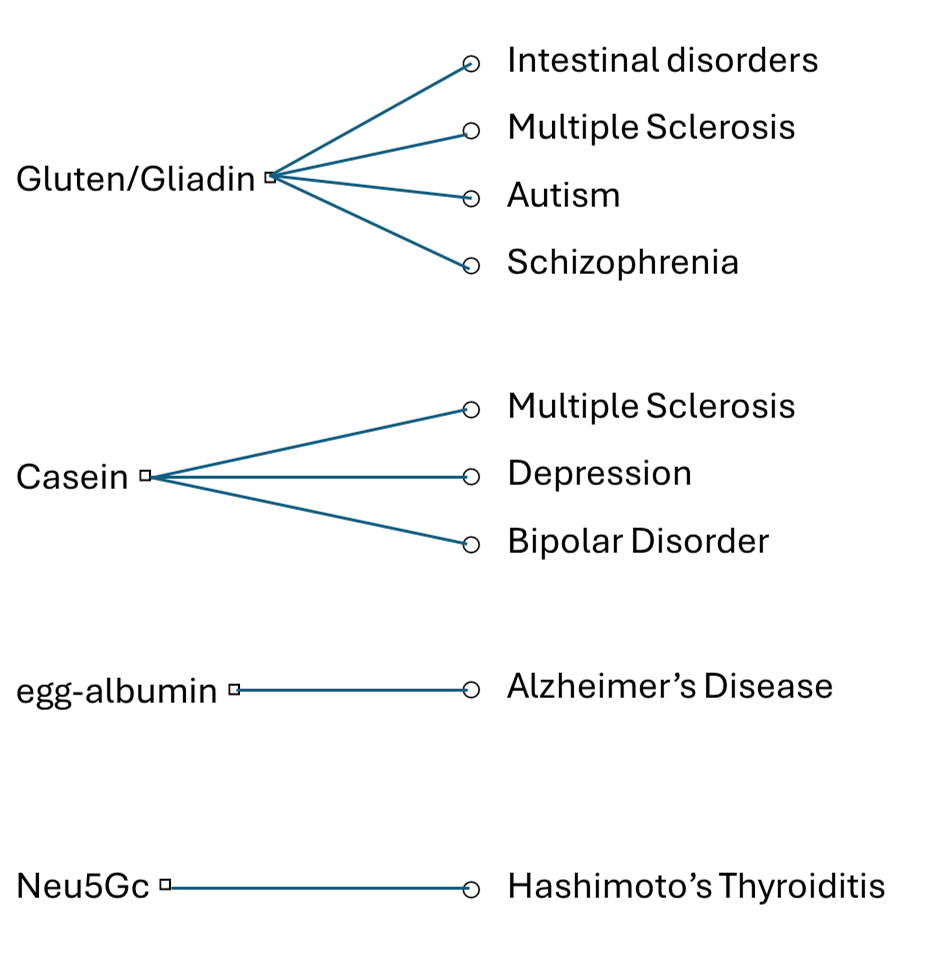
Figure 1. The most common food incedients related to diseases
Specific dietary recommendations, based on the food-antigen specific antibodies relieved the symptoms of the diseases in some cases [8][21][22][23][24][25][26]. However, antibodies against specific food antigens are usually present only in a subgroup of patients in all related diseases, suggesting that such immune responses represent one of multiple contributing factors in disease onset or progression.
In the recent study of Amanatidou D., Eleftheriou P. et al [27], antibodies against egg-albumin, bovine-casein and N-Glycolyl-Neuraminic acid (Neu5Gc) were measured in the cerebrospinal fluid (CSF) and serum of the patients (Figure 2A) using enzyme-linked immunosorbent assay (ELISA). Zero anti-Neu5Gc and low concentration of anti-casein antibodies were detected. However, increased anti-native egg-albumin antibodies were present in the serum of patients of all stages with 65% positivity (p<0.001) in mild disease and a higher percentage in females (81.9%, p<0.001). Lower serum positivity to anti-denatured egg albumin antibodies was observed showing gradual increase with severity and higher prevalence also in females. In the CSF, anti-native and anti-denatured egg-albumin antibodies were mainly observed in severely ill patients with accumulative positivity to either antigen reaching 61.8% in severe vs 15% in mild disease (p<0.001). Increased values were mainly observed in males (Figure 2B) [27].
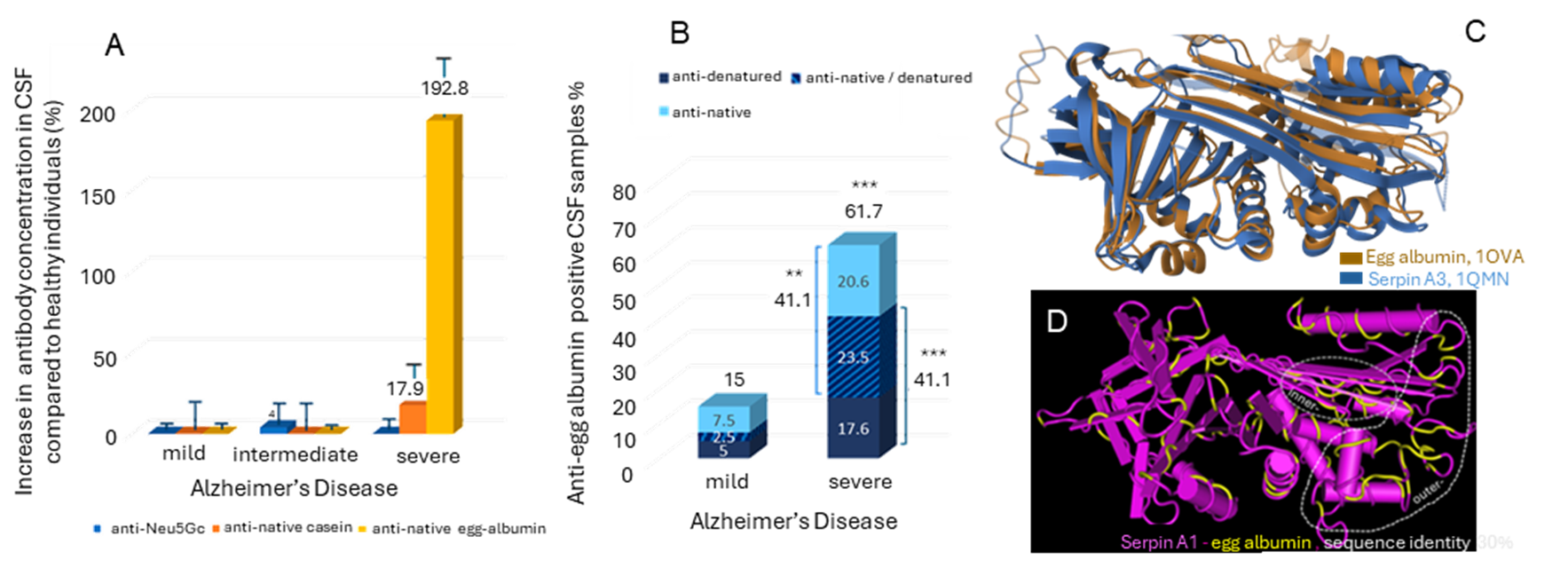
Figure 2. A. Increase in anti-Neu5Gc, anti-native casein and anti-native egg-albumin antibody concentration in the CSF of AD patients with mild, intermediate and severe disease compared to healthy individuals (%). B. Percentage of CSF samples % of anti-native egg albumin positive, anti-denatured egg albumin positive and mixed positive samples in AD patients with mild and severe disease. C. Structural alignment of egg-albumin (PDB ID: 1OVA) with Serpin A3 (PDB: 1QMN). D. Structure of serpin A1 (a1-antitrypsin) which presents 30%sequence identity with egg-albumin. The identical amino-acids are shown in yellow [27].
As already mentioned, increased concentrations of antibodies against food-derived antigens like egg-albumin presuppose an intestinal or immunologic disturbance such as leaky intestine. However, increased Blood Brain Barrier (BBB) permeability may contribute to the high antibody concentration in Cerebrospinal Fluid. Both intestinal and BBB disturbances are common in AD patients [12]. Immune system impairment may also characterize some of the patients as it is common characteristic of aging [28]. In Alzheimer’s Disease, bacterial dysbiosis, the main cause of impaired intestinal permeability [29] is also a common and gradually progressive characteristic, affecting both colon and small intestine [30][31] with colon dysfunction detected in the first stages of the disease [32]. Dysbiosis is probably involved in oxidative and inflammatory conditions in AD patients [33] and may be implicated in protein modification and disregulation of digestion process. The sex differences, known to exist in intestinal microbiota [34][35], oxidative conditions [36][37][38] and intestinal permeability as well as the increased male susceptibility in BBB disfunction [39] may contribute to the sex differences observed anti-egg albumin antibodies in the serum and CSF. Although events of the disease may justify the observed results, experimental work is needed to further evaluate the probable correlation.
Anti-egg-albumin antibodies may be implicated in Disease mechanism through sequence/structural similarity with human proteins, mainly serpins (Figure 2C,D) and it would be worth of consideration in further investigations and therapeutic strategies. BLAST research revealed 21-42% similarity of ovalbumin with 35 human serine protease inhibitors (serpins) and with three non-serpin proteins: the Serpin-like minor histocompatibility protein HMSD, the phosphatidylinositol 5-phosphate 4-kinase type-2 gamma (PIP4K2C) and the N-acylglucosamine 2-epimerase (RENBP) [27]. Many of these molecules are related to the nervus system physiology or AD linked mechanisms and relevant disorders. Of great interest is the sequence/structural similarity with human serpins, involved in processes which exhibit impairment in AD such as intestinal permeability and inflammation (serpins A1, A2), Blood Brain Barrier (serpin A8, known as angiotensin, AGT, and its active metabolite, angiotensin II, AngII), and Central Nervus System (CNS) function (serpins A1, A3, A8, B1, E1, F1, I1) [27].
References
- Uzunismail, H.; Cengiz, M.; Uzun, H.; Ozbakir, F.; Goksel, S.; Demirdag, F.; Can, G.; Balci, H. The effects of provocation by foods with raised IgG antibodies and additives on the course of Crohn’s disease: a pilot study. Turk J Gastroenterol. 2012, 23(1), 19–27.
- Cappelletti, M.; Tognon, E.; Vona, L.; Basello, K.; Costanzi, A.; Speciani, MC.; Speciani, A.F. Food-specific serum IgG and symptom reduction with a personalized, unrestricted-calorie diet of six weeks in Irritable Bowel Syndrome (IBS). Nutr Metab (Lond) 2020, 17(1).
- Eleftheriou, P.; Kynigopoulos, S.; Giovou, A.; Mazmanidi, A.; Yovos, J.; Skepastianos P.; Vagdatli, E.; Petrou, C.; Papara, D.; Efterpiou, M. Prevalence of anti-Neu5Gc antibodies in patients with hypothyroidism. Research International, 2014, Article ID 963230, 1-9. http://dx.doi.org/10.1155/2014/963230.
- Padler-Karavani, V.; Hurtado-Ziola, N.; Pu, M.; Yu, H.; Huang, S.; Muthana, S.; Chokhawala, H.A.; Cao, H.; Secrest, P.; Friedmann-Morvinski, D.; Singer, O.; Ghaderi, D.; Verma, I.M.; Liu, Y-T.; Messer, K.; Chen, X.; Varki, A.; Schwab. R. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. 2011, 71(9), 3352-3363.
- Geiselman, JF. The clinical use of IgG food sensitivity testing with migraine headache patients: a literature review. Curr Pain Headache Rep. 2019, 23(11),
- Chundera, R.; Weiera, A.; Maurerb, H.; Luberb, N.; Endersa, M.; Luberc, G.; Heiderd, T.; Spitzere, A.; Tackeb, S.; Becker-Gototf, J.; Kurtsf, C.; Iyerg, R.; Hoh, P.P.; Robinsong, W.H.; Lanzg, T.V.; Kuertena S. Antibody cross-reactivity between casein and myelin-associated glycoprotein results in central nervous system demyelination. PNAS 2022, 119(10) e2117034119.
- Hadjivassiliou, M.; Sanders, D.S.; Grünewald, R.A.; Woodroofe, N.; Boscolo, S.; Aeschlimann, D. Gluten sensitivity: from gut to brain. Lancet Neurol 2010, 9(3), 318-330.
- Versino, M.; Biagi, F.; Bianchi, P. I.; Zardini, E.; Colnaghi, S.; Moglia, A.; Corazza, G. R.; Franciotta, D. Gluten sensitivity and the CNS: diagnosis and treatment. The Lancet Neurology 2010,9(7).
- Karakula-Juchnowicz, H.; Galecka, M.; Rog, J.; Bartnicka, A.; Lukaszewicz, Z.; Krukow, P.; Morylowska-Topolska, J.; Skonieczna-Zydecka, K.; Krajka, T.; Jonak, K.; Juchnowicz, D. The food-specific serum IgG reactivity in major depressive disorder patients, irritable bowel syndrome patients and healthy controls. 2018, 10(5), 548.
- Severance, EG.; Dupont, D.; Dickerson, FB.; Stallings, CR.; Origoni, AE.; Krivogorsky, B.; Yang, S.; Haasnoot, W.; Yolken, R.H. Immune activation by casein dietary antigens in bipolar disorder. Bipolar Disord. 2010, 12(8), 834–842.
- Karakula-Juchnowicz, H.; Szachta, P.; Opolska, A.; Morylowska-Topolska, J.; Galecka, M.; Juchnowicz, D.; Krukow, P.; Lasik, Z. The role of IgG hypersensitivity in the pathogenesis and therapy of depressive disorders. Nutr Neurosci. 2017, 20(2), 110–118.
- Vojdani, A. Reaction of food-specific antibodies with different tissue antigens. IJFST 2020, 55, 1800–1815.
- Adams, D.H.; Eksteen, B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Rev. Immunol. 2006, 6, 244–251.
- Molberg, Ø.; Sollid, L.M. A gut feeling for joint inflammation – using coeliac disease to understand rheumatoid arthritis. Trends Immunol. 2006, 27, 188–194.
- Vojdani, A. A potential link between environmental triggers and autoimmunity. Autoimmune Dis. 2014, ID 437231, 1-18.
- Vojdani, A.; O'Bryan, T.; Green, J.A.; Mccandless, J.; Woeller, K.N.; Vojdani, E.; Nourian, A.A.; Cooper, E.L. Immune response to dietary proteins, gliadin and cerebellar peptides in children with autism. Nutr Neurosci. 2004, 7(3), 151-161.
- Cie´ sli´ nska, A.; Fiedorowicz, E.; Rozmus, D.; Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Kami´ nski, S. Does a Little Difference Make a Big Difference? Bovine-Casein A1 and A2Variants and Human Health—An Update. J. Mol. Sci. 2022, 23, 15637.
- De Magistris, L.; Picardi, A.; Siniscalco, D.; Riccio, M.P.; Sapone, A.; Cariello, R.; Abbadessa, S.; Medici, N.; Lammers, K.M.; Schiraldi, C.; Iardino, P.; Marotta, R.; Tolone, C.; Fasano, A.; Pascotto, A.; Bravaccio, C. Antibodies against Food Antigens in Patients with Autistic Spectrum Disorders. BioMed Research International, 2013, ID 729349, http://dx.doi.org/10.1155/2013/729349.
- Rowland, LM.; Demyanovich H.K.; Wijtenburg, S.A.; Eaton, W.W.; Rodriguez, K.; Gaston, F.; Cihakova, D.; Talor, M.V.; Liu, F.; McMahon, R.R.; Elliot Hong, L.; Kelly, D.L. Antigliadin Antibodies (AgA IgG) Are Related to Neurochemistry in Schizophrenia. Psychiatry 2017, 8, 104.
- Yang, W.; Jiang, Y.; Guo, Q.; Tian, Z.; Cheng, Z. Aberrant N-glycolylneuraminic acid in breast MCF-7 cancer cells and cancer stem cells. Front Mol Biosci. 2022, 7, 9, 1047672. doi: 10.3389/fmolb.2022.1047672. eCollection 2022.
- Bentz, S.; Hausmann, M.; Piberger, H.; Kellermeier, S.; Paul, S.; Held, L.; Falk, W.; Obermeier, F.; Fried, M.; Schölmerich, J.; Rogler, G; Clinical relevance of IgG antibodies against food antigens in Crohn’s disease: a double-blind cross-over diet intervention study. Digestion 2010, 81(4), 252–264.
- Atkinson, W.; Sheldon, T.A.; Shaath, N.; Whorwell, P.J. Food elimination based on IgG anti-bodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004, 53(10), 1459–1464.
- Mitchell, N.; Hewitt, C.E.; Jayakody, S.; Islam, M.; Adamson, J.; Watt, I.; Torgerson D.J. Randomised con-trolled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr J. 2011, 10:85.
- Xie, Y.; Zhou, G.; Xu, Y.; He, B.; Wang, Y.; Ma, R.; et al. Chang, Y.; He, D.; Xu, C.; Xiao Z. Effects of diet based on IgG elimination combined with probiotics on migraine plus irritable bowel syndrome. Pain Res Manag. 2019, 2019:7890461.
- Jian, L.; Anqi, H.; Gang, L.; Litian, W.; Yanyan, X.; Mengdi, W.; Tong, L. Food exclusion based on IgG antibodies alleviates symptoms in ulcerative colitis: a prospective study. Inflamm Bowel Dis. 2018, 24(9), 1918–1925.
- Gunasekeera, V.; Mendall, M.A.; Chan, D.; Kumar, D. Treatment of Crohn’s disease with an IgG4-guided exclusion diet: a randomized controlled trial. Dig Dis Sci. 2016, 61(4), 1148–57.
- Amanatidou, D.; Tsolaki, M.; Fouskas, V.; Gavriilidis, I.; Myriouni, M.; Anastasiou, A.; Papageorgiou, A.; Porfyriadou, D.; Parcharidi, Z.; Papasavva, E.; et al. Increased Concentration of Anti-Egg Albumin Antibodies in Cerebrospinal Fluid and Serum of Patients with Alzheimer’s Disease—Discussion on Human Serpins’ Similarity and Probable Involvement in the Disease Mechanism. Biomolecules 2025, 15, 1085. https://doi.org/10.3390/biom15081085.
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247.
- Arrieta, M.C.; Bistritz, L.; Meddings, J.B. Recent advances in clinical practice. Alterations in intestinal permeability. Gut 2006, 55, 1512–1520.
- Heston, M.B.; Hanslik, K.L.; Zarbock, K.R.; Harding, S.J.; Davenport‑Sis, N.J.; Kerby, R.L.; Chin, N.; Sun, Y.; Hoeft, A.; Deming, Y.; Vogt, N.M.; Betthauser, T.J.; Johnson, S.C.; Asthana, S.; Kollmorgen, G.; Suridjan, I.; Wild, N.; Zetterberg, H.; Blennow, K.; Rey, F.E.; Bendlin, B.B.; Ulland, T.K. Gut inflammation associated with age and Alzheimer’s disease pathology: a human cohort study. Sci. Rep. 2023, 13, 18924.
- Li, Z.; Zhu, H.; Zhang, L.; Qin, C. The intestinal microbiome and Alzheimer's disease: A review. Animal Models Exp Med. 2018, 1, 180–188.
- Pellegrini, C.; Daniele, S.; Antonioli, L.; Benvenuti, L.; D’Antongiovanni, V.; Piccarducci, R.; Pietrobono, D.; Citi, V.; Piragine, E.; Flori, L.; Ippolito, C.; Segnani, C.; Palazon-Riquelme, P.; Lopez-Castejon, G.; Martelli , A.; Colucci, R.; Bernardini, N.; Trincavelli, M.L.; Calderone, V.; Martini, C.; Blandizzi, C.; Fornai, M. Prodromal Intestinal Events in Alzheimer’s Disease (AD): Colonic Dysmotility and Inflammation Are Associated with Enteric AD-Related Protein Deposition. J. Mol. Sci. 2020, 21, 3523-3551.
- Tushar, K.; Ganesh, D.; Ganesh, B.P. Interlink between the gut microbiota and inflammation in the context of oxidative stress in Alzheimer’s disease progression, Gut Microbes 2023, 15(1), 2206504, DOI: 10.1080/19490976.2023.2206504.
- Yoon, K.; Kim, N. Roles of Sex Hormones and Gender in the Gut Microbiota. J Neurogastroenterol Motil. 2021, 27(3).
- Kim, Y.S.; Unno, T.; Kim, B-Y.; Park. M.-S. Sex Differences in Gut Microbiota. World J Mens Health 2020 38(1), 48-60.
- Towera, J.; Pomattod, L.C.D.; Daviesa, K.J.A.. Sex differences in the response to oxidative and proteolytic stress. Redox Biol. 2020, 31.
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. Cell. Mol. Med. 2017, 21(5), 1024-1032.
- Tiberi, J.; Cesarini, V.; Stefanelli, R.; Canterini, S.; Fiorenza, M.T.; La Rosa, P. Sex differences in antioxidant defense and the regulation of redox homeostasis in physiology and pathology. Mech Ageing Dev 2023, 211.
- Castellazzi, M.; Morotti, A.; Tamborino, C.; Alessi, F.; Pilotto, S.; Baldi, E.; Caniatti, L.M.; Trentini, A.; Casetta, I.; Granieri, E.; Pugliatti, M.; Fainardi E.; Bellini, T. Increased age and male sex are independently associated with higher frequency of blood–cerebrospinal fluid barrier dysfunction using the albumin quotient. Fluids Barriers CNS 2020, 17, 14 https://doi.org/10.1186/s12987-020-0173-2.




