
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lukasz Szymanski | + 1834 word(s) | 1834 | 2020-12-16 07:35:50 | | | |
| 2 | Vivi Li | + 3 word(s) | 1837 | 2020-12-28 03:17:13 | | |
Video Upload Options
The retinoids are a group of compounds including vitamin A and its active metabolite all-trans-retinoic acid (ATRA). Retinoids regulate a variety of physiological functions in multiple organ systems, are essential for normal immune competence, and are involved in the regulation of cell growth and differentiation. Vitamin A derivatives have held promise in cancer treatment and ATRA is used in differentiation therapy of acute promyelocytic leukemia (APL). ATRA and other retinoids have also been successfully applied in a variety of dermatological conditions such as skin cancer, psoriasis, acne, and ichthyosis. Moreover, modulation of retinoic acid receptors and retinoid X (or rexinoid) receptors function may affect dermal cells. The studies using complex genetic models with various combinations of retinoic acid receptors (RARs) and retinoid X (or rexinoid) receptors (RXRs) indicate that retinoic acid and its derivatives have therapeutic potential for a variety of serious dermatological disorders including some malignant conditions.
1. Introduction
Retinoids are defined as synthetic or natural derivatives of vitamin A that were first discovered in 1913. Retinol and retinyl ester are dietary forms of what is commonly known as vitamin A. These forms of vitamin A are not biologically active and require transformation, by cytosolic alcohol dehydrogenases (ADHs) and microsomal retinol dehydrogenases (MDHs), to become retinaldehyde, and subsequent oxidation by retinaldehyde dehydrogenases RALDH1, RALDH2, and RALDH3 to retinoic acid (RA) [1]. While the reverse transformation of retinaldehyde to retinol is possible through the DHRS3 enzyme activity, the retinaldehyde to RA conversion is irreversible [2]. RA exists in several isoforms of which the most common are all-trans retinoic acid and 9-cis retinoic acid [2]. All-trans-retinoic acid (ATRA) is also one of the main physiologically active metabolites of vitamin A. Retinoids, which are hydrophobic compounds, require retinoid-binding proteins for stabilization in aqueous media. Depending on the localization, the stabilization of retinoids is achieved by binding to different proteins such as cellular retinol-binding proteins (CRBPs) or cellular retinoic acid-binding proteins (CRABPs), the interstitial retinol-binding protein (RBP 3), and plasma-retinol binding protein (RBP 4) [3]. The half-life of RA is around 1 h because it is rapidly metabolized by the cytochrome P450 enzymes (CYP26s) [2]. CYPs are involved in ATRA hydroxylation and thus inactivation of its function. RAs are not only substrates for CYP26 enzymes but are also potent CYP26 inducers, therefore creating a negative feedback loop [4].
It is believed that ATRA deficiency, mediated by CYPs or other mechanisms, is associated with cancer progression and various dermatological diseases [5].
Retinoids are usually classified in one of the three generations; however, some researchers consider pyranones derivatives as the fourth generation. Briefly, naturally occurring, non-aromatic retinoids are classified as the first generation, monoaromatic vitamin A derivatives are the second generation, whereas retinoids containing a cyclic polyene side-chain are the third generation [3]. More detailed information about retinoic acid and its signaling pathways may be found in a recent review by Ghyselinck and Duester (2019) [2].
2. Retinoic Acid Receptors and Molecular Mechanism of Their Action
Retinoic acid receptors are key developmental regulators and, as such, function as a molecular switch in many developmental processes including skin development. In the absence of a ligand, RARs repress transcription through recruiting histone deacetylases (HDAC) complexes such as HDAC-N-CoR (negative co-regulator) or HDAC-SMRT (silencing mediator for retinoid and thyroid hormone receptors) (Figure 1) [6].
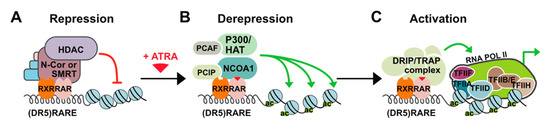
Figure 1. Mechanism of all-trans-retinoic acid (ATRA) action. (A) In the absence of a ligand, retinoid X (or rexinoid) receptors and retinoic acid receptors (RXR-RAR) heterodimers bind to retinoic acid response elements (RAREs) in the regulatory regions of target genes and repress transcription through recruiting histone deacetylases (HDAC)/co-repressor (negative co-regulator (N-CoR) or silencing mediator for retinoid and thyroid hormone receptors (SMRT)) complexes. HDACs remove acetyl groups from nucleosomal histones causing chromatin condensation that precludes binding of other factors and ultimately results in the silencing of gene expression. (B) Upon ligand binding, RAR undergoes structural change leading to dissociation of the co-repressor complex and association of co-activators with histone acetyltransferase (HAT) activities (e.g., P300 and NCOA1) that cause decondensation of chromatin by adding acetyl groups to nucleosomal histones. (C) Additional multiprotein complexes called vitamin D3 receptor-interacting proteins or thyroid hormone receptor-associated proteins (DRIP or TRAP) may also be recruited for activation of transcription through interaction with basal transcriptional.
In the presence of a ligand, change in the position of helix 12 occurs and co-repressors are replaced with co-activators such as DRIP/TRAP/ARC (Vitamin D3 receptor-interacting proteins/Thyroid hormone receptor-associated proteins/Activator-recruited complex) which leads to gene-targeted transcription activation through chromatin decompression. Three different RAR coding genes (-α, -β, and -γ) and retinoid X (or rexinoid) receptor (RXR) genes have been characterized. Each of these genes encodes multiple N-terminal protein isoforms (differing in their N-terminal regions) which can be produced by differential promoter usage and alternative splicing [7]. RXRs serve as the obligatory heterodimerization partners for RARs and several other nuclear receptors including those for thyroid hormones and vitamin D3, thus integrating different signaling pathways [8]. Like other RAR genes, RARα encodes two major isoforms: RARα1 and RARα2. These isoforms differ in their A regions that are generated by alternative splicing and differential promoter usage and are identical in their B to F region sequences, which contain a ligand (LBD) and a DNA (DBD) binding domain. The isoforms are also identical in terms of structural motifs responsible for dimerization, co-repressor interaction, and ligand-dependent trans-activation. The difference in the A regions contributes to different transcriptional regulation in a cell promoter-specific and ligand-independent manner [9]. Expression of the isoforms RARα2, RARβ2, and RARγ2 is induced by ATRA and is controlled by the promoters containing RAREs (retinoic acid response elements) sequences. RARs dependent transcription activation is intrinsically linked to their proteasome-mediated degradation. At the same time, ATRA upregulates the expression of RARα2, RARβ2, and RARγ2 isoforms, and induces proteasome-dependent degradation of retinoic acid receptor, which is related to this ubiquitination as described for many other proteins. Therefore, it might be possible that activation of transcription by RARs related to proteasome-mediated degradation and upregulation of RARs expression by ATRA was evolutionarily favored to sustain the expression of a given receptor and maintain gene regulation and physiological effects of ATRA over an extended time [10].
Physiologically, to interact with its nuclear receptor, ATRA is transported into the nucleus by the CRABP that binds ATRA with high affinity. CRABP are divided into two subtypes, CRABP I, and CRABP II that is more abundant in the skin [11]. CRABP II has been proposed to be a marker of RA activity in human skin. Expression of CRABP II is induced by ATRA and reduced in aging human and mouse skin. Research suggests a role of CRABP II in skin aging. Knock-out of the CRABP II gene in mice causes, among other things, the reduction of keratinocyte layers, degree of proliferation and differentiation, and skin thickness. Loss of the CRABP II gene leads to the reduction and loosening of collagen bundles, which contributes to premature and severe skin aging [12].
RARα, RARγ, and RXR are highly expressed in the fibroblasts and keratinocytes with RARα being more abundant in the fibroblasts. RARβ is either not present or expressed at a low level; however, exposure to retinoic acid rapidly induces its expression [13]. In the human epidermis, the abundance of the given type of heterodimer complex depends on the keratinocyte differentiation state, and so the RARα/RXRα complex is dominant in the basal layer whereas the RARγ/RXRα complex is most common in the suprabasal layer [14].
Upon binding of ATRA to the LBD, the RARs exhibit a conformational change allowing for heterodimerization with an RXR. However, the RAR is not an exclusive heterodimerization partner for RXR. Thyroid hormone receptor, estrogen receptor, constitutive androstane receptor, vitamin D receptor, and many other nuclear receptors can act as a heterodimeric partner for RXR. Therefore, the broad range of RA effects (through the 9-cis RA) is not induced solely by the RAR/RXR mechanism of action but also by the physiological effects of activation of any RXR heterodimerization partners [7]. ATRA and its isomers, 9-cis-RA and 13-cis-RA, are the naturally occurring ligands for the RARs. They can bind three RARs with different affinities. The 9-cis-RA is a high-affinity ligand for RXR, and a selective agonist of RARα, RARβ, and RARγ. The RARα is bound with the highest affinity by the 9-cis-RA followed by the 13-cis-RA and ATRA, whereas for RARβ and RARγ, the order of affinity is reversed [15][16]. A detailed description of the retinoic acid receptors and their functions is presented in Das et al. [17].
3. Retinoic Acid in the Skin
Retinoids, besides their success in APL (acute promyelocytic leukemia) therapy, are also widely used in the treatment of skin diseases such as skin cancer, psoriasis, acne, ichthyosis, and even wrinkles because of their effects on cell differentiation, proliferation, and apoptosis [18][19][20]. The usefulness of ATRA in the skin appears to be partially limited due to ATRA-mediated resistance caused by a variety of multifactorial mechanisms. The reason that ATRA has limited efficacy in the clinics may be linked to CYPs activity. Since RAs act towards normalization of the skin by regulating the keratin’s expression, an unregulated degradation of ATRA by CYPs can cause a RA deficiency state related to the progression of hyperkeratinization, desquamation in the context of acne, psoriasis, and ichthyosis [14][21][22]. To overcome these limitations, novel strategies associated with exogenous ATRA therapy have been proposed. These strategies are based on modulation and increasing levels of endogenous ATRA by inhibiting ATRA-4-hydroxylase enzymes, which are responsible for ATRA metabolism. These inhibitors are also referred to as retinoic acid metabolism-blocking agents (RAMBAs) and they are considered crucial in ATRA-mediated therapy [23]. The main mechanism of RAMBAs action is associated with inhibition or blocking of 4-hydroxylation of all-trans-retinoic acids, which depend on cytochrome P450-dependent enzymes (CYPs). This in turn results in an increase of the intracellular all-trans-retinoic acids [24]. Nowadays, there are a large number of RAMBAs which may modulate the activity of ATRA. This blocking aging may be divided into several groups of compounds which are structurally or chemically similar, i.e., to liarozole (LiazalTM) and related compounds—R115866 and R116010; azolyl retinoids and related compounds, benzene acetic acid derivatives, 2,6-disubstituted naphthalenes, or miscellaneous structures [22]. Regulation of endogenous ATRA concentration and its natural stereoisomers with the use of new RAMBAs may bring out additional cancer therapy strategies and treatments of dermatological diseases [22]. Ketoconazole (from azole) was the first RAMAB compound described by Van Wauwe et al. in 1988 [25]. In high doses (400 mg three times a day), ketoconazole affected the androgenic hormone, which was used in the treatment of prostatic cancer. In dermatology, the drug is used mainly as an antifungal agent used for the treatment of superficial and systemic fungal infections. The most studied RAMBA compound is liarozole. It has been proven that liarozole exhibits anti-tumor properties against prostate and breast cancers [26]. In dermatology, liarozole is used in treatment of psoriasis and ichthyosis [27]. In RAMBAs’ history, there were also examples of very promising but not widely used drugs. One of them—talarazol—was carefully investigated in the past decade for the treatment of acne, psoriasis, and other keratinization disorders; however, despite its properties, it was withdrawn from the market after the results of clinical trials [24].
References
- Niederreither, K.; Dollé, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553.
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019.
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A Journey from the Molecular Structures and Mechanisms of Action to Clinical Uses in Dermatology and Adverse Effects. J. Dermatol. Treat. 2017, 28, 684–696.
- Ross, A.C.; Zolfaghari, R. Cytochrome P450s in the Regulation of Cellular Retinoic Acid Metabolism. Annu. Rev. Nutr. 2011, 31, 65–87.
- Stevison, F.; Jing, J.; Tripathy, S.; Isoherranen, N. Role of retinoic acid metabolizing cytochrome P450s, CYP26, in inflammation and cancer. In Advances in Pharmacology; Academic Press: Cambridge, MA, USA, 2015; Volume 74, pp. 373–412.
- Watson, P.J.; Fairall, L.; Schwabe, J.W.R. Nuclear hormone receptor co-repressors: Structure and function. Mol. Cell. Endocrinol. 2012, 348–135, 440–449.
- Huang, P.; Chandra, V.; Rastinejad, F. Retinoic Acid Actions Through Mammalian Nuclear Receptors. Chem. Rev. 2014, 114, 233–254.
- Di Martino, O.; Welch, J.S. Retinoic Acid Receptors in Acute Myeloid Leukemia Therapy. Cancers 2019, 11, 1915.
- Leid, M.; Kastner, P.; Chambon, P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem. Sci. 1992, 17, 427–433.
- Schenk, T.; Stengel, S.; Zelent, A. Unlocking the potential of retinoic acid in anticancer therapy. Br. J. Cancer 2014, 111, 2039–2045.
- Bushue, N.; Wan, Y.-J.Y. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 1285–1298.
- Aging | Cellular Retinoic Acid Binding Protein-II Expression and Its Potential Role in Skin Aging. Available online: https://www.aging-us.com/article/101813 (accessed on 27 November 2020).
- Redfern, C.P.; Todd, C. Retinoic acid receptor expression in human skin keratinocytes and dermal fibroblasts in vitro. J. Cell Sci. 1992, 102, 113–121.
- Törmä, H. Regulation of keratin expression by retinoids. Dermatoendocrinology 2011, 3, 136–140.
- Idres, N.; Marill, J.; Flexor, M.A.; Chabot, G.G. Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers. J. Biol. Chem. 2002, 277, 31491–31498.
- Di Masi, A.; Leboffe, L.; De Marinis, E.; Pagano, F.; Cicconi, L.; Rochette-Egly, C.; Lo-Coco, F.; Ascenzi, P.; Nervi, C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Asp. Med. 2015, 41, 1–115.
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.-C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014, 22, 673–683.
- Coombs, C.C.; Tavakkoli, M.; Tallman, M.S. Acute promyelocytic leukemia: Where did we start, where are we now, and the future. Blood Cancer J. 2015, 5, e304.
- Lee, D.-D.; Stojadinovic, O.; Krzyzanowska, A.; Vouthounis, C.; Blumenberg, M.; Tomic-Canic, M. Retinoid-Responsive Transcriptional Changes in Epidermal Keratinocytes. J. Cell. Physiol. 2009, 220, 427–439.
- Castleberry, S.A.; Hammond, P.T.; Quadir, M.A. Nano-Fibular Nanoparticle Polymer-Drug Conjugate for Sustained Dermal Delivery of Retinoids. U.S. Patent 0185513 A1, 5 July 2018.
- Orfanos, C.E.; Zouboulis, C.C.; Almond-Roesler, B.; Geilen, C.C. Current use and future potential role of retinoids in dermatology. Drugs 1997, 53, 358–388.
- Njar, V.C.O.; Gediya, L.; Purushottamachar, P.; Chopra, P.; Vasaitis, T.S.; Khandelwal, A.; Mehta, J.; Huynh, C.; Belosay, A.; Patel, J. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg. Med. Chem. 2006, 14, 4323–4340.
- Purushottamachar, P.; Patel, J.B.; Gediya, L.K.; Clement, O.O.; Njar, V.C.O. First chemical feature-based pharmacophore modeling of potent retinoidal retinoic acid metabolism blocking agents (RAMBAs): Identification of novel RAMBA scaffolds. Eur. J. Med. Chem. 2012, 47, 412–423.
- Verfaille, C.J.; Borgers, M.; van Steensel, M.A.M. Retinoic acid metabolism blocking agents (RAMBAs): A new paradigm in the treatment of hyperkeratotic disorders. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2008, 6, 355–364.
- Ketoconazole Inhibits the in Vitro and in Vivo Metabolism of All-Trans-Retinoic Acid. | Journal of Pharmacology and Experimental Therapeutics. Available online: https://jpet.aspetjournals.org/content/245/2/718 (accessed on 27 November 2020).
- Vijjan, V.; Dubey, D. New therapeutic targets in the treatment of prostate cancer. Indian J. Urol. IJU J. Urol. Soc. India 2007, 23, 61–66.
- Vahlquist, A.; Blockhuys, S.; Steijlen, P.; van Rossem, K.; Didona, B.; Blanco, D.; Traupe, H. Oral liarozole in the treatment of patients with moderate/severe lamellar ichthyosis: Results of a randomized, double-blind, multinational, placebo-controlled phase II/III trial. Br. J. Dermatol. 2014, 170, 173–181.




