Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Margarita L. Martinez-Fierro | -- | 2164 | 2025-04-03 05:51:42 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; Martinez-Fierro, M.L.; González-Delgado, S. Therapeutic Effects of Coumarins with Different Substitution Patterns. Encyclopedia. Available online: https://encyclopedia.pub/entry/58071 (accessed on 28 February 2026).
Flores-Morales V, Villasana-Ruíz AP, Garza-Veloz I, Martinez-Fierro ML, González-Delgado S. Therapeutic Effects of Coumarins with Different Substitution Patterns. Encyclopedia. Available at: https://encyclopedia.pub/entry/58071. Accessed February 28, 2026.
Flores-Morales, Virginia, Ana P. Villasana-Ruíz, Idalia Garza-Veloz, Margarita L. Martinez-Fierro, Samantha González-Delgado. "Therapeutic Effects of Coumarins with Different Substitution Patterns" Encyclopedia, https://encyclopedia.pub/entry/58071 (accessed February 28, 2026).
Flores-Morales, V., Villasana-Ruíz, A.P., Garza-Veloz, I., Martinez-Fierro, M.L., & González-Delgado, S. (2025, April 03). Therapeutic Effects of Coumarins with Different Substitution Patterns. In Encyclopedia. https://encyclopedia.pub/entry/58071
Flores-Morales, Virginia, et al. "Therapeutic Effects of Coumarins with Different Substitution Patterns." Encyclopedia. Web. 03 April, 2025.
Copy Citation
The use of derivatives of natural and synthetic origin has gained attention because of their therapeutic effects against human diseases. Coumarins are one of the most common organic molecules and are used in medicine for their pharmacological and biological effects, such as anti-inflammatory, anticoagulant, antihypertensive, anticonvulsant, antioxidant, antimicrobial, and neuroprotective, among others.
coumarin
antineoplastic
cancer therapy
docking
1. Introduction
The medicinal use of compounds that are derived from natural sources such as fungi, plants, and animals precede documented human history as an ancient tradition by perhaps thousands of years [1]. These compounds have often been used to treat diseases and injuries with an important role in drug discovery and drug development thanks to the modern tools of molecular biology, biochemistry, and chemistry, which allow us to dissect biological effects on the human body, as well as possible interactions [2]. These advances put natural compounds in the spotlight as potential new therapies against many complex diseases, including inflammatory, vascular, hypertensive, infectious, and neurodegenerative diseases, as well as cancer [3].
Among the most studied natural compounds in human health are coumarins, which are molecular compounds of natural and synthetic origin. They constitute a family of heterocyclic compounds and have been extensively studied in the biochemical and pharmaceutical fields [4].
2. Coumarins
Coumarins have been studied for more than 200 years. Their name derives from the species Coumarouna odorata Aube (Dipteryx odorata), from which they were isolated for the first time [5]. Coumarin is a secondary metabolite found naturally in various plant families and in essential oils. The basic nucleus of coumarin (Figure 1) corresponds to the compound benzo-a-pyrone (2H-1-benzopyran-2-one), whose nomenclature was established by the International Union of Pure and Applied Chemistry (IUPAC). In addition to the isolated coumarins of natural origin, the synthetic derivatives due to the substituents at several positions in the chemical structures have increased the number of coumarins currently known, with interesting biological effects [1,5].
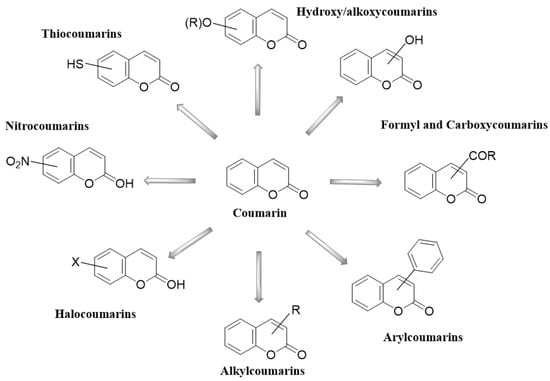
Figure 1. Coumarin as a basic nucleus for obtaining simple coumarins. Taken from Borges, F. et al. (2005) [6]. Accessed date 28 November 2022.
The coumarin 2H-chromen-2-one, 1,2-benzopyrone, being a semi-volatile lactone of low molecular weight, has a sweet odor and is present in more than 60 plants [7]. It was also identified that coumarins are present in tobacco, but in relatively low amounts, something that explains the cytotoxic of tobacco toxicants [8].
Coumarins and their metabolites have been useful in the pharmaceutical industry; they are part of the basic nucleus of many compounds with therapeutic purposes due to the great potential they have. Coumarins are present in various types of plants, among which we can mention Rutaceae, Apiaceae, Asteraceae, Leguminoceae, and Thymelaeaceae. Among the various therapeutic effects that have been identified, it was found that they present antimicrobial, antiarrhythmic, anti-HIV, and antineoplastic activity. Recent research has described the structure and biological function of analogues derived from coumarins synthesized from plants. Coumarins are classified into six main groups: simple coumarins [9], furanocoumarins [10], dihydrofuranocoumarins [11], pyranocoumarins [12], phenylcoumarins [13], and biscoumarins [14]. The six types of coumarins are shown in Table 1, showing some characteristic examples.
Table 1. Main structures of coumarins.
| Type of Coumarin | Structure | Examples of Coumarins | Reference |
|---|---|---|---|
| Single coumarin | 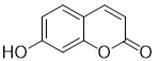 Umbelliferone (7-hydroxycoumarin) |
Esculetin | [15] |
| Ostrutin | [16] | ||
| Osthole | [17] | ||
| Novobiocin | [18] | ||
| Coumermycin | [19] | ||
| Umbelliferone | [20] | ||
| Fraxidine | [21] | ||
| Ferudenol | [22] | ||
| Furanocoumarin |  Imperatorin |
Isopimpinellin | [23] |
| Psoralen | [24] | ||
| Bergaptene | [25] | ||
| Methoxsalene | [26] | ||
| Marmelosin | [27] | ||
| Pyranocoumarin |  Nordentatin |
1. Linear type: | [28] |
| Grandivitin | [29] | ||
| Agasyllin | [30] | ||
| Aegelinol de Benzonatate | |||
| Xanthylethine | [31] | ||
| 2. Angular type: | |||
| Inophyllum A, B, C, E, P, G1 and G2 | [32] | ||
| Calanolide A, B and F | [33] | ||
| (+)-Dihydrocalanolide A and B | |||
| Pseudocordatolide | [34] | ||
| Biscoumarin | 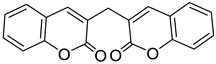 Dicoumarol |
Biscoumarin | [35] |
| Dihydrofuranocoumarin | 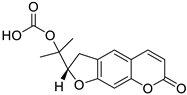 Felamidine |
Anthogenol | [36] |
| Marmesin | [37] | ||
| Rutaretin | [38] | ||
| Phenycoumarins | 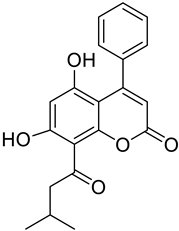 Isodispare B |
Dispardiol B | [39] |
| Mammea A/AB | [40] | ||
| Disparinol D | [41] | ||
| Disparpropylinol B | [42] |
The different derivatives of coumarins have characteristic properties that allow them to possess diverse therapeutic effects and therefore multiple applications in several areas of medicine showing to be useful as anticancer, carbonic anhydrase inhibitors, anti-HIV, anti-inflammatory, and anticoagulant agents, as biomarkers, and in green chemistry [43,44].
One of the main factors to which the biosynthesis of coumarin derivatives has been resorted is the resistance generated by the administration of some drugs, which has become a great inconvenience for the development and treatment of different types of cancer. Derived from this, compounds of natural origin and certain synthetic molecules to assess the possible resistance generated at the time of administration, have demonstrated significant potency in the inhibition of multidrug resistance (MDR) in patients with cancer [45].
3. Coumarins and Their Pharmacological Activity
Coumarins present diverse biological activities derived from their substitution pattern, which has an impact on their physicochemical properties and pharmacological application [46]. Crum-Brown and Frasser, through their research, proposed a theory by which they demonstrated that the mechanism of action and the pharmacological effect of a substance depends on the chemical composition [47].
Coumarins have shown hepatic metabolism where cytochrome P450 acts as an inducer of the oxidative processes of coumarins. It has also been demonstrated that the intestinal microbiome plays an important role. Coumarins are rapidly absorbed and distributed throughout the body with high concentrations detected in the liver and kidneys. Significant tissue accumulation of coumarin or its metabolites by the oral route has not been determined. The route of elimination depends on the administration and type of coumarin [48]. The main therapeutic effects of coumarins are described in the following paragraphs (Figure 2).
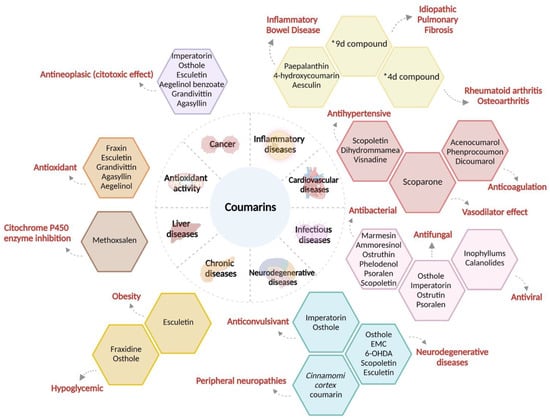
Figure 2. Properties and pharmacological effects of coumarins and coumarin derivatives against human diseases. A wide range of effects and pharmacological properties of cumarins have been reported; as we can see, scopoletin, esculetin, and osthole have been the most described coumarins to be involved in the modulation of different systems such as cardiovascular and neurological system. A great knowledge of coumarins is their variety number of antineoplastic properties, acting as cytotoxic compounds in different kinds of cancer. *9d compound: (N-(4-((2-((2-oxo-2H-chromen-4-yl)oxy)phenyl)-2-(piperidin-1-yl)acetamide); *4d compound: 3-(1H-benzo[d]imidazol-2-yl)-6-chloro-2H-chromen-2-one.
3.1. Therapeutic Use of Coumarins in Inflammatory Diseases
Coumarin (Figure 3A) has shown a therapeutic effect against edema, eliminating proteins and fluid from injured tissue by activating mechanisms such as phagocytosis, enzyme release and proteolysis [49]. Research in the field of pharmacology has indicated that imperatorin (Figure 3B) has an anti-inflammatory effect in lipopolysaccharide-stimulated mouse macrophages (RAW264.7) in an in vitro model of edema, as it inhibits the protein expression of nitric oxide synthase (NOS) and cyclooxygenase-2 (COX-2) [50]. Rabe et al. identified the anti-inflammatory and immunomodulatory effect of terpenoid coumarins (umbelliprenin and methyl galbanate) (Figure 2C,D) in vivo and in vitro demonstrating proliferative effects and the release of interleukin 4 (IL-4) and interferon (INF)-γ upon COX-2 inhibition [51,52].
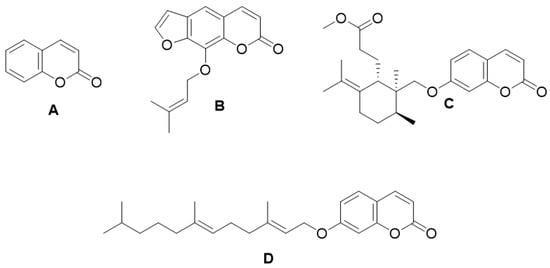
Figure 3. Chemical structures of coumarin (A), imperatorin (B), methyl galbanate (C), and umbelliprenin (D).
3.2. Coumarins in Cardiovascular Disease
Coumarin derivatives have demonstrated pharmacological effects based on the substituents, e.g., coumarins with different heterocycles based on the cyclization of 2-ethoxy-3-phenylpropanoic acid and 2-benzylmalonic acid have been investigated as lipid-lowering agents to inhibit the formation of atheroma caused by the accumulation of triglycerides and cholesterol in the walls of arteries or blood vessels, thereby decreasing the incidence of cardiovascular disease [63]. Coumarin 7,8-dihydroxy-3-(4-methylphenyl)coumarin was assessed for its lipid-lowering effects in a murine model of hypercholesterolemia, and led to a significant reduction in serum cholesterol levels [64].
3.3. Coumarins in Infectious Diseases
Coumarins with antimicrobial effects has been evaluated in several reports. They may have anti-bacterial, antifungal, and/or antiviral activities.
3.4. Coumarins in Neurodegenerative Diseases
Neurodegenerative diseases are a heterogeneous group of disorders that are characterized by the progressive degeneration of the structure and function of the central nervous system or peripheral nervous system, and are an important public health problem in older adults. This group of diseases includes Alzheimer’s disease [82], Parkinson’s disease and Huntington’s disease, among others. The greatest known risk factor for many neurodegenerative disorders is age. These figures are likely to rise as the population ages, making neurodegenerative disorders a growing healthcare concern. Coumarins are considered to be promising molecules against several neurodegenerative diseases because they have been shown to ameliorate clinical manifestations and symptoms.
3.5. Coumarins in Chronic Degenerative Diseases
A degenerative disease is a disease in that in which the function or structure of affected tissues or organs worsens over time. The pharmacological properties of coumarins have also been tested with the aim of lessening the signs and symptoms of degenerative diseases, including the complications of obesity [98], metabolic syndrome [99,100], and type 2 diabetes mellitus (T2DM) [101,102] among others.
3.6. Coumarins in Liver Diseases
A coumarin derivative that showed a microsomal inhibitory effect against the cytochrome P450 enzyme was methoxsalen (8-methoxypsoralen) (Figure 20B) synthesized from the seeds of Ammi majus (Umbelliferae family). This compound at a single dose was tested in vitro and found to be effective and selective against hepatic CYP2A6, having a clinical utility against the metabolism of this enzyme [103,104,105].
3.7. Coumarins with Antioxidant Activity
Fraxin (Figure 20C), a coumarin derivative, demonstrated an antioxidant effect through an in vitro model in cells induced with H2O2-mediated OS showing a protective scavenging effect against free radicals at a high concentration of 0.5 mM [106]. Another coumarin that also demonstrated an antioxidant effect was esculetin (6,7-dihydroxycoumarin) through an in vitro model in Chinese Hamster lung cells (V79-4) inhibiting H2O2-induced damage of fibroblasts [107]. The pyranocoumarins grandivitin, agasilin and aegelinol benzoate, isolated from the roots of Ferulago campestris (Apiaceae), showed an antioxidant effect by evaluating the effect on human whole blood leukocytes and on isolated polymorphonuclear cells by chemiluminescence [108].
3.8. Coumarins as Phytoalexins
Phytoalexin is synthesized from plants in response to a fungal infection, physical damage, chemical injury, or pathogenic damage. Through various signaling mechanisms, phytoalexin inhibits foreign agents such as bacteria, insects, and even, viruses. One phytoalexin is ayapin (6,7-methylenedioxycoumarin) (Figure 20D), isolated from Eupatorium ayapana (Asteraceae) [109].
3.9. Main Biological Targets of Coumarin Derivatives According to In Vitro, In Vivo, and Structure-Activity Relationship Analyses
The therapeutic activity of coumarin compounds is largely linked to the substitution pattern in their structure. In addition, it has been reported that unsubstituted coumarins tend to be toxic when metabolized [110]. The introduction of one or several substituents has electronic effects on the structure, which is associated with the biological properties that it may possess, resulting in being very attractive in the design of new drugs, and expanding the development of bioactive molecules. In this sense, coumarins present a wide variety of biological activities as antibiotic agents, antioxidant [111], antiviral [112], antimalarial [113], antineoplastic [114], antimicrobial, anti-inflammatory, and anticoagulant.
The biological activity, as well as its possible therapeutic application, are related to the substitution patterns that the coumarin nucleus present. In the case of anti-inflammatory activity, in general, the derivatives substituted in positions 3 and 4 contain highly variable groups, ranging from aliphatic chains, aromatic rings to heterocycles, especially those containing nitrogen atoms (4-hydroxycoumarin, 3-(1H-benzo[d]imidazol-2-yl)-6-chloro-2H-chromen-2-one) [115,116]. There are few reports on substituted derivatives at position 5, when this occurs a hydroxyl or methoxy group is generally found ((N-(4-((2-((2-oxo-2H-chromen-4-yl)oxy)phenyl)-2-(piperidin-1-yl)acetamide)). On the other hand, position 6 is generally substituted with halogens such as chlorine and bromine mainly, since it is observed that they increase the activity of the molecule; positions 7 and 8 can be hydroxyl groups, aliphatic chains, aromatic systems, and heterocycles (paelanthin and aesculin) [117]. Most of the coumarins used for these type of conditions are inhibitors of the production of NO and COX-2 (Figure 21).
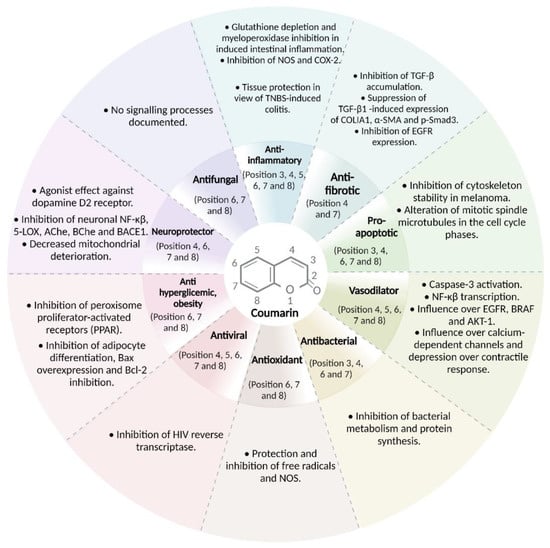
Figure 21. Coumarins substitution patterns and effects on the cell and molecular processes. Depending on position of the substituent (from 1 to 8) on the coumarin ring, the coumarins possess the ability to regulate biological and pathological processes, as seen pathological processes such as inflammation and/or infectious diseases. Coumarins with substituents on 3, 4, 7, and 8 positions have been the most described to have influence over these pathological processes.
Regarding the coumarins used as anticoagulants (acenocoumarol, phenprocoumon, and dicoumarol) and antihypertensives (dihydrommamea), they share substituents at positions 3, 4, and 5 of the coumarin ring. Coumarins with substituents in positions 6 and 7 (scopoletin, scoparone) and substitutions 7 and 8 (visnadine) show antihypertensive activity. Dicoumarol‘s mechanism of action is that it is a selective vitamin K antagonist, while scoparone shows its effect through the activation of calcium channels.
Coumarins have an important action in infectious diseases, either as antibacterial, antifungal, or antiviral. Their function is carried out through inhibition of metabolism, reproduction, or protein synthesis. Most of the coumarins with antibacterial and antifungal activity share substituents at positions 6 and 7 of the basic coumarin nucleus (marmesin, ostruthin, phelodenol, scopoletin, among others). While ammoresinol (substituents in positions 3,4 and 7) and inophyllums (substituents in 4, 5, 6, 7, and 8) have more complex substitution patterns.
The activity of coumarins has also been tested in neurodegenerative diseases, observing that structurally coumarins have substituents in positions 6 and 7, as is the case with 6-OHDA, scopoletin and esculetin. Acting anticholineterase activity (scopoletin) or inhibiting the activation of NK-kB via 5-LOX.
The study of coumarins in the field of chronic diseases has also been developed. Esculetin, with a 6,7-substitution pattern, showed activity against obesity activity by inhibiting adipocyte differentiation. While fraxidine (6,7,8 substitution) and osthole (7,8 substitution) have hypoglycemic activity by inhibiting peroxisome proliferation-receptor activation (PPAR).
The antioxidant function of coumarins is widely reported and their substitution pattern corresponds to positions 6 and 7. Examples are fraxin, esculetin, grandivittin, agasyllin and aegelinol; which exhibit a free radical scavenging effect H2O2-mediated OS.
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
632
Entry Collection:
Common Diseases
Revision:
1 time
(View History)
Update Date:
03 Apr 2025
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





