Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ashutosh Pareek | -- | 4830 | 2024-04-02 12:08:22 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pareek, A.; Kumari, L.; Pareek, A.; Chaudhary, S.; Ratan, Y.; Janmeda, P.; Chuturgoon, S.; Chuturgoon, A. Emerging Therapies and Therapeutics for Atopic Dermatitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/56544 (accessed on 07 February 2026).
Pareek A, Kumari L, Pareek A, Chaudhary S, Ratan Y, Janmeda P, et al. Emerging Therapies and Therapeutics for Atopic Dermatitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/56544. Accessed February 07, 2026.
Pareek, Ashutosh, Lipika Kumari, Aaushi Pareek, Simran Chaudhary, Yashumati Ratan, Pracheta Janmeda, Sanam Chuturgoon, Anil Chuturgoon. "Emerging Therapies and Therapeutics for Atopic Dermatitis" Encyclopedia, https://encyclopedia.pub/entry/56544 (accessed February 07, 2026).
Pareek, A., Kumari, L., Pareek, A., Chaudhary, S., Ratan, Y., Janmeda, P., Chuturgoon, S., & Chuturgoon, A. (2024, April 02). Emerging Therapies and Therapeutics for Atopic Dermatitis. In Encyclopedia. https://encyclopedia.pub/entry/56544
Pareek, Ashutosh, et al. "Emerging Therapies and Therapeutics for Atopic Dermatitis." Encyclopedia. Web. 02 April, 2024.
Copy Citation
Atopic dermatitis (AD) is an inflammatory skin condition that frequently develops before the onset of allergic rhinitis or asthma. More than 10% of children are affected by this serious skin condition, which is painful for the sufferers. Recent research has connected the environment, genetics, the skin barrier, drugs, psychological factors, and the immune system to the onset and severity of AD. The causes and consequences of AD and its cellular and molecular origins are reviewed in this paper. The exploration of interleukins and their influence on the immunological pathway in AD has been facilitated by using relevant biomarkers in clinical trials.
atopic dermatitis
therapeutics
interleukins
interferon
T helper cells
1. Microbiome Modulation
Altering microbiota diversity helps in modulating the development of AD. Different strategies have been proposed for the modulation of the skin’s microbiome. Two clinical trial products (MSB-01 and DB-001) are currently being used to investigate microbiome transplantation and bacterial modulation [35]. However, there are currently no conclusive research findings supporting this theory. The skin microbiome of AD patients has a greater abundance of Gram-negative bacteria in the Rosemonas mucosa membranes as compared to healthy skin, which is more composed of Gram-positive bacteria [36]. A mucosal erythrocyte series (FB-401), whose therapeutic capabilities may include activating tissue healing and inhibiting Toll-like receptor 5 (TLR5) signaling and tumor necrosis factor receptor (TNFR), has been developed [37]. The topical treatment of Staphylococcus hominis A9 (ShA9), involving the use of a similar bacteriotherapeutic approach, kills S. aureus and inhibits S. aureus-produced toxins, allowing the microbiome to recover [38]. The bacterium Nitrosomonas eutropha (B244), which can produce nitric oxide by oxidizing ammonia, has been utilized to treat AD [39]. A synthetic antimicrobial cationic peptide called omeganan pentachloride (CLS-001) is now undergoing clinical testing as a possible topical treatment for managing dysbiosis [40]. Several microbiome modifiers designed for oral use are currently in phase I trials, including EDP1815, STMC-103H, and KBL69 [41]. Future strategies are likely to focus on gaining a better understanding of how bacterial quorum sensing and host immune responses influence the modulation of the skin microbiome. It may be possible to use interventions targeting the skin microbiome, possibly during infancy, to address this disease at an early stage. Determining the most effective timing for intervention could be crucial for enhancing disease management and potentially restoring a healthy adaptive immune response against S. aureus [42].
2. Targeting the Function of the Epidermal Barrier
There are two strategies aimed at restoring the epidermal barrier function in AD. The first involves developing products that specifically target the biochemical changes associated with this condition. However, the lack of understanding regarding the functional genetics of the various structures involved presents a significant obstacle to this approach. The second strategy focuses on effectively managing the underlying inflammatory response, although this may not completely restore barrier function. As a result, the current approach involves individualized and empirically adapted skincare using emollients or moisturizers alongside inflammation control [43,44]. This combined approach remains the primary method for improving barrier function, alleviating dryness, and reducing water loss and is considered fundamental therapy [43]. Supporting this idea, even basic products like petrolatum have been found to enhance antimicrobial activity and improve epidermal barrier function [44]. While initial studies showed promise in using emollients and moisturizers to prevent AD in high-risk newborns, a recent report has raised doubts about the effectiveness of this strategy [45].
3. Controlling Immunological Response
3.1. Innate Immune Response
The role of the innate immune system was demonstrated using animal models in the early phase of AD [46].
-
Aryl-hydrocarbon receptor (AhR)
A ligand-activated transcriptional factor, known as the AhR, plays a dual role in the development of numerous types of skin inflammation, including AD [47]. AhR can be an excellent option for a pharmacological strategy as it involves keratinocyte expression and epidermal DCs’ residence. Immunohistochemistry and transcriptome studies have shown that coal tar binds to AhR and restores FLG expression [48].
Tapinarof (benvitimod), which is a natural agonist of AhR, decreases inflammatory reactions when topically administered in both animal models and human skin. The most common adverse events were upper-respiratory-tract infections and folliculitis. The data indicate that this compound shows potential as a new and promising choice for treating AD and psoriasis, both chronic inflammatory skin conditions [49].
-
SPINK5
SPINK5 is mostly prevalent in keratinocytes and is associated with skin differentiation. When inflammation occurs, it infiltrates TSLP, IL-33, and IL-25, activating Th2 cells [50]. There are possible therapeutic targets that include these interleukins. Th2 activation is enhanced by the cytokine TSLP, which is produced in response to pro-inflammatory stimuli. These stimuli promote the production of IL-4, IL-5, IL-13, and TNF-α, affecting mast cells, DCs, and natural killer cells, among other immune cells. In patients with acute or chronic AD, keratinocytes exhibit elevated expression of TSLP [46,51].
Patients with chronic and unmanageable allergic asthma responded favorably to the anti-TSLP antibody tezepelumab (AMG 157) [52,53]. In contrast, a phase IIa trial involving patients with AD revealed that 64.7% of patients achieved the EASI50 endpoint, which can be compared to 48.2% in the placebo group. However, interpreting these results is challenging because all the patients were permitted to use topical corticosteroids (TCSs) [54].
-
IL-36R
IL-36 is part of the innate immune system and is increased in the skin of both psoriasis and AD patients. Notably, in mice, infection with S. aureus leads to inflammation that relies on IL-36R and IL-1R. As a result, a treatment called spesolimab, which targets IL-36R and has been effective in treating a rare type of psoriasis, is being studied in a phase-II trial involving 51 AD patients [55].
It is still unclear which specific pathways within the innate immune system play a significant role in the early stages of AD in infancy and throughout the progression of this disorder. Understanding this aspect is essential for developing effective targeted treatments [42].
-
IL-1
IL-1, a crucial cytokine for innate immunity, induces inflammation. IL-1R and IL-1 are both highly produced in skin cells and need to be balanced for epidermal homeostasis to be maintained [56]. IL-1 expression is raised in patients with inflammatory cutaneous diseases such as psoriasis, alopecia areata, and AD. Through an increase in Th1 expression, IL-1 leads to the formation of Th17 and Th2 cells as well as the chronification of AD lesions [57].
Phase-II, open-label, dose-escalation trials have been conducted on the anti-IL-1 monoclonal antibody bermekimab (MABP1). The corresponding study compared two groups receiving different doses of bermekimab: 200 mg and 400 mg. The 400 mg group showed greater improvements in Eczema Area and Severity Index (EASI) scores, along with other severity measures like IGA, pain, and itchiness, compared to the 200 mg group [58].
-
IL-33
In AD, keratinocytes release IL-33, which causes the skin barrier to deteriorate and become inflamed; when the skin is exposed to allergens or staphylococcal toxins, it generates excessive amounts of IL-33. IL-33 activates Th2 responses and increases the production of IL-4, IL-5, and IL-13 [59]. A single application of Etokimab was administered in a proof-of-concept study using 12 patients, and the improvement was observed to last for 140 days [60]. The G1k humanized anti-IL-33 monoclonal antibody etokimab did not meet its primary objective in a 16-week phase II b clinical study on treating AD and is no longer being studied. Itepekimab (REGR3500), another IL-33 inhibitor, proved insignificant in a phase II clinical trial [61].
3.2. Adaptive Immune Response
The etiology of AD is excessively complex, reflected in the variability of its clinical phenomenology. The “march of the adaptive immune system,” which affects several pathways and offers a variety of therapeutic options, begins with the introduction of antigens [62]. Antigen presentation is the starting point for the fundamental “march that comprises the adaptive immune system”, which regulates several pathways and gives rise to various treatment options [32,63]. Apart from targeting Th2 immune response, which involves IgE, IL-4, IL-5, IL-13, IL-31, IL-18, IL-37, Janus kinase (JAK), OX 40, and IL-4R (a common receptor chain between IL-13 and IL-4), there are other potential targets, including mediators associated with conditions like psoriasis, such as IL-17, IL-36, or IL-22, which are being explored in clinical studies [63,64].
-
IL-4 and IL-13
The cytokines IL-4 and IL-13, which are crucial in the pathophysiology of AD, appear to be the primary determinants of the Th2 immune axis. In mouse studies, they enhance S. aureus infections, resulting in pruritus, xerosis, inflammation, and an epidermal phenotype like AD [65]. IL-13 and IL-4 impair the barrier in AD skin and decrease the generation of proteins necessary for terminal differentiation [65].
The IgG4k IL-13 antagonist tralokinumab prevents IL-13 from interacting with IL-13R1 and IL-13R2. Tralokinumab was FDA-approved in December 2021 for treating adult patients with moderate-to-severe AD. Three phase-III clinical trials remain ongoing, while six have already been completed. Conjunctivitis and upper respiratory tract infections were the most often-reported side effects [66]. Similar to this, lebrikizumab is an IgG4k monoclonal antibody that selectively binds to IL-13, blocking the heterodimerization of IL-13Rα1/IL-4Rα and the ensuing signaling, which ultimately stops the proliferation of AD [67,68].
-
IL-5
IL-5 causes eosinophils to migrate, which is important in relation to atopic diseases such as eosinophilic esophagitis and asthma. In AD patients, blood eosinophil levels are typically higher and appear to be correlated with the severity of this condition [69]. Patients with extrinsic AD and concomitant respiratory allergy illness had higher blood levels of eosinophils [70,71].
Benralizumab and mepolizumab inhibit the action of IL-5 by inhibiting IL5R. Two phase-II studies, including on benralizumab, have been conducted; one is now complete, although the findings have not yet been made public [72,73]. A phase-II clinical trial involving the IgG1k IL-5 inhibitor mepolizumab was eventually stopped because the criteria used and met were ineffective. Mepolizumab significantly reduced the quantity of peripheral blood eosinophils during 16 weeks of treatment. However, it did not meet the primary goals of clinical improvements [72].
-
IL-31
Since this cytokine induces pruritus symptoms in AD patients, IL-31 is known as the “itch cytokine” [74]. Activated macrophages, epidermal keratinocytes, eosinophils, basophils, DCs, and cutaneous peripheral nerves are among the immune cells that express IL-31RA, which is the target of the humanized monoclonal antibody nemolizumab. Through the inhibition of IL-31RA, AD inflammation and pruritus may be controlled [75,76]. Nemolizumab can fail to treat eczematous plaques as rapidly or effectively as conventional immunobiologics or JAK inhibitors despite reducing itching by blocking IL-31 [77].
-
JAK
For several cytokines, including interleukins like IL-4, IL-13, and IL-31, the JAK/STAT signaling pathway serves as a classical cascade. [78]. The complete blocking of JAK signaling, which is necessary for immune function and homeostasis, causes severe immunodeficiency [79]. JAK inhibitors exert limited and reversible effects by limiting competition to lower intracellular signal transmission, in contrast to biologics intended to change cytokine signaling pathways [80]. When cytokines, including TSLP, IL-4, IL-13, IL-22, and IL-31, bind to JAK1 heterodimeric receptors in AD patients, this causes the receptors to be activated, which, in turn, causes Th2 cell differentiation and itching. Three JAK inhibitors, upadacitinib [81], abrocitinib [82], and baricitinib [83], have just acquired licenses for treating AD. Despite being biologics, which are large molecules administered parenterally, JAK inhibitors are small substances that may be used orally. Tyrosine kinase 2 (TYK2) and JAK1/2/3 inhibitors, such as delgocitinib, have played a significant role in AD treatment. Following studies conducted by Nakagawa et al. [84] in Japan, delgocitinib 0.5% ointment received a license for AD treatment for adults and children, alongside a 0.25% ointment. Notable small molecules like ruxolitinib [85], cerdulatinib [86], and brepocitinib [87] function as selective JAK inhibitors, contributing to AD therapy.
Biological molecules, including dupilumab, tralokinumab, omalizumab, and nemolizumab, have demonstrated significant efficacy in regulating the JAK/STAT pathway and inhibiting JAK. This is particularly relevant as the JAK/STAT pathway encompasses interleukins such as IL-4, IL-13, and IL-31 [88].
-
OX40
OX40 functions primarily as a costimulatory receptor and is a member of the TNF receptor superfamily. It expresses when T-cells, including effector and regulatory T-cells (Tregs), become stimulated. When there is inflammation, TSLP activates antigen-presenting cells, including endothelial cells and DCs, causing them to produce OX40L [89]. Additionally, it increases T-cell adhesion and migration and encourages and maintains the growth of Th2 central memory cells. By preventing further Th22 pathway activation after Th2 activation, blocking this receptor–ligand interaction may also promote the proliferation of Tregs and T-cell tolerance [90]. An anti-OX40 monoclonal IgG1 antibody (GBR830) [91] passed phase IIa testing, and the findings are encouraging. Amlitelimab [92], an anti-OX40L monoclonal antibody, reduced IL-22 serum levels; however, there was no difference in IL-22 baseline serum levels between responders and nonresponders. Rocatinlimab (KHK4083) [93] is a monoclonal antibody against OX40 that yielded positive results during a 16-week phase-II experiment. A novel selective phosphodiesterase 4 (PDE4) inhibitor, difamilast, is currently undergoing phase-3 trials. Difamilast achieved a significant milestone in 2021 by becoming the first PDE4 inhibitor to obtain manufacturing and marketing approval in Japan for treating both adult and pediatric AD patients, including those ≥2 years old [94].
-
IL-4R
An excellent target is IL-4R, which encourages the signaling of IL-4 and IL-13. The FDA has recently authorized the use of dupilumab, an IgG4 monoclonal antibody that explicitly targets IL-4R, for the treatment of AD for patients aged six months and older [95].
-
IL-17A
In a pilot study (NCT02594098), secukinumab, an anti-IL-17A antibody presently used to treat plaque psoriasis, was explored with regard to AD treatment [96]. The findings showed that there were no discernible differences in clinical improvements (alterations in SCORAD and the EASI score from the beginning of the study) between the secukinumab group and the placebo group at week 16. These findings suggest that focusing on IL-17 by itself is insufficient to effectively cure AD. Although another phase II-trial is complete, the results have not yet been released [97].
-
IL-22
IL-22 plays a vital role in the proliferation and downregulation of FLG in keratinocytes [98]. This seems an attractive therapeutic approach for AD treatment. Anti-IL-22 fezakinumab can be used for the downregulation of IL-22. Furthermore, it was shown that fezakinumab-induced IL-22 inhibition could cause the AD genomic profile to revert [99].
-
IL-18 and IL-37
A recently identified anti-inflammatory member within the IL-1 cytokine family is interleukin 37 (IL-37) [100]. Notably, children with AD exhibit significantly lower levels of IL-37 in their skin barrier. It is crucial to highlight the relevance of the cytokine IL-18 in relation to IL-37 [101]. Compared to IL-37, IL-18 provides a 50-fold-higher receptor affinity for IL-18Rα [102]. As biological drugs and JAK become more widely accessible, there is a strong focus on finding further treatments that directly adjust inflammatory processes and specifically target immune pathways or substances involved in AD’s development.
4. Treatment Strategies for AD and a Recently Developed Novel Drug Delivery System Based on Nanotechnology
The current research emphasizes therapeutic approaches that maximize formulation efficacy while minimizing adverse effects [106]. Treatment safety has been improved via numerous research efforts, including using novel drug delivery systems, combined therapy, and specific delivery systems (patch, liposome, nanoparticle, etc.) [107]. AD treatment plans center on lowering inflammation when necessary, healing skin, and eliminating itching. As a result, effective management and therapy make it feasible to apply a multi-targeted strategy that calls for caretaker and patient awareness (Figure 4). Additionally, it has been advised that taking care of the skin is necessary, entailing using topical calcineurin inhibitors (TCIs) for anti-inflammatory therapy, taking corticosteroids, and treating any skin infections. In cases of severity, systemic corticosteroids may also be used [108].
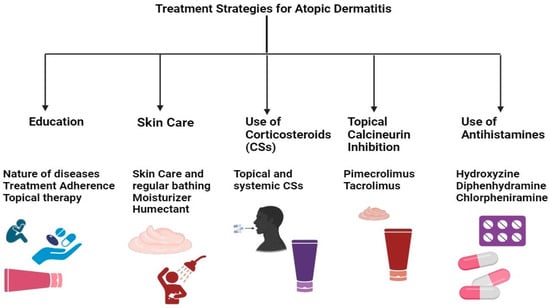
Figure 4. Multi-targeted treatment strategies for AD treatment.
The treatment of AD encompasses skincare routines, topical solutions, systemic treatments, and preventive measures. Besides preventive measures and topical therapy, conventional systemic therapies include the use of drugs like cyclosporine, methotrexate, and azathioprine, alongside novel systemic medications such as biological agents and JAK inhibitors [34]. Notably, drugs targeting IL-4, IL-13, and IL-33, including dupilumab, and JAK inhibitors like baricitinib, abrocitinib, and upadacitinib have been approved or are pending approval in certain regions for moderate to severe AD. These treatments, especially JAK inhibitors, have demonstrated the ability to provide rapid itch relief and improve conditions, enhancing patients’ quality of life and presenting a generally favorable safety profile, though long-term safety data are still needed. Additionally, tralokinumab, a recently approved biological drug that specifically targets IL-13, has shown safety and efficacy, particularly when used with topical corticosteroids. Despite the effectiveness of these therapies, their high cost can limit access in some countries. The economic impact of these treatments and their affordability continue to be significant considerations, given the chronic nature of AD and its impact on quality of life. Measures such as EASI, the Dermatology Life Quality Index (DLQI), and the Pruritus/Itch Numeric Rating Scale are utilized to evaluate disease severity and treatment impact, underscoring the potential value of these therapies despite their expense. However, traditional biologics like rituximab, omalizumab, and ustekinumab are not recommended for treating AD, with mepolizumab being reserved for cases unresponsive to standard therapies [109].
Nanotechnology offers a safer and more effective method for treating various skin conditions, like AD, psoriasis, eczema, and cancer. Although nano-cosmetics are available, their potential for treating skin conditions still requires investigation. Nano-based drug delivery systems allow drugs to be precisely delivered to the skin with controlled release and diffusion, reducing off-target side effects. Nanoparticles can also overcome the skin’s natural barriers and poor drug solubility, enhancing drug delivery. Different nanoparticle formulations, including antibiotics, corticosteroids, herbal, synthetic, and combinations of these drugs, have been developed for treating AD through topical application [110,111,112].
In AD, ceramide levels drop, trans-epidermal water loss increases, and the skin barrier is compromised. As a result, surface engineering for vehicles has received much greater attention. API-loaded particles with the ideal charge, size, and surface transformation can treat skin conditions successfully [113,114]. Different nanosized systems, starting with liposomes, have been developed to improve the deep absorption of drugs into the skin. Polymeric particulates, nanoparticles made of lipids, dendrimers, and dendritic-core multi-shell nano transporters are some of the carriers that have been examined [115,116].
Lipid nanoparticles serve as carriers in place of liposomes, emulsions, and polymeric nanoparticles. The lipid NPs that showed the most potential for cutaneous application were solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs).
Yu et al. [117] developed chitosan-based nanoparticles (NPs) containing tacrolimus (FK506) and nicotinamide (NIC) as a hydrotropic agent. These NP exhibited high entrapment efficiency and stability in vitro. In vivo studies demonstrated sustained skin permeation for up to 24 hours in Sprague Dawley rats. BABL/c mice with DNCB-induced AD-like skin lesions treated with NPs showed lower dermatitis scores compared to the induced group. Additionally, measurements of ear skin thickness and splenic weight were conducted. In the DNCB group, the thickness was significantly higher than that for the treated mice, and spleen index elevation post-AD-induction suggested immune activation in the AD murine model. Treatment with NIC-CS-NPs loaded with FK506 suppressed activated immunity and decreased spleen index values, further reducing spleen index values compared to Protopic. The anti-inflammatory and immunosuppressive effects of NIC–CS–NPs were evident. In the histopathology of the DNCB-induced AD skin lesions, mast cell infiltration in DNCB-induced AD skin lesions was significantly decreased in the treatment group. Modified nano lipidic carrier-loaded gels showed noticeably improved release and penetration and greater bioavailability. Moisturizers that include nicotinamide (NIC) have successfully been used to treat AD [118].
Fan et al. [119] designed hyaluronic acid (HA)-decorated TAC-loaded NPs that were evaluated in vitro and found the drug’s effectiveness (there was a reduction in TEWL, skin integrity was maintained, and the histopathology results showed restoration of skin integrity), penetration, and release kinetics were sustained and controlled. These NPs had enhanced skin retention, anti-AD efficacy, and drug penetration capabilities. For the rationalized management of AD, NPs may be a useful therapeutic strategy, especially for adults and children with steroid phobia.
Niosomes are nonionic, drug-containing vesicular systems used for delivery developed using self-assembling hydrated surfactant monomers and used to improve drug solubility, bioavailability, and encapsulation effectiveness [120]. On the other hand, because of their vast surface area, extreme smallness, and excellent encapsulation effectiveness, polymeric nanoparticles are emerging as intriguing candidates for the topical administration of medicinal compounds. Chitosan is a polysaccharide made of chitin with a cationic charge used in tissue engineering and targeted medication delivery [121,122]. Due to the need for local medication delivery, it is more appropriate to create topical formulations for fatigued skin.
Betamethasone valerate (BMV)-loaded chitosan NPs adorned with HA were developed by Pandey and colleagues [123]. These particles possessed a 300 nm nanosize, a 58 mV positive zeta potential, an 86.56 entrapment efficiency, and a 34.72 loading capacity. Their drug dispersion and penetration efficiency were found to be suitable for treating AD.
Using Carbopol®980 (Surfachem Group Ltd., West Yorkshire, UK) as a gel, Tessema and associates [124] investigated phytoceramides produced from oats that are included in nanocarriers, such as starch- and lecithin-based microemulsions and NPs. The delivery system exhibited essential physical-chemical characteristics. The microemulsion gel enhanced the absorption of oat ceramides into the deeper layers of the skin. Overall, the gel formulations proved effective in concentrating oat ceramides within the SC precisely where they were required.
Espinoza et al. [125] developed a nanoemulsion of pioglitazone (PGZ) as a topical cream; PGZ, recognized for its anti-diabetic properties, demonstrated efficacy in modulating inflammatory responses, establishing itself as a potential therapeutic candidate for various skin diseases. The results obtained using an animal model demonstrated that the PGZ-loaded nanoemulsion suppressed inflammatory cytosines and reduced redness. This formulation has been proven to reduce the levels of inflammatory cytokines such as IL-6, IL-1β, and TNF-α. Histopathological studies have shown improved structural features of SC and reduced inflammatory cell penetration and thickness in the dermis.
Alam et al. [126] designed a nanoemulsion for topical application by using clobetasol propionate (CP) as a therapeutic agent, employed for the treatment of skin disease, resulting in a significant inhibition of edema when compared to marketed cream (Glevate®, Dygen Pharma Distribution Corporation 1754E, Quezon City, Philippines); studies did not show evidence of irritation. These NCs, whose particle sizes typically range between 10 and 200 nm, deliver water-insoluble drugs to the skin’s deeper layers while reducing side effects by reducing dosage.
When liposomes encapsulating BMV and diflucortolone valerate (DFV) were produced and added to chitosan gel, Eroglu et al. [127] observed extended drug retention, strong anti-inflammatory activity (evaluated using the carrageenan-induced paw edema method), decreased erythema, and quick lesion healing in a rat model. In vivo studies on Albino Wistar rats with DNFB-induced conditions showed that the corresponding treatment improved skin barrier function (evidenced by reduced TEWL), supported skin barrier recovery, and promoted hair regrowth. Visual skin examinations and histopathology revealed decreased mast cell activity, which is important in attenuating AD progression.
El-Menshawe et al. [128] designed thermally sensitive ethosomal gels with varying ratios of polymers to increase entrapment efficiency and vesicle deformability for topical nicotinamide (Vitamin B3) delivery. In induced rats (Wistar rat), the topical administration of optimized ethosomal gels diminished inflammation and corneocyte maturation, exhibiting an enhanced anti-inflammatory effect compared with Betaderm® (TARO Pharmaceuticals Industries Ltd, Brampton, Canada) (0.1% Betamethasone valerate cream), decreases in TEWL, increased skin hydration, a reduction in histamine levels, and decreased IgE titers.
Chauhan et al. [129] developed a transfersome that was loaded with glycyrrhizic acid (GA) and incorporated into hydrogel to evaluate its anti-inflammatory efficacy concerning the topical treatment of AD. In BALB/c mice induced with DNCB, the GA-Trans loaded gel induced the most significant reduction in scratching and erythema scores compared to other groups, showcasing the hydrogel formulation’s superior performance. The results highlight the substantial decline in in-vivo scratching and erythema scores with the GA-Trans loaded gel and underscore this formulation’s safety and efficacy with respect to addressing atopic dermatitis.
Carreras et al. [130] pioneered the development of ultra-flexible lipid vesicles designed for the topical administration of cyclosporine A (CsA), an immunosuppressive medication utilized in the treatment of AD. The ability to cross the human epidermis was assessed in Franz diffusion cells, and these liposomal formulations effectively delivered CsA in the epidermis, according to the in vitro results. Nevertheless, in vivo studies are necessary to authenticate the anti-inflammatory effect.
Kang et al. [131] investigated the use of thermosensitive solid lipid nanoparticles (NPs) for TAC delivery. These NPs showed high drug loading efficiency and achieved deeper skin penetration than commercial TAC ointments in an in vivo AD model, improving skin histopathology. Skin irritation tests conducted on rabbits using the Draize test revealed no irritation and only weak reddening after 24 hours of TAC-SLN application, indicating superior safety and effectiveness.
Nagaich et al. [132] developed nanostructured lipid carriers (NLCs) for delivering betamethasone dipropionate (BMD). Comparing BMD-loaded NLC gel with traditional BMD gel on rat skin, the cited study showed that the NLC gel provided extended anti-inflammatory effects, suggesting its suitability as a once-daily treatment for AD. The NLC-based W/O ointment with BMD proved safe and effective for topical application, causing no skin inflammation or edema, minimizing systemic absorption side effects, and enhancing skin retention.
Eiras et al. [133] developed NLCs that incorporated vitamin E (VE) into the hydrogel, and the results indicated the formulation’s adequate pharmaceutical properties. NLCs are well-known systems that demonstrate effectiveness in improving skin moisture and are recommended for cosmetic and dermatological usage. Regarding vitamin E and NLCs’ potential to increase skin hydration, it has been suggested that HG-NLCVE could be utilized in cosmetic applications (e.g., moisturizers and anti-aging) or treating dermatological illnesses such as AD.
Ferreira et al. [134] developed Methotrexate (MTX)-loaded NLCs to enhance MTX bioavailability and dermal penetration. In vitro studies demonstrated an initial burst release followed by sustained release, reducing the need for frequent application. NLCs emerged as an innovative topical treatment for AD, holding promise in relation to improving drug safety, efficacy, and bioavailability with limited skin bioavailability, like MTX.
Beclomethasone dipropionate (BDP) was used as a topical medication for treating AD via the formulation of nanocrystals, serving as an anti-inflammatory agent; the corresponding results revealed that when BDP-loaded nanocrystals were compared with Beclozone® cream (Teva Pharmaceutical Industries, Parsippany, NJ, USA) (0.25% BDP cream), the optimized formulation showed higher drug deposition through mouse skin, despite reduced flux and low systemic exposure of the drug [135].
To address the issue of skin penetration, Pan et al. [136] formulated HA and cholesterol-based polymeric nanoparticles (NPs) and encapsulated TAC. In order to improve TAC and NIC’s solubility, a hydrotropic solution (20% w/v) was utilized. In vitro studies indicated that NIC, when incorporated into hyaluronic-acid/cholesterol-based nanoparticles (HA/Chol-based NPs), improves skin penetration and tacrolimus (TAC) deposition to a greater extent than Protopic® 0.1% TAC ointment (Astellas Pharma Tech Co., Ltd., Toyama, Japan). Cell uptake experiments conducted using HaCaT cells and confocal laser scanning microscopy, using C6 as a fluorescent marker, showed that C6-loaded HA–Chol–NPs with NIC exhibited similar green fluorescence to HA–Chol–NPs alone. This suggests NIC does not affect the nanoparticles’ cellular uptake.
Boisgard et al. [137] introduced innovative semi-solid formulations (Avicel and Viscarin) subsequent to the development of fluorescent polylactic acid (PLA)-based NPs for anti-inflammatory purposes. The assessment of the NP suspension included the evaluation of spreadability and rheological behavior while maintaining the structure of the PLA-based NPs. This highlights the substantial potential of these formulations, incorporating PLA-based NPs, for targeted and topical SC administration with minimal systemic absorption. An in vivo skin irritation test (BALB/c mice) was performed, in which data on skin inflammation scoring, ear thickness, and histology were obtained to analyze inflammation severity. It was found there was no sign of inflammation, no visible ear skin inflammation, and no increase in ear thickness. The histological results showed a decrease in inflammatory cells upon filtration in the treatment group.
Zabihi et al. [138] developed polylactide-co-glycerol (PLG) NPs for the topical delivery of TAC. After topical administration, the biodegradability of PLG was verified by incubating it with skin lysates, which lowers the possibility of toxicity and inflammation. Upon comparison with NPs that were developed using PLG to be marketed as Protopic® (0.03% TAC cream), higher TAC levels were seen in the SC, epidermis, and dermis. Research conducted in vitro verified that TAC-loaded PLG-based NPs could reduce IL-2 expression in a manner comparable to marketed cream. Additionally, TSLP showed an unanticipated considerable reduction with the topical administration of PLG-based NPs compared to the ointment.
A topical anhydrous formulation of NPs, which were PLGA-based and loaded with CsA, was developed by Badihi et al. [139]. Ex vivo studies showed that PLGA-based nanoparticles (NPs) enhance CsA penetration into deeper skin layers and significantly lower pro-inflammatory cytokine production, with IL-6 and IL-8 levels reduced by approximately 50%, indicating potent anti-inflammatory effects. In vivo studies conducted using an Ovalbumin-induced AD animal model revealed that the treated group had markedly lower levels of INF-γ, IL-4, and IL-5; reduced TEWL; and decreased OVA-IgE serum levels, alongside improvements in skin integrity and reduced skin thickness. These findings suggest that drug-loaded nanoparticles can be an effective alternative to systemic CsA delivery, offering deep skin penetration with minimal side effects, showcasing their potential as a topical drug delivery method.
BMV was encapsulated in PLGA or lecithin (LEC)/chitosan-based NPs by Ozcan et al. [140]. To achieve a suitable viscosity for easy skin application, NPs were added to chitosan gel formulations. The results indicate that the NP-loaded chitosan gel formulations enhanced BMV accumulation compared to marketed 0.1% BMV cream, prolonged skin residence, and minimized systemic toxicity. The NP-loaded chitosan gel provided greater anti-inflammatory effects than the cream, although it contained only 1/10 of the BMV concentration. The gel formulation exhibited good skin-whitening ability and does not cause any changes to the skin’s integrity after use.
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
439
Revision:
1 time
(View History)
Update Date:
02 Apr 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




