
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Minako Yamaoka-Tojo | + 1576 word(s) | 1576 | 2020-12-29 04:16:47 | | | |
| 2 | Camila Xu | Meta information modification | 1576 | 2020-12-30 09:46:27 | | |
Video Upload Options
Vascular endothelial cells are a monolayer of cells that comprise the innermost layer of cells in the vascular system, including arteries, veins, and capillaries, and serve a barrier function for the blood vessels surrounding all organs and in direct contact with the blood flowing through the vascular lumen.
1. Vascular Endothelial Glycocalyx (VEGLX)
The glycocalyx is a complex gel-like layer of glycosylated lipid-protein mixtures that covers the surface of all living cells, and is known to serve as a physical protective layer as well as a buffer region between cells and the extracellular matrix to control various cellular functions [1]. Vascular endothelial cells are a monolayer of cells that comprise the innermost layer of cells in the vascular system, including arteries, veins, and capillaries, and serve a barrier function for the blood vessels surrounding all organs and in direct contact with the blood flowing through the vascular lumen. As shown in Figure 1, for example, the VEGLX plays an important role in regulating various vascular endothelial cell functions such as coagulation, inflammation, vasoconstriction and relaxation, vascular permeability, and angiogenesis [2][3].
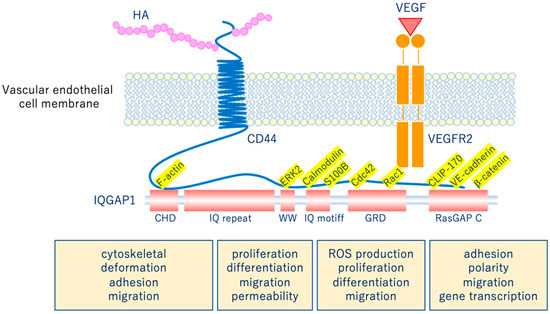
Figure 1. Vascular endothelial glycocalyx regulates cellular functions. Hyaluronic acid (HA) activates the HA/CD44 system by binding to CD44 and regulates various intracellular signaling through the multifunctional platform IQGAP1, as well as the vascular endothelial growth factor VEGF/VEGFR2 system. VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; CD44, cluster of differentiation 44; CHD, calponin homology domain; IQ repeat, IQGAP specific repeat; ERK, extracellular-signal-regulated kinase; WW, region containing two tryptophans; S100B, S100 calcium-binding protein B; IQ motif, calmodulin-binding motif; Cdc42, cell division cycle 42; Rac1, Rac family small GTPase 1; GRD, Ras GTPase-activating protein-related domain; CLIP-170, cytoplasmic linker protein 170; RasGAP C, Ras GTPase-activating protein C terminus; ROS, reactive oxygen species.
As shown in Figure 2, glycocalyx consists of sialic acid-containing glycoproteins, core proteins consisting of membrane-bound proteoglycans (syndecan, glypican, etc.), glycosaminoglycan side chains (heparan sulfate, chondroitin sulfate, etc.), and long-chain hyaluronic acid (HA) [4][5]. VEGLX is stabilized by shear stress [6] and this stabilization is crucial for nitric oxide (NO) production in vascular endothelial cells [7][8]. Although glycosaminoglycans are constantly degraded by enzymes, their levels are maintained by newly synthesized glycosaminoglycans supplied from vesicles in the golgi apparatus, regulating their homeostatic balance [9].
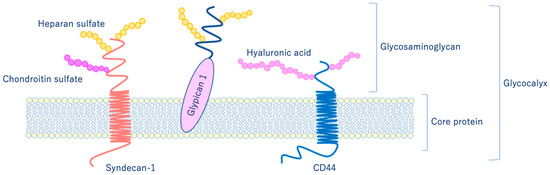
Figure 2. Schematic representation of the vascular endothelial glycocalyx, which is present in the vascular endothelial cell membrane. The vascular endothelial glycocalyx is composed of core proteins and highly water-retaining glycosaminoglycans that bind to the cell membrane.
VEGLX protects vascular endothelial cells from the turbulence caused by blood flow and is involved in the regulatory function of the vascular permeability barrier. In addition, it plays an important role in endothelial function, especially in microvascular endothelial function, as it controls vascular reactivity and is involved in regulating the interaction between vascular endothelial cells and blood components [10]. Vascular endothelial cells in a healthy state covered by the VEGLX (Figure 3) contain a variety of cytokines, chemokines, receptors, growth factors, gap-binding proteins, extracellular superoxide dismutase (ecSOD), and endothelial nitric oxide synthase (eNOS). In addition, lipoprotein lipases are expressed that play a variety of functions required for homeostasis. Owing to its negative charge, VEGLX is also thought to be a determinant of salt sensitivity, and neutralizes cell surface charges by retaining plasma sodium in the glycocalyx layer [11].
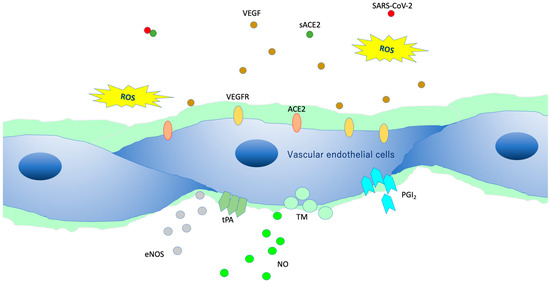
Figure 3. Intact vascular endothelial glycocalyx (VEGLX). When vascular endothelial cells are covered enough with healthy VEGLX, even if severe acute respiratory coronavirus 2 (SARS-CoV-2) enters the body, it could be neutralized by the effects of appropriate reactive oxygen species (ROS) and soluble angiotensin-converting enzyme 2 (sACE2), and it may be possible to prevent the virus entry into the vascular endothelium. VEGF, vascular endothelial growth factor; VEGFR, VEGF receptors; NO, nitric oxide; eNOS, endothelial NO synthase; TM, thrombomodulin; tPA, tissue plasminogen activator; PGI2, prostacyclin.
2. Damage of VEGLX
As shown in Figure 4, VEGLX shedding and degradation are known to be caused by a variety of cellular stresses [12]. Specifically, ischemia/reperfusion injury [13], endotoxin [14], inflammatory mediators [15], atrial natriuretic peptide, hypoxia, excessive reactive oxygen species (ROS), uric acid [16], hyperglycemia [17][18], hypernatremia [19], excessive fluid infusion, dehydration, decreased vascular wall shear stress, oxidized low-density lipoprotein (ox-LDL) [20], and others can cause VEGLX damage. In addition, this damage is known to be sex-specific, mostly observed in men. Systemic shedding of VEGLX has been associated with serious infections [21], Kawasaki disease [22], gestational hypertensive nephropathy (preeclampsia) [23], gestational diabetes [24], sepsis [25], acute lung injury (ALI)/ARDS [26], trauma [27], ischemic cerebral embolism [28], acute coronary syndrome [29], and shock [30]. The term shock-induced endothelial glycocalyx disturbance, called shock-induced endotheliopathy (SHINE), is an indicator of poor prognosis in fairly serious conditions, such as severe trauma, sepsis, myocardial infarction, and cardiac arrest syndrome [31]. Under these severe conditions, VEGLX is degraded via inflammatory mechanisms that promote tissue degradation, including reactive activation of metalloproteinases, heparanases, and hyaluronidases [32][33]. In patients with these acute diseases, high concentrations of fragmented VEGLX, such as syndecan-1 (soluble CD138), syndecan-4, hyaluronic acid, and heparan sulfate, can be detected in the blood. The degraded VEGLX detaches from the surface of vascular endothelial cells, thinning the glycocalyx layer, and inducing extravascular leakage in microvessels with excessive vascular permeability, contributing to further pathological deterioration by causing interstitial edema in various organs [33][34].
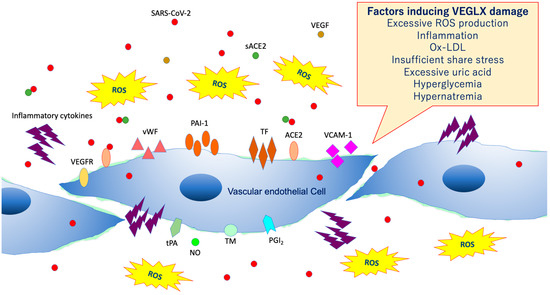
Figure 4. Damaged vascular endothelial glycocalyx (VEGLX). VEGLX damage is associated with vascular endothelial dysfunction, which induces reduced nitric oxide (NO) bioavailability, increased excessive reactive oxygen species (ROS) production, inflammatory cytokine release, platelet adherence, coagulation, and leukocyte adhesion. SARS-CoV-2, severe acute respiratory coronavirus 2; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; ACE2, angiotensin-converting enzyme 2; sACE2, soluble ACE2; PAI-1, plasminogen activator inhibitor-1; TF, tissue factor; vWF, von Willebrand factor; ox-LDL, oxidized low-density lipoprotein; MMPs, matrix metalloproteases; tPA, tissue plasminogen activator; PGI2, prostacyclin; TM, thrombomodulin.
3. Evaluation of VEGLX In Vivo
Elevated concentrations of soluble VEGLX fragments in the blood of patients with sepsis and diabetes have been previously reported and qualitatively assessed using electron micrographic imaging of the microvascular VEGLX in animal models and autopsy cases. The endothelial glycocalyx measurement device has emerged as a simple and reproducible instrument for measuring VEGLX by monitoring the sublingual microcirculation of the VEGLX vulnerable region as an indicator of the perfusion boundary region (PBR). Evaluation of the VEGLX using GlycoCheck® (Microvascular Health Solutions, UT, USA) has been used [35]. The VEGLX is composed of a tight glycocalyx layer and a coarse glycocalyx layer. The latter allows red blood cells to bounce freely within the layer, which is observed as an increased PBR (Figure 5).
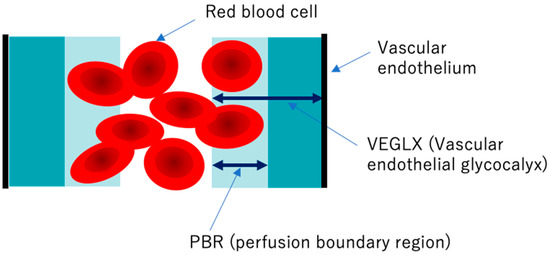
Figure 5. Conceptual diagram of the vascular endothelial glycocalyx (VEGLX) vulnerable region (PBR). The VEGLX, which covers the surface of vascular endothelial cells, can be broadly divided into a region of dense glycans on the vascular endothelial side and a region covered by fragile glycans on the vascular lumen side. In disorders of the VEGLX, the fragile region is known to be enlarged.
To detect VEGLX damage, PBR of the sublingual arterial microvessels is measured as a noninvasive test. Higher PBR values are thought to indicate an increase in this vulnerable area of the VEGLX and represent a thinning of the tight healthy VEGLX. In addition to reports on physiological changes in the VEGLX in healthy subjects, there have been reports on PBR related to a variety of diseases. A higher PBR was observed in elderly people, women, people with low body mass index (BMI), and patients with diastolic hypertension and diabetes [36][37][38]. In the context of racial differences, one report found no obvious racial differences between 472 Chinese and 254 Flemish residents [39], while a cross-sectional report of 6169 people found racial differences in allowing for increased PBR among blacks of African descent and whites of European descent compared to Asians and Arabs [36].
In diseases associated with complications of severe coronavirus, infections include sepsis, chronic heart failure [40], cerebrovascular disease [41], microvascular angina [42], and ischemic heart disease [41], PBR has been reported to be associated with the severity of these diseases, suggesting the usefulness of VEGLX measurements [43][44].
4. Virus Infections and VEGLX
SARS-CoV-2, the virus responsible for COVID-19, is a positive-sense single-stranded RNA virus that is classified as a (+)ssRNA virus. The genome of positive-stranded RNA viruses also acts as mRNA and is translated into proteins in the host cell. These viruses are categorized into Group IV of the Baltimore Classification of Viruses, and are classified as dengue fever virus, and include SARS-CoV-1, MERS-CoV2, hepatitis C virus, yellow fever virus, Japanese encephalitis viruses, and rhinoviruses.
In case of viral infections, research on dengue fever and VEGLX has been reported. Dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) is characterized by microvascular barrier dysfunction and shock [45]. Dengue virus nonstructural protein 1 (NS1) has been identified as the only membrane-associated protein that anchors the viral replication complex to the vascular endothelial cell membrane. Increased blood levels of VEGLX components such as hyaluronic acid, heparin sulfate, claudin-5, and syndecan-1, suggesting shedding of the VEGLX and extravascular leakage of plasma components, have been associated with the development and exacerbation of severe dengue infection [46][47]. Such viral entry into and proliferation of vascular endothelial cells in severe viral infections is responsible for a variety of microvascular disorders, including ARDS and DIC [48][49].
The susceptibility of influenza A virus to host cell infection is defined by the density, glycosylation state, and length of the glycocalyx, which constitutes a protective barrier called mucin on the surface of the cell [50]. Dense mucin is the most important factor in the ability of influenza virus to penetrate the cell surface and inhibit binding to glycolipid receptors. The rate at which the virus fuses to the cell is found slower in a concentration-dependent manner with respect to mucin [50]. Several flu drugs target the interaction between the influenza virus and mucin-like proteins [50].
References
- Butler, P.J.; Bhatnagar, A. Mechanobiology of the abluminal glycocalyx. Biorheology 2019, 56, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, K.B.; Arkill, K.P.; Neal, C.R.; Harper, S.J.; Foster, R.R.; Satchell, S.C.; Bates, D.O.; Salmon, A.H.J. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J. Physiol. 2017, 595, 5015–5035. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Ramnath, R.; Kadoya, H.; Desposito, D.; Riquier-Brison, A.; Ferguson, J.K.; Onions, K.L.; Ogier, A.S.; ElHegni, H.; Coward, R.J.; et al. Aldosterone induces albuminuria via matrix metalloproteinase-dependent damage of the endothelial glycocalyx. Kidney Int. 2019, 95, 94–107. [Google Scholar] [CrossRef]
- Curry, F.R. Microvascular solute and water transport. Microcirculation 2005, 12, 17–31. [Google Scholar] [CrossRef]
- Curry, F.E. Layer upon layer: The functional consequences of disrupting the glycocalyx-endothelial barrier in vivo and in vitro. Cardiovasc. Res. 2017, 113, 559–561. [Google Scholar] [CrossRef]
- Ueda, A.; Shimomura, M.; Ikeda, M.; Yamaguchi, R.; Tanishita, K. Effect of glycocalyx on shear-dependent albumin uptake in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2287–H2294. [Google Scholar] [CrossRef]
- Thi, M.M.; Tarbell, J.M.; Weinbaum, S.; Spray, D.C. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: A “bumper-car” model. Proc. Natl. Acad. Sci. USA 2004, 101, 16483–16488. [Google Scholar] [CrossRef]
- Koo, A.; Dewey, C.F., Jr.; Garcia-Cardena, G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am. J. Physiol. Cell Physiol. 2013, 304, C137–C146. [Google Scholar] [CrossRef] [PubMed]
- Prydz, K. Determinants of Glycosaminoglycan (GAG) Structure. Biomolecules 2015, 5, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Bar, A.; Targosz-Korecka, M.; Suraj, J.; Proniewski, B.; Jasztal, A.; Marczyk, B.; Sternak, M.; Przybylo, M.; Kurpinska, A.; Walczak, M.; et al. Degradation of Glycocalyx and Multiple Manifestations of Endothelial Dysfunction Coincide in the Early Phase of Endothelial Dysfunction Before Atherosclerotic Plaque Development in Apolipoprotein E/Low-Density Lipoprotein Receptor-Deficient Mice. J. Am. Heart Assoc. 2019, 8, e011171. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H.; Wilhelmi, M. Vascular glycocalyx sodium store—Determinant of salt sensitivity? Blood Purif. 2015, 39, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Brands, J.; Spaan, J.A.; Van den Berg, B.M.; Vink, H.; VanTeeffelen, J.W. Acute attenuation of glycocalyx barrier properties increases coronary blood volume independently of coronary flow reserve. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H515–H523. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gayosso, I.; Platts, S.H.; Duling, B.R. Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2247–H2256. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Meuwese, M.C.; Vink, H.; Hoekstra, J.B.; Kastelein, J.J.; Stroes, E.S. The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr. Opin. Lipidol. 2005, 16, 507–511. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Meuwese, M.C.; Mooij, H.L.; van Lieshout, M.H.; Hayden, A.; Levi, M.; Meijers, J.C.; Ince, C.; Kastelein, J.J.; Vink, H.; et al. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis 2009, 202, 296–303. [Google Scholar] [CrossRef]
- Ko, J.; Kang, H.J.; Kim, D.A.; Kim, M.J.; Ryu, E.S.; Lee, S.; Ryu, J.H.; Roncal, C.; Johnson, R.J.; Kang, D.H. Uric acid induced the phenotype transition of vascular endothelial cells via induction of oxidative stress and glycocalyx shedding. FASEB J. 2019, 33, 13334–13345. [Google Scholar] [CrossRef]
- Zuurbier, C.J.; Demirci, C.; Koeman, A.; Vink, H.; Ince, C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J. Appl. Physiol. 2005, 99, 1471–1476. [Google Scholar] [CrossRef]
- Pahwa, R.; Nallasamy, P.; Jialal, I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J. Diabetes Complicat. 2016, 30, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Rorije, N.M.G.; Rademaker, E.; Schrooten, E.M.; Wouda, R.D.; Homan Van Der Heide, J.J.; Van Den Born, B.H.; Vogt, L. High-salt intake affects sublingual microcirculation and is linked to body weight change in healthy volunteers: A randomized cross-over trial. J. Hypertens. 2019, 37, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Vink, H.; Constantinescu, A.A.; Spaan, J.A. Oxidized lipoproteins degrade the endothelial surface layer: Implications for platelet-endothelial cell adhesion. Circulation 2000, 101, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.R.; Gaini, S.; Pedersen, C.; Johansson, P.I. Sympathoadrenal activation and endothelial damage in patients with varying degrees of acute infectious disease: An observational study. J. Crit. Care 2015, 30, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Yasudo, H.; Suzuki, Y.; Furuta, T.; Matsuguma, C.; Azuma, Y.; Miyake, A.; Okada, S.; Ichihara, K.; Ohga, S.; et al. Circulating endothelial glycocalyx components as a predictive marker of coronary artery lesions in Kawasaki disease. Int. J. Cardiol. 2019, 292, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Garcia-Valencia, O.; Milic, N.M.; Codsi, E.; Cubro, H.; Nath, M.C.; White, W.M.; Nath, K.A.; Garovic, V.D. Early Onset Preeclampsia Is Associated With Glycocalyx Degradation and Reduced Microvascular Perfusion. J. Am. Heart Assoc. 2019, 8, e010647. [Google Scholar] [CrossRef]
- Long, D.S.; Hou, W.; Taylor, R.S.; McCowan, L.M. Serum levels of endothelial glycocalyx constituents in women at 20 weeks’ gestation who later develop gestational diabetes mellitus compared to matched controls: A pilot study. BMJ Open 2016, 6, e011244. [Google Scholar] [CrossRef]
- Steppan, J.; Hofer, S.; Funke, B.; Brenner, T.; Henrich, M.; Martin, E.; Weitz, J.; Hofmann, U.; Weigand, M.A. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J. Surg. Res. 2011, 165, 136–141. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Overdier, K.H.; Sun, X.; Lin, L.; Liu, X.; Yang, Y.; Ammons, L.A.; Hiller, T.D.; Suflita, M.A.; Yu, Y.; et al. Urinary Glycosaminoglycans Predict Outcomes in Septic Shock and Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2016, 194, 439–449. [Google Scholar] [CrossRef]
- Chignalia, A.Z.; Yetimakman, F.; Christiaans, S.C.; Unal, S.; Bayrakci, B.; Wagener, B.M.; Russell, R.T.; Kerby, J.D.; Pittet, J.F.; Dull, R.O. The Glycocalyx and Trauma: A Review. Shock 2016, 45, 338–348. [Google Scholar] [CrossRef]
- DellaValle, B.; Hasseldam, H.; Johansen, F.F.; Iversen, H.K.; Rungby, J.; Hempel, C. Multiple Soluble Components of the Glycocalyx Are Increased in Patient Plasma After Ischemic Stroke. Stroke 2019, 50, 2948–2951. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.H.; de Carvalho Borges, M.; Schmidt, A.; Marin-Neto, J.A.; Pazin-Filho, A. Evaluation of the endothelial glycocalyx damage in patients with acute coronary syndrome. Atherosclerosis 2016, 247, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Marechal, X.; Favory, R.; Joulin, O.; Montaigne, D.; Hassoun, S.; Decoster, B.; Zerimech, F.; Neviere, R. Endothelial glycocalyx damage during endotoxemia coincides with microcirculatory dysfunction and vascular oxidative stress. Shock 2008, 29, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care 2017, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Chelazzi, C.; Villa, G.; Mancinelli, P.; De Gaudio, A.R.; Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 2015, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Rodriguez, E.; Cardenas, J.C.; Cox, C.S.; Kitagawa, R.S.; Stensballe, J.; Holcomb, J.B.; Johansson, P.I.; Wade, C.E. Traumatic brain injury is associated with increased syndecan-1 shedding in severely injured patients. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 102. [Google Scholar] [CrossRef]
- Lee, D.H.; Dane, M.J.; van den Berg, B.M.; Boels, M.G.; van Teeffelen, J.W.; de Mutsert, R.; den Heijer, M.; Rosendaal, F.R.; van der Vlag, J.; van Zonneveld, A.J.; et al. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE 2014, 9, e96477. [Google Scholar] [CrossRef]
- Valerio, L.; Peters, R.J.; Zwinderman, A.H.; Pinto-Sietsma, S.J. Sublingual endothelial glycocalyx and atherosclerosis. A cross-sectional study. PLoS ONE 2019, 14, e0213097. [Google Scholar] [CrossRef]
- Broekhuizen, L.N.; Lemkes, B.A.; Mooij, H.L.; Meuwese, M.C.; Verberne, H.; Holleman, F.; Schlingemann, R.O.; Nieuwdorp, M.; Stroes, E.S.; Vink, H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010, 53, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Groen, B.B.; Hamer, H.M.; Snijders, T.; van Kranenburg, J.; Frijns, D.; Vink, H.; van Loon, L.J. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J. Appl. Physiol. 2014, 116, 998–1005. [Google Scholar] [CrossRef]
- Gu, Y.M.; Wang, S.; Zhang, L.; Liu, Y.P.; Thijs, L.; Petit, T.; Zhang, Z.; Wei, F.F.; Kang, Y.Y.; Huang, Q.F.; et al. Characteristics and determinants of the sublingual microcirculation in populations of different ethnicity. Hypertension 2015, 65, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Hulsmann, M.; Schorgenhofer, C.; Lang, I.M.; Wurm, R.; Gremmel, T.; Koppensteiner, R.; Steinlechner, B.; Schwameis, M.; Jilma, B. Sublingual functional capillary rarefaction in chronic heart failure. Eur. J. Clin. Invest. 2018, 48. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, A.Y.; Klimushina, M.V.; Boytsov, S.A.; Kots, A.Y.; Gumanova, N.G. Increase in perfused boundary region of endothelial glycocalyx is associated with higher prevalence of ischemic heart disease and lesions of microcirculation and vascular wall. Microcirculation 2018, 25, e12454. [Google Scholar] [CrossRef]
- Jaarsma, C.; Vink, H.; van Haare, J.; Bekkers, S.; van Rooijen, B.D.; Backes, W.H.; Wildberger, J.E.; Crijns, H.J.; van Teeffelen, J.; Schalla, S. Non-invasive assessment of microvascular dysfunction in patients with microvascular angina. Int. J. Cardiol. 2017, 248, 433–439. [Google Scholar] [CrossRef]
- Yen, W.; Cai, B.; Yang, J.; Zhang, L.; Zeng, M.; Tarbell, J.M.; Fu, B.M. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS ONE 2015, 10, e0117133. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Lambadiari, V.; Kousathana, F.; Varoudi, M.; Spanoudi, F.; Maratou, E.; Parissis, J.; Triantafyllidi, H.; Dimitriadis, G.; et al. Early detection of left ventricular dysfunction in first-degree relatives of diabetic patients by myocardial deformation imaging: The role of endothelial glycocalyx damage. Int. J. Cardiol. 2017, 233, 105–112. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Tang, T.H.; Alonso, S.; Ng, L.F.; Thein, T.L.; Pang, V.J.; Leo, Y.S.; Lye, D.C.; Yeo, T.W. Increased Serum Hyaluronic Acid and Heparan Sulfate in Dengue Fever: Association with Plasma Leakage and Disease Severity. Sci. Rep. 2017, 7, 46191. [Google Scholar] [CrossRef]
- Suwarto, S.; Sasmono, R.T.; Sinto, R.; Ibrahim, E.; Suryamin, M. Association of Endothelial Glycocalyx and Tight and Adherens Junctions With Severity of Plasma Leakage in Dengue Infection. J. Infect. Dis. 2017, 215, 992–999. [Google Scholar] [CrossRef]
- Grandoch, M.; Bollyky, P.L.; Fischer, J.W. Hyaluronan: A Master Switch Between Vascular Homeostasis and Inflammation. Circ. Res. 2018, 122, 1341–1343. [Google Scholar] [CrossRef]
- Cioffi, D.L.; Pandey, S.; Alvarez, D.F.; Cioffi, E.A. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L1067–L1077. [Google Scholar] [CrossRef] [PubMed]
- Delaveris, C.S.; Webster, E.R.; Banik, S.M.; Boxer, S.G.; Bertozzi, C.R. Membrane-tethered mucin-like polypeptides sterically inhibit binding and slow fusion kinetics of influenza A virus. Proc. Natl. Acad. Sci. USA 2020, 117, 12643–12650. [Google Scholar] [CrossRef] [PubMed]




