
Video Upload Options
Microbial cell factories, renowned for their economic and environmental benefits, have emerged as a key trend in academic and industrial areas, particularly in the fermentation of natural compounds. Among these, plant-derived terpenes stand out as a significant class of bioactive natural products. The large-scale production of such terpenes, exemplified by artemisinic acid—a crucial precursor to artemisinin—is now feasible through microbial cell factories. In the fermentation of terpenes, two-phase fermentation technology has been widely applied due to its unique advantages. It facilitates in situ product extraction or adsorption, effectively mitigating the detrimental impact of product accumulation on microbial cells, thereby significantly bolstering the efficiency of microbial production of plant-derived terpenes.
1. Types of Two-Phase fermentation (TPF) Systems
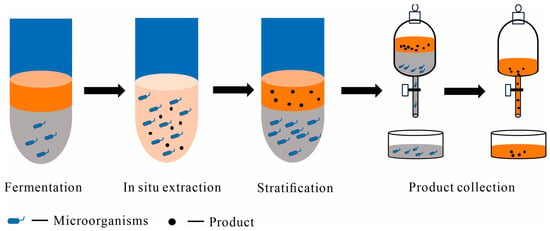
1.1. Liquid–Liquid TPF Systems
1.1.1. Aqueous-Organic TPF Systems
1.1.2. Aqueous Two-Phase System
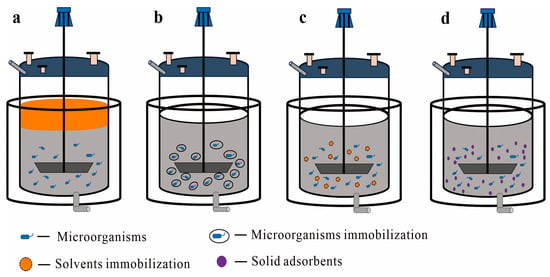
1.2. Liquid–Solid TPF Systems
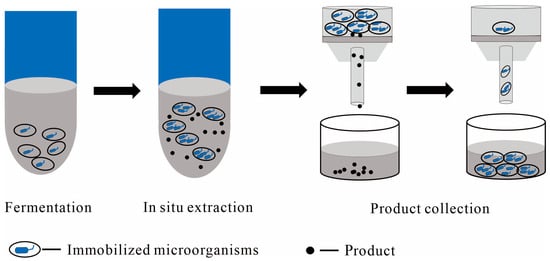
1.2.1. Immobilized Cells as the Solid Phase
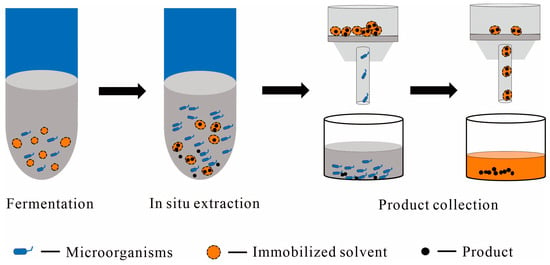
1.2.2. Immobilized Solvent as the Solid Phase
1.2.3. Solid Adsorbents as the Solid Phase
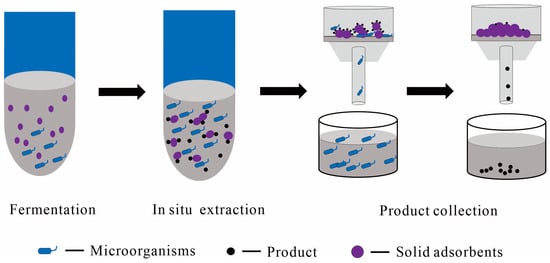
2. The Advantages of TPF Systems
2.1. Enhance Productivity
2.1.1. Reducing Toxicity to Microbial Cells
2.1.2. Alleviating Feedback Inhibition
2.1.3. Preventing Product Degradation and Loss
2.2. Industrial Application: Cost-Effective and Downstream Processing
2.2.1. Increase in Cell Biomass and Recycling of the Second Phase
2.2.2. Reduction of Post-Processing Steps
3. Applications of TPF in Microbial Production of Terpenes
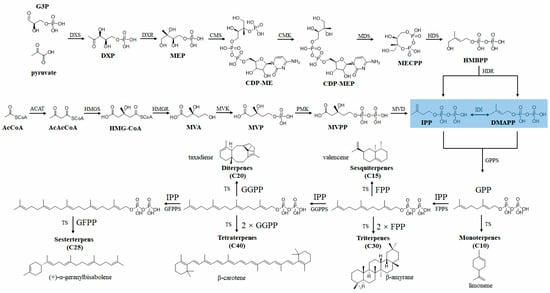
3.1. Microbial Production of Plant-Derived Terpenes
3.1.1. Monoterpenes
3.1.2. Sesquiterpenes
3.1.3. Diterpenes
3.1.4. Triterpenes and Tetraterpenes
References
- Daugulis, A.J. Partitioning bioreactors. Curr. Opin. Biotechnol. 1997, 8, 169–174.
- Malinowski, J.J. Two-phase partitioning bioreactors in fermentation technology. Biotechnol. Adv. 2001, 19, 525–538.
- Quijano, G.; Hernandez, M.; Thalasso, F.; Muñoz, R.; Villaverde, S. Two-phase partitioning bioreactors in environmental biotechnology. Appl. Microbiol. Biotechnol. 2009, 84, 829–846.
- Malik, S.; Hossein Mirjalili, M.; Fett-Neto, A.G.; Mazzafera, P.; Bonfill, M. Living between two worlds: Two-phase culture systems for producing plant secondary metabolites. Crit. Rev. Biotechnol. 2012, 33, 1–22.
- Drouin, C.M.; Cooper, D.G. Biosurfactants and aqueous two-phase fermentation. Biotechnol. Bioeng. 2004, 40, 86–90.
- Aguilar, O.; Rito-Palomares, M. Aqueous two-phase systems strategies for the recovery and characterization of biological products from plants. J. Sci. Food Agric. 2010, 90, 1385–1392.
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18.
- Freeman, A.; Woodley, J.M.; Lilly, M.D. In situ product removal as a tool for bioprocessing. Bio/Technology 1993, 11, 1007–1012.
- Rosinha Grundtvig, I.P.; Heintz, S.; Krühne, U.; Gernaey, K.V.; Adlercreutz, P.; Hayler, J.D.; Wells, A.S.; Woodley, J.M. Screening of organic solvents for bioprocesses using aqueous-organic two-phase systems. Biotechnol. Adv. 2018, 36, 1801–1814.
- Inoue, A.; Horikoshi, K. A Pseudomonas thrives in high concentrations of toluene. Nature 1989, 338, 264–266.
- Verhoef, S.; Wierckx, N.; Westerhof, R.G.M.; de Winde, J.H.; Ruijssenaars, H.J. Bioproduction of p-Hydroxystyrene from Glucose by the Solvent-Tolerant Bacterium Pseudomonas putida S12 in a Two-Phase Water-Decanol Fermentation. Appl. Environ. Microbiol. 2009, 75, 931–936.
- Tan, N.; Ong, L.; Shukal, S.; Chen, X.; Zhang, C. High-Yield Biosynthesis of trans-Nerolidol from Sugar and Glycerol. J. Agric. Food Chem. 2023, 71, 8479–8487.
- Freire, M.G.; Cláudio, A.F.M.; Araújo, J.M.M.; Coutinho, J.A.P.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N. Aqueous biphasic systems: A boost brought about by using ionic liquids. Chem. Soc. Rev. 2012, 41, 4966–4995.
- Phong, W.N.; Show, P.L.; Chow, Y.H.; Ling, T.C. Recovery of biotechnological products using aqueous two phase systems. J. Biosci. Bioeng. 2018, 126, 273–281.
- He, A.; Dong, B.; Feng, X.; Yao, S. Extraction of bioactive ginseng saponins using aqueous two-phase systems of ionic liquids and salts. Sep. Purif. Technol. 2018, 196, 270–280.
- González-Valdez, J.; Mayolo-Deloisa, K.; Rito-Palomares, M. Novel aspects and future trends in the use of aqueous two-phase systems as a bioengineering tool. J. Chem. Technol. Biotechnol. 2017, 93, 1836–1844.
- González-Peñas, H.; Lu-Chau, T.A.; Moreira, M.T.; Lema, J.M. Solvent screening methodology for in situ ABE extractive fermentation. Appl. Microbiol. Biotechnol. 2014, 98, 5915–5924.
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Liu, J.; Liu, Y.; Xu, Q.; Chen, N. Effect of a new ionic liguid on L-valine fermentation. Bull. Ferment. Sci. Technol. 2020, 49, 207–229.
- Badhwar, P.; Kumar, P.; Dubey, K.K. Extractive Fermentation for Process integration and amplified pullulan production by Apullulans in Aqueous Two Phase Systems. Sci. Rep. 2019, 9, 32.
- Haykir, N.I.; Nizan Shikh Zahari, S.M.S.; Harirchi, S.; Sar, T.; Awasthi, M.K.; Taherzadeh, M.J. Applications of ionic liquids for the biochemical transformation of lignocellulosic biomass into biofuels and biochemicals: A critical review. Biochem. Eng. J. 2023, 193, 108850.
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565.
- Pedro, A.Q.; Coutinho, J.A.P.; Freire, M.G. Immobilization of Ionic Liquids, Types of Materials, and Applications. In Encyclopedia of Ionic Liquids; Springer Nature: Singapore, 2019; pp. 1–12.
- Hou, D.; Yu, W.; Zhang, D.; Zhao, L.; Liu, X.; Ma, X. Culture of yeast cells immobilized by alginate-chitosan microcapsules in aqueous-organic solvent biphasic system. J. Oceanol. Limnol. 2019, 37, 863–870.
- Plieva, F.M.; Oknianska, A.; Degerman, E.; Mattiasson, B. Macroporous gel particles as robust macroporous matrices for cell immobilization. Biotechnol. J. 2008, 3, 410–417.
- Lu, J.; Peng, W.; Lv, Y.; Jiang, Y.; Xu, B.; Zhang, W.; Zhou, J.; Dong, W.; Xin, F.; Jiang, M. Application of Cell Immobilization Technology in Microbial Cocultivation Systems for Biochemicals Production. Ind. Eng. Chem. Res. 2020, 59, 17026–17034.
- Brányik, T.; Vicente, A.; Oliveira, R.; Teixeira, J. Physicochemical surface properties of brewing yeast influencing their immobilization onto spent grains in a continuous reactor. Biotechnol. Bioeng. 2004, 88, 84–93.
- Dulieu, C.; Poncelet, D.; Neufeld, R.J. Encapsulation and Immobilization Techniques. In Cell Encapsulation Technology and Therapeutics; Kühtreiber, W.M., Lanza, R.P., Chick, W.L., Eds.; Birkhäuser: Boston, MA, USA, 1999; pp. 3–17.
- Coradello, G.; Tirelli, N. Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules 2021, 26, 3123.
- Rathore, S.; Desai, P.M.; Liew, C.V.; Chan, L.W.; Heng, P.W.S. Microencapsulation of microbial cells. J. Food Eng. 2013, 116, 369–381.
- El-Sayed, E.-S.R.; Ahmed, A.S.; Hassan, I.A.; Ismaiel, A.A.; Karam El-Din, A.-Z.A. Strain improvement and immobilization technique for enhanced production of the anticancer drug paclitaxel by Aspergillus fumigatus and Alternaria tenuissima. Appl. Microbiol. Biotechnol. 2019, 103, 8923–8935.
- Darmayanti, R.F.; Tashiro, Y.; Noguchi, T.; Gao, M.; Sakai, K.; Sonomoto, K. Novel biobutanol fermentation at a large extractant volume ratio using immobilized Clostridium saccharoperbutylacetonicum N1-4. J. Biosci. Bioeng. 2018, 126, 750–757.
- Ham, S.; Kim, H.J.; Shin, N.; Hwang, J.H.; Oh, S.J.; Park, J.Y.; Joo, J.C.; Kim, H.T.; Bhatia, S.K.; Yang, Y.-H. Continuous production of gamma aminobutyric acid by engineered and immobilized Escherichia coli whole-cells in a small-scale reactor system. Enzym. Microb. Technol. 2023, 168, 110258.
- de Souza, W.F.C.; Pereira, I.; de Lucena, F.A.; Martins, L.P.; Furtado, R.F.; da Castro, R.J.S.; Sato, H.H. A new system of Erwinia sp. D12 cells immobilized in a matrix of alginate and algaroba gum (Prosopis juliflora): An efficient way to improve isomaltulose production. Process Biochem. 2022, 114, 52–58.
- Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast. Pharmaceuticals 2021, 14, 295.
- Dusséaux, S.; Wajn, W.T.; Liu, Y.; Ignea, C.; Kampranis, S.C. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids. Proc. Natl. Acad. Sci. USA 2020, 117, 31789–31799.
- Li, H.; Bhadury, P.S.; Song, B.; Yang, S. Immobilized functional ionic liquids: Efficient, green, and reusable catalysts. RSC Adv. 2012, 2, 12525–12551.
- Lee, C.; Sandig, B.; Buchmeiser, M.R.; Haumann, M. Supported ionic liquid phase (SILP) facilitated gas-phase enzyme catalysis—CALB catalyzed transesterification of vinyl propionate. Catal. Sci. Technol. 2018, 8, 2460–2466.
- Wang, M.; Wang, M.; Zhang, S.; Chen, J. Pickering gel emulsion stabilized by enzyme immobilized polymeric nanoparticles: A robust and recyclable biocatalyst system for biphasic catalysis. React. Chem. Eng. 2019, 4, 1459–1465.
- Wiese, S.; Spiess, A.C.; Richtering, W. Microgel-Stabilized Smart Emulsions for Biocatalysis. Angew. Chem. Int. Ed. 2012, 52, 576–579.
- Phillips, T.; Chase, M.; Wagner, S.; Renzi, C.; Powell, M.; DeAngelo, J.; Michels, P. Use of in situ solid-phase adsorption in microbial natural product fermentation development. J. Ind. Microbiol. Biotechnol. 2013, 40, 411–425.
- Xu, L.-J.; Liu, Y.-S.; Zhou, L.-G.; Wu, J.-Y. Enhanced beauvericin production with in situ adsorption in mycelial liquid culture of Fusarium redolens Dzf2. Process Biochem. 2009, 44, 1063–1067.
- Qi, H.; Zhao, S.; Fu, H.; Wen, J.; Jia, X. Enhancement of ascomycin production in Streptomyces hygroscopicus var. ascomyceticus by combining resin HP20 addition and metabolic profiling analysis. J. Ind. Microbiol. Biotechnol. 2014, 41, 1365–1374.
- Fordjour, E.; Liu, C.L.; Hao, Y.P.; Sackey, I.; Yang, Y.K.; Liu, X.X.; Li, Y.; Tan, T.W.; Bai, Z.H. Engineering Escherichia coli BL21 (DE3) for high-yield production of germacrene A, a precursor of β-elemene via combinatorial metabolic engineering strategies. Biotechnol. Bioeng. 2023, 120, 3039–3056.
- Liu, W.; Xu, X.; Zhang, R.; Cheng, T.; Cao, Y.; Li, X.; Guo, J.; Liu, H.; Xian, M. Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture. Biotechnol. Biofuels 2016, 9, 58.
- Engels, B.; Dahm, P.; Jennewein, S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab. Eng. 2008, 10, 201–206.
- Tsueng, G.; Lam, K.S. Stabilization effect of resin on the production of potent proteasome inhibitor NPI-0052 during submerged fermentation of Salinispora tropica. J. Antibiot. 2007, 60, 469–472.
- Pemberton, T.A.; Chen, M.B.; Harris, G.G.; Chou, W.K.W.; Duan, L.; Köksal, M.; Genshaft, A.S.; Cane, D.E.; Christianson, D.W. Exploring the Influence of Domain Architecture on the Catalytic Function of Diterpene Synthases. Biochemistry 2017, 56, 2010–2023.
- Fan, M.; Yuan, S.Q.; Li, L.S.; Zheng, J.; Zhao, D.; Wang, C.J.; Wang, H.; Liu, X.; Liu, J. Application of Terpenoid Compounds in Food and Pharmaceutical Products. Fermentation 2023, 9, 119.
- Jiang, H.; Wang, X. Biosynthesis of monoterpenoid and sesquiterpenoid as natural flavors and fragrances. Biotechnol. Adv. 2023, 65, 22.
- Ajikumar, P.K.; Tyo, K.; Carlsen, S.; Mucha, O.; Phon, T.H.; Stephanopoulos, G. Terpenoids: Opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 2008, 5, 167–190.
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457.
- Belcher, M.S.; Mahinthakumar, J.; Keasling, J.D. New frontiers: Harnessing pivotal advances in microbial engineering for the biosynthesis of plant-derived terpenoids. Curr. Opin. Biotechnol. 2020, 65, 88–93.
- Ren, Y.Y.; Liu, S.S.; Jin, G.J.; Yang, X.B.; Zhou, Y.J.J. Microbial production of limonene and its derivatives: Achievements and perspectives. Biotechnol. Adv. 2020, 44, 16.
- Daletos, G.; Katsimpouras, C.; Stephanopoulos, G. Novel Strategies and Platforms for Industrial Isoprenoid Engineering. Trends Biotechnol. 2020, 38, 811–822.
- Marienhagen, J.; Bott, M. Metabolic engineering of microorganisms for the synthesis of plant natural products. J. Biotechnol. 2013, 163, 166–178.
- Moser, S.; Pichler, H. Identifying and engineering the ideal microbial terpenoid production host. Appl. Microbiol. Biotechnol. 2019, 103, 5501–5516.
- Chen, X.X.; Zhang, C.Q.; Lindley, N.D. Metabolic Engineering Strategies for Sustainable Terpenoid Flavor and Fragrance Synthesis. J. Agric. Food Chem. 2020, 68, 10252–10264.
- Abdel-Mawgoud, A.M.; Markham, K.A.; Palmer, C.M.; Liu, N.; Stephanopoulos, G.; Alper, H.S. Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng. 2018, 50, 192–208.
- Groenewald, M.; Boekhout, T.; Neuvéglise, C.; Gaillardin, C.; van Dijck, P.W.M.; Wyss, M. Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 2014, 40, 187–206.
- Geiselman, G.M.; Kirby, J.; Landera, A.; Otoupal, P.; Papa, G.; Barcelos, C.; Sundstrom, E.R.; Das, L.; Magurudeniya, H.D.; Wehrs, M.; et al. Conversion of poplar biomass into high-energy density tricyclic sesquiterpene jet fuel blendstocks. Microb. Cell Factories 2020, 19, 16.
- Liu, Q.; Zhang, G.; Su, L.Q.; Liu, P.; Jia, S.R.; Wang, Q.H.; Dai, Z.J. Reprogramming the metabolism of oleaginous yeast for sustainably biosynthesizing the anticarcinogen precursor germacrene A. Green Chem. 2023, 25, 7988–7997.
- Özaydin, B.; Burd, H.; Lee, T.S.; Keasling, J.D. Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production. Metab. Eng. 2013, 15, 174–183.
- Wang, X.; Xiao, L.J.; Zhang, X.Y.; Zhang, J.; Zhang, Y.; Wang, F.; Li, X. Combined bioderivatization and engineering approach to improve the efficiency of geraniol production. Green Chem. 2022, 24, 864–876.
- Zhou, J.; Wang, C.L.; Yang, L.Y.; Choi, E.S.; Kim, S.W. Geranyl diphosphate synthase: An important regulation point in balancing a recombinant monoterpene pathway in Escherichia coli. Enzym. Microb. Technol. 2015, 68, 50–55.
- Zhao, J.Z.; Li, C.; Zhang, Y.; Shen, Y.; Hou, J.; Bao, X.M. Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae. Microb. Cell Factories 2017, 16, 11.
- Hoshino, Y.; Moriya, M.; Matsudaira, A.; Katashkina, J.I.; Nitta, N.; Nishio, Y.; Usuda, Y. Stereospecific linalool production utilizing two-phase cultivation system in Pantoea ananatis. J. Biotechnol. 2020, 324, 21–27.
- Chuck, C.J.; Donnelly, J. The compatibility of potential bioderived fuels with Jet A-1 aviation kerosene. Appl. Energy 2014, 118, 83–91.
- Zhang, J.; Zhao, C. A new approach for bio-jet fuel generation from palm oil and limonene in the absence of hydrogen. Chem. Commun. 2015, 51, 17249–17252.
- Willrodt, C.; David, C.C.; Cornelissen, S.; Bühler, B.; Julsing, M.K.; Schmid, A. Engineering the productivity of recombinant Escherichia coli for limonene formation from glycerol in minimal media. Biotechnol. J. 2014, 9, 1000–1012.
- Rolf, J.; Julsing, M.K.; Rosenthal, K.; Lütz, S. A Gram-Scale Limonene Production Process with Engineered Escherichia coli. Molecules 2020, 25, 1881.
- Schewe, H.; Holtmann, D.; Schrader, J. P450 BM-3-catalyzed whole-cell biotransformation of α-pinene with recombinant Escherichia coli in an aqueous-organic two-phase system. Appl. Microbiol. Biotechnol. 2009, 83, 849–857.
- Alonso-Gutiérrez, J.; Chan, R.; Batth, T.S.; Adams, P.D.; Keasling, J.D.; Petzold, C.J.; Lee, T.S. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 2013, 19, 33–41.
- Shi, Q.Q.; Tang, J.J.; Gao, J.M. Picrotoxane sesquiterpenoids: Chemistry, chemo- and bio-syntheses and biological activities. Nat. Product Rep. 2022, 39, 2096–2131.
- Martin, V.J.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003, 21, 796–802.
- Westfall, P.J.; Pitera, D.J.; Lenihan, J.R.; Eng, D.; Woolard, F.X.; Regentin, R.; Horning, T.; Tsuruta, H.; Melis, D.J.; Owens, A.; et al. Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc. Natl. Acad. Sci. USA 2012, 109, E111–E118.
- Crock, J.; Wildung, M.; Croteau, R. Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha x piperita, L.) that produces the aphid alarm pheromone (E)-beta-farnesene. Proc. Natl. Acad. Sci. USA 1997, 94, 12833–12838.
- Wang, C.; Yoon, S.H.; Jang, H.J.; Chung, Y.R.; Kim, J.Y.; Choi, E.S.; Kim, S.W. Metabolic engineering of Escherichia coli for α-farnesene production. Metab. Eng. 2011, 13, 648–655.
- Zhu, F.; Zhong, X.; Hu, M.; Lu, L.; Deng, Z.; Liu, T. In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli. Biotechnol. Bioeng. 2014, 111, 1396–1405.
- Tippmann, S.; Anfelt, J.; David, F.; Rand, J.M.; Siewers, V.; Uhlen, M.; Nielsen, J.; Hudson, E.P. Affibody Scaffolds Improve Sesquiterpene Production in Saccharomyces cerevisiae. ACS Synth. Biol. 2017, 6, 19–28.
- Tippmann, S.; Ferreira, R.; Siewers, V.; Nielsen, J.; Chen, Y. Effects of acetoacetyl-CoA synthase expression on production of farnesene in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2017, 44, 911–922.
- You, S.P.; Yin, Q.D.A.; Zhang, J.Y.; Zhang, C.Y.; Qi, W.; Gao, L.; Tao, Z.P.; Su, R.X.; He, Z.M. Utilization of biodiesel by-product as substrate for high-production of β-farnesene via relatively balanced mevalonate pathway in Escherichia coli. Bioresour. Technol. 2017, 243, 228–236.
- Wang, J.H.; Jiang, W.; Liang, C.J.; Zhu, L.H.; Li, Y.R.; Mo, Q.; Xu, S.; Chu, A.; Zhang, L.; Ding, Z.Y.; et al. Overproduction of α-Farnesene in Saccharomyces cerevisiae by Farnesene Synthase Screening and Metabolic Engineering. J. Agric. Food Chem. 2021, 69, 3103–3113.
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697.
- Liu, Y.; Jiang, X.; Cui, Z.; Wang, Z.; Qi, Q.; Hou, J. Engineering the oleaginous yeast Yarrowia lipolytica for production of α-farnesene. Biotechnol. Biofuels 2019, 12, 296.
- Halfmann, C.; Gu, L.P.; Gibbons, W.; Zhou, R.B. Genetically engineering cyanobacteria to convert CO2, water, and light into the long-chain hydrocarbon farnesene. Appl. Microbiol. Biotechnol. 2014, 98, 9869–9877.
- Adio, A.M. (-)trans-β-Elemene and related compounds: Occurrence, synthesis, and anticancer activity. Tetrahedron 2009, 65, 5145–5159.
- Gao, Y. The Study of Microbial Synthesis of Germacrene A the Precursor of β-Elemene. Master’s Thesis, Hangzhou Normal University, Hangzhou, China, 2012.
- Dai, Z.; Liu, Y.; Huang, L.; Zhang, X. Production of miltiradiene by metabolically engineered Saccharomyces cerevisiae. Biotechnol. Bioeng. 2012, 109, 2845–2853.
- Ajikumar, P.K.; Xiao, W.H.; Tyo, K.E.J.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid Pathway Optimization for Taxol Precursor Overproduction in Escherichia coli. Science 2010, 330, 70–74.
- Nowrouzi, B.; Li, R.A.; Walls, L.E.; d’Espaux, L.; Malci, K.; Liang, L.G.; Jonguitud-Borrego, N.; Lerma-Escalera, A.I.; Morones-Ramirez, J.R.; Keasling, J.D.; et al. Enhanced production of taxadiene in Saccharomyces cerevisiae. Microb. Cell Factories 2020, 19, 12.
- Hu, T.Y.; Zhou, J.W.; Tong, Y.R.; Su, P.; Li, X.L.; Liu, Y.; Liu, N.; Wu, X.Y.; Zhang, Y.F.; Wang, J.D.; et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast. Metab. Eng. 2020, 60, 87–96.
- Cao, X.; Yu, W.; Chen, Y.; Yang, S.; Zhao, Z.K.; Nielsen, J.; Luan, H.W.; Zhou, Y.J. Engineering yeast for high-level production of diterpenoid sclareol. Metab. Eng. 2023, 75, 19–28.
- Yendo, A.C.A.; de Costa, F.; Gosmann, G.; Fett-Neto, A.G. Production of Plant Bioactive Triterpenoid Saponins: Elicitation Strategies and Target Genes to Improve Yields. Mol. Biotechnol. 2010, 46, 94–104.
- Zhao, Y.-j.; Li, C. Biosynthesis of Plant Triterpenoid Saponins in Microbial Cell Factories. J. Agric. Food Chem. 2018, 66, 12155–12165.
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.-W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12.
- Dai, Z.B.; Liu, Y.; Zhang, X.A.; Shi, M.Y.; Wang, B.B.; Wang, D.; Huang, L.Q.; Zhang, X.L. Metabolic engineering of Saccharomyces cerevisiae for production of ginsenosides. Metab. Eng. 2013, 20, 146–156.




