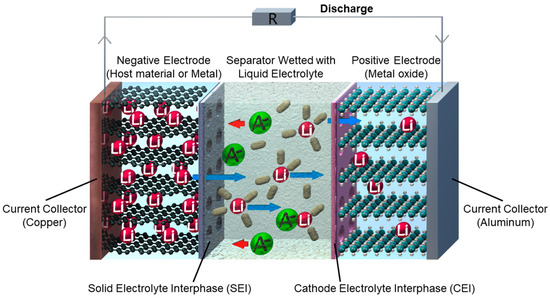
Each battery cell consists of three main components: the anode, the cathode, and the separator soaked with liquid electrolyte, the medium in the battery that allows charged ions to move between the two electrodes. Besides a wide electrochemical stability window and good compatibility with both electrodes, the electrolyte should also be safe, thermally stable and environmentally benign, showing a high ionic conductivity of the charge-carrying Li ions and finally a low price. This unique combination of properties is impossible to achieve with a simple salt–solvent mixture and usually requires a combination of different electrolyte components, i.e., several liquid solvents and additives and one or more conducting salt(s). For lithium-based batteries, which are the most common electrochemical energy storage devices today, a solution based on lithium hexafluorophosphate (LiPF6) in a mixture of organic carbonates as the solvent is used. Usually, the conducting salt concentrations used for lithium-based electrolytes are in the range of ≈1 to 1.2 M, but recently, electrolytes with much higher conducting salt concentrations of 5 M and even over 10 M have been investigated as they offer several benefits ranging from increased safety to a broadened electrochemical stability window, thus enabling cheap and safe solvents, even water.
Lithium-based batteries, such as lithium-ion batteries (LIBs) and lithium metal batteries (LMBs), for electrochemical energy storage and conversion have reshaped the technology sector by powering devices ranging from portable electronics, to electric vehicles to stationary (“grid”) storage. LIBs are commercially available in various sizes and chemistries, usually distinguished by their performance characteristics. For a battery cell consisting of two electrodes separated by an insulating membrane
[1], the electrolyte is the key factor for ion transport and the overall battery performance. It enables the lithium ions to move between the electrodes during charging and discharging without short-circuiting the battery (
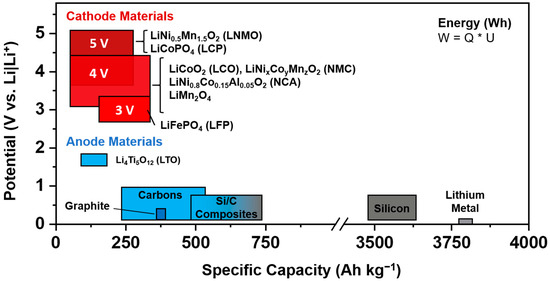
Figure 1). A selection of common materials for the positive and negative electrode characterized by their operating potential range against Li|Li
+ and specific capacity is shown in
Figure 2.
Figure 1. Schematic battery setup and working principle of a lithium-based battery during the discharge process.
Figure 2. Common electrode materials for lithium-based batteries and their capacities and potentials.
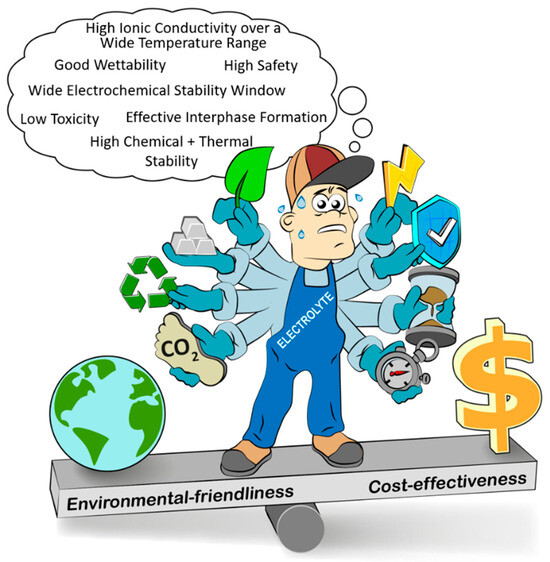
Due to the electrolyte’s unique role as ion transport medium, it has to simultaneously provide a wide range of properties: high ionic conductivity (>1 mS cm−2) in a wide temperature range (−20 to 60 °C), high chemical and thermal stability, broad (kinetic) electrochemical stability window (ESW) (0 to up to 5 V vs. Li|Li+), sufficient wettability, compatibility with further battery components, including particularly electrodes and an effective interphase formation as well as environmental friendliness, high safety, low toxicity and cost-effectiveness (Figure 3).
Figure 3. Properties that an ideal electrolyte for lithium-based batteries should provide.
In commercial lithium-based batteries, the state-of-the-art electrolytes are non-aqueous liquid-based systems. Commonly, the lithium conducting salt, in most cases lithium hexafluorophosphate (LiPF
6), is dissolved in concentrations ≈1 to 1.2 mol L
−1 (1 to 1.2 M) in a mixture of aprotic solvents such as linear and cyclic organic carbonates, like dimethyl carbonate (DMC), ethyl methyl carbonate (EMC) or ethylene carbonate (EC)
[2][3]. Customarily, (multi)-functional additives are introduced to these electrolyte formulations to form effective and robust interphases
[4]. The solid electrode interphase (SEI) at the anode side and the cathode electrolyte interphase (CEI) at the cathode side are rather solid layers formed by multiple electrolyte decomposition products at the electrode–electrolyte interface
[5][6]. In the ideal case, this layer is lithium-ion-conducting and protects the electrolyte from ongoing decomposition. The benefits of moderate-concentration organic carbonate-based electrolytes are their high ionic conductivity, good wettability, compatibility with the electrode materials and sufficient electrochemical stability. Nevertheless, the chemical instability against moisture, the volatility and flammability of these electrolytes are accompanied by considerable safety risks and a limited operating temperature range for the battery cell
[2]. Additionally, the performance of moderate-concentration standard organic carbonate-based electrolytes in combination with LMB based electrode chemistries is typically quite poor
[7].
Increasing the conducting salt concentration in the electrolyte very much beyond 1.2 M leads to a new class of concentrated liquid electrolytes, high-concentration electrolytes (HCEs), also called solvent-in-salt electrolytes
[8]. Compared to the aforementioned liquid moderate-concentration electrolytes (MCEs) with conducting salt concentrations in the range of 1 to 1.2 mol L
−1, HCEs have a conducting salt concentration of up to 5 M or even exceeding 10 M. Often, the conducting salt concentration for HCEs is no longer given as molar concentration, but as molal concentration. Molarity is defined as the amount of dissolved salt ions per liter of solvent (mol L
−1, M). In contrast, the molality is specified as the number of dissolved salt ions per kilogram of solvent (mol kg
−1, m), which makes the molal concentration independent of temperature and pressure. The increase in conducting salt concentration goes along with a change in the coordination environment and solvation structure in the electrolyte, thus causing thermally and electrochemically different properties. Moreover, the composition of SEI and CEI is changing, too. The advantages of an HCE over an MCE comprise an increased electrochemical stability at both electrodes, enhanced Li
+ transport properties in the electrolyte, lower volatility and higher thermal stability, which improve the battery’s safety aspects. However, a disadvantage for HCEs is their higher viscosity, which results in a lower ionic conductivity compared to the MCEs. Besides, due to the higher costs for the conducting salt, the cost-effectiveness of such electrolytes is decreasing in relation to the state-of-the-art electrolytes.
In line with this, finding the balance between the advantages and disadvantages of HCEs is the highest aim for designing an ideal electrolyte. Herein, the history of the development of HCEs for lithium-based batteries with non-aqueous and aqueous electrolytes up to today’s newest research results will be illuminated and the electrolyte properties will be discussed. In “beyond lithium chemistries” such as sodium-, potassium-, magnesium- or zinc-ion batteries, HCEs are also reported in the literature
[9], but will not be considered here.