Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Grzegorz Porebski | -- | 1496 | 2024-03-25 12:08:05 | | | |
| 2 | Fanny Huang | Meta information modification | 1496 | 2024-03-27 09:29:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dziadowiec, A.; Popiolek, I.; Kwitniewski, M.; Porebski, G. Pathophysiological Basis of Mas-Related G Protein-Coupled Receptor X2. Encyclopedia. Available online: https://encyclopedia.pub/entry/56474 (accessed on 07 February 2026).
Dziadowiec A, Popiolek I, Kwitniewski M, Porebski G. Pathophysiological Basis of Mas-Related G Protein-Coupled Receptor X2. Encyclopedia. Available at: https://encyclopedia.pub/entry/56474. Accessed February 07, 2026.
Dziadowiec, Alicja, Iwona Popiolek, Mateusz Kwitniewski, Grzegorz Porebski. "Pathophysiological Basis of Mas-Related G Protein-Coupled Receptor X2" Encyclopedia, https://encyclopedia.pub/entry/56474 (accessed February 07, 2026).
Dziadowiec, A., Popiolek, I., Kwitniewski, M., & Porebski, G. (2024, March 25). Pathophysiological Basis of Mas-Related G Protein-Coupled Receptor X2. In Encyclopedia. https://encyclopedia.pub/entry/56474
Dziadowiec, Alicja, et al. "Pathophysiological Basis of Mas-Related G Protein-Coupled Receptor X2." Encyclopedia. Web. 25 March, 2024.
Copy Citation
Mast cells (MCs) are immune cells that reside in tissues; particularly in the skin, and in the gastrointestinal and respiratory tracts. In recent years, there has been considerable interest in the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2), which is present on the surface of MCs and can be targeted by multiple exogenous and endogenous ligands. It is potentially implicated in non-IgE-mediated pseudoallergic reactions and inflammatory conditions such as asthma or atopic dermatitis.
xenobiotic
Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2)
mast cell
1. Introduction
Mast cells (MCs) are, among a number of other functions, the primary initiators of allergic and allergic-like symptoms; they swiftly release numerous mediators upon activation. Allergic MC activation occurs via an IgE-dependent pathway, in which the allergen is matched to a specific IgE that binds to a high-affinity IgE receptor (FcεRI) on the cell surface [1]. However, there are also clinical reactions that resemble allergy and develop after exposure to a variety of xenobiotic compounds for which IgE-mediated mechanisms have not been demonstrated and are therefore termed pseudoallergic or anaphylactoid [2]. After a period of uncertainty regarding the responsible pathway, the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2) was proposed to be one of the possible IgE-independent MC activation pathways [3]. McNeil et al. demonstrated that MRGPRX2 can be activated by xenobiotics, including fluoroquinolones, neuromuscular blocking agents, and peptidergic therapeutics (e.g., icatibant, leuprolide), in addition to previously known endogenous ligands such as neuropeptides and substance P (SP) [4]. Since the publication of McNeil’s seminal paper in 2015, the number of publications addressing xenobiotic triggering of MRGPRX2 has increased rapidly. The hypothesis that drug hypersensitivity reactions are induced via an MRGPRX2-dependent pathway, mainly by drugs from the muscle relaxant and flouroquinolone antibiotic groups, has attracted much attention from the scientific community [5]. However, many studies have also been devoted to other xenobiotics—including those found in medicinal plants—and these analyse their association with the MRGPRX2 receptor.
2. Pathophysiological Basis
2.1. Mast Cell Characteristics
MCs are immune cells that are present in almost all tissues of the body but are particularly abundant in those tissues directly exposed to the external environment [6]. While MCs are primarily associated with allergic reactions, they also play a significant role in various physiological and pathological processes [7][8][9][10][11].
All MCs contain intracellular granules and express the high-affinity IgE receptor FcεRI on their surface [12]. The cross-linking of FcεRI receptors upon antigen-IgE binding is the most recognized pathway of MC activation, playing a crucial role in potentially fatal reactions such as anaphylaxis [1]. MC stimulation leads to degranulation and the release of granule contents, which is a primary cause of hypersensitivity manifestations [1]. The granules store a wide range of preformed mediators, including histamine [13], proteases such as tryptases and chymases [13][14], and also some cytokines; mainly tumor necrosis factor alpha (TNF-α) [15]. These substances cause various biological effects, such as increasing vascular permeability, smooth muscle contraction and activation of immune cells, which are associated with symptoms of allergic inflammation [16]. In addition to the immediate release of preformed mediators, MCs also secrete de novo synthesized compounds that are produced after MC stimulation [13]. These include lipid mediators—such as prostaglandin D2 (PGD2), which are rapidly produced and released [17]—and cytokines, which are produced and secreted over a longer period of time (hours rather than minutes) [18][19][20].
In humans, MCs are generally categorized into one of three subtypes, based on the content of specific proteases. MCs that contain only tryptase (MCT) are found in the mucosa of the small intestine and in the alveolar septa [21]. MCs that contain only chymase (MCC) are commonly found in synovial tissue. MCs, which contain both tryptase and chymase (MCTC), are predominantly found in the skin, submucosal layers of the small intestine, and tonsils [22]. However, at the transcriptional level, the protease content displays more tissue-specific variability, which is evident both between and within tissues [12]. Cutting-edge advancements in single-cell profiling technologies have opened new avenues to unravel the complexity and diversity of MCs. These breakthroughs shed light on previously unseen heterogeneity among MCs across various tissues, which is distinct from other cell types. In humans, transcriptomic analysis unveiled the existence of seven distinct MC subsets (MC1–7) distributed across 12 organs, each with unique transcriptomic core signatures [23].
All MCs express FcεRI, but there is controversy regarding whether MCT and MCC express MRGPRX2, despite the known expression of MRGPRX2 in skin MCTC [24][25][26]. Furthermore, even among skin MCTC, only a small percentage of cells exhibit MRGPRX2 expression under steady-state conditions [24][25].
2.2. Structure and Regulation of MRGPRX2 Function
MRGPRX2 is a G protein-coupled receptor (GPCR) that was first reported to be expressed mainly on MCs and sensory neurons [3][27]. The receptor has low affinity and low selectivity with respect to ligand binding. MRGPRX2 has been shown to be activated by a wide range of endogenous and exogenous compounds, primarily by small cationic molecules and peptides that have amphipathic properties, or share a motif of tetrahydroisoquinoline (THIQ) or a similar motif [4][5]. Endogenous ligands of MRGPRX2 include neuropeptides such as SP, PAMP-12, and cortistatin-14 (CST-14), as well as antimicrobial host defense peptides such as cathelicidin LL-37, hBD2, and eosinophil granule proteins (e.g., MBP). Exogenous ligands of MRGPRX2 include the cationic polymer compound 48/80 (C48/80), which is commonly used in receptor functional assays, and a variety of drugs approved by the Food and Drug Administration (FDA), such as fluoroquinolones (e.g., ciprofloxacin), neuromuscular blocking agents (e.g., rocuronium, atracurium), opioids (e.g., morphine), and many others [4][9][28]. MRGPRX2 can also be activated or inhibited by other exogenous agents, such as bacterial quorum sensing proteins, insect venoms [3][29][30], or many different plant xenobiotics (Figure 1) [31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59].
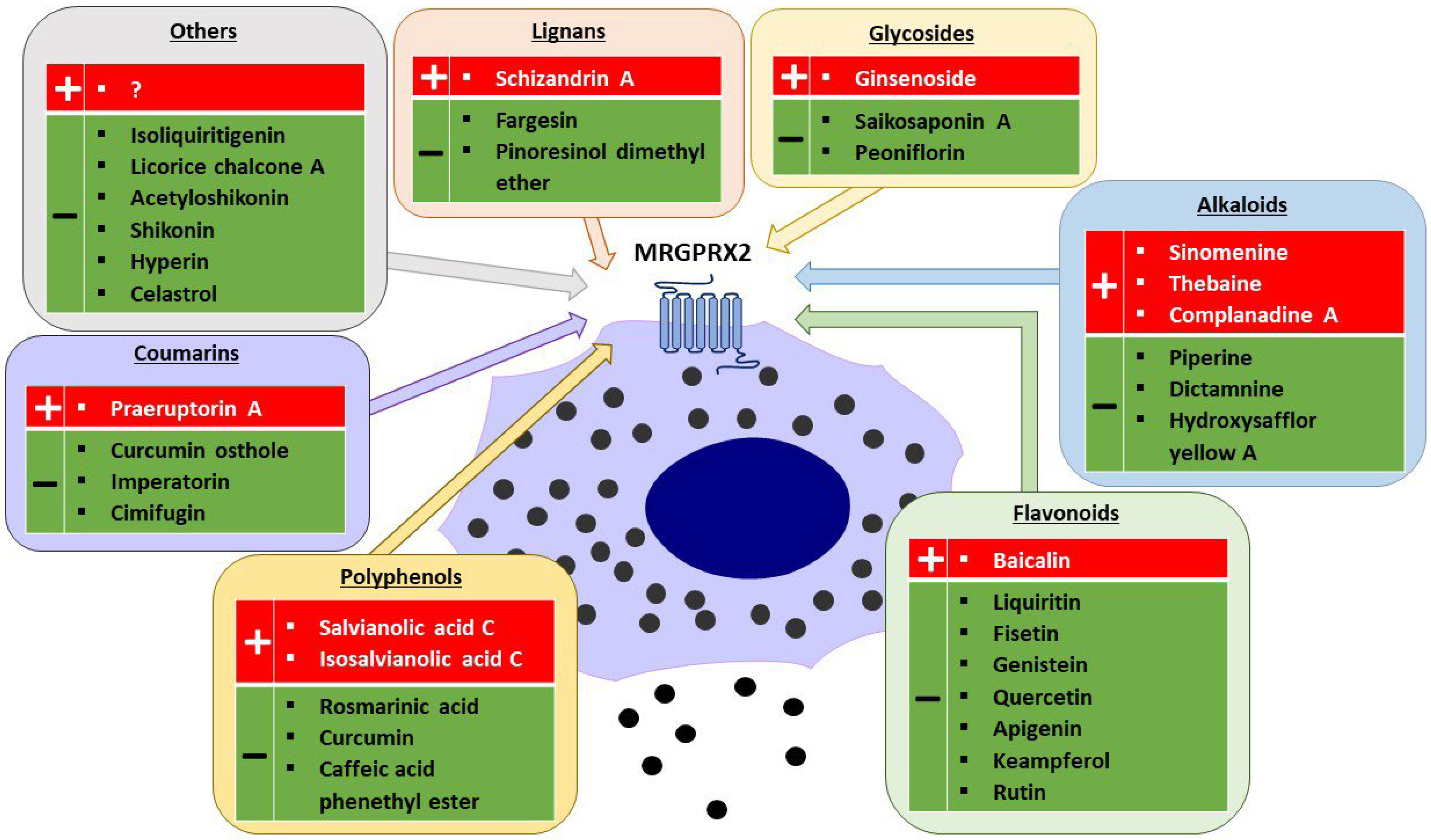
Figure 1. Plant-derived agonists (+) and inhibitors (−) of MRGPRX2 (created with Motifolio, Motifolio Inc., Elliocott City, MD, USA).
As a GPCR, MRGPRX2 shares the structure of seven transmembrane (TM) α-helices connected by three extracellular loops (ECLs) and three intracellular loops (ICLs) [60]. The ECL region contains the N-terminus responsible for ligand binding, whereas the ICL region contains the C-terminus involved in G protein coupling, β-arrestin recruitment, and downstream signalling [61][62][63][64]. The extracellular binding of ligands to MRGPRX2 promotes the conformational changes in the transmembrane domains, resulting in structural changes on the cytoplasmic side of the membrane and activation of G proteins, and subsequent MC degranulation [65]. Conversely, some ligands can induce intracellular β-arrestin recruitment, leading to receptor desensitization and internalization [62]. The downstream signalling pathways of MRGPRX2 involve the activation of the phospholipase C pathway (PLC-PKC-IP3R), which result in intracellular Ca2+ influx and MC degranulation. Additionally, the MAP kinase (ERK-P38-JNK), PI3K-AKT, and NF-κB pathways are activated, leading to cytokines and PGD2 synthesis in MCs [7].
2.3. Role of MRGPRX2 in MC-Driven Skin Diseases
To date, the exact role of MRGPRX2 in MCs has not been fully understood [9]. Numerous in vivo and in vitro studies have been conducted on the receptor (and its mouse ortholog, MrgprB2 [4]), indicating its potential involvement in various physiological and pathological processes. With its ability to bind to a diverse range of ligands, MRGPRX2 has been implicated in drug pseudoallergic reactions, neurogenic inflammation, and a wide array of inflammatory diseases such as allergic contact dermatitis (ACD), chronic urticaria (CU), rosacea, rheumatoid arthritis, atopic dermatitis (AD), mastocytosis, ulcerative colitis, and allergic asthma [8][9][10][11]. However, conclusive evidence regarding MRGPRX2′s involvement in these conditions in humans is still lacking.
Endogenous peptides considered to be MRGPRX2 ligand play an important role in the development of inflammatory skin diseases. The neuropeptide SP and the host defense peptide cathelicidin LL-37 are key players in the pathogenesis of rosacea and AD, and are upregulated in the skin of patients [8][10]. Both peptides in vitro were shown to activate MCs via MRGPRX2, leading to MC degranulation and release of pro-inflammatory mediators, including histamine and cytokines (i.e., TNFα) [66][67]. It was proposed that the released mediators can subsequently act on sensory neurons and vascular endothelial cells to promote neurogenic inflammation, resulting in itching, erythema, swelling, and pain that exacerbate disease symptoms [8][10]. In addition, MC-derived mediators recruit immune cells into the inflamed tissue and stimulate both neurons and immune cells (such as neutrophils) to secrete more SP and LL-37, which then could again activate MCs [8][10][11]. Similar mechanisms involving SP and MCs are also present in ACD and CU [8][10][68]. Another neuropeptide ligand of MRGPRX2 involved in the development of neurogenic skin inflammation, such as the non-histaminergic pruritus associated with ACD, is CST-14 [8][10][69][70][71]. The skin conditions are also characterized by elevated levels of proinflammatory cytokines such as IL-13 and IL-31 [72][73][74]. It is noteworthy that in all these diseases, except CU, an increased number of MCs has been reported in the skin of patients compared to healthy controls [8][10]. Additionally, the expression of MRGPRX on cutaneous MCs is higher in patients with CU [24]. Therefore, the involvement of MRGPRX2 in inflammatory skin diseases is suggested [8][10][11].
In several of these diseases, the usual treatment with antihistamines and other first-line drugs has been reported to be ineffective [75][76][77]. With the current generation of H1-antihistamines, sedation has become a minor concern, as the use up to fourfold normal doses are minimally or non-sedating [77][78][79]. However, due to incomplete efficacy in all patients, the search for other medications remains a priority.
References
- Dispenza, M.C.; Metcalfe, D.D.; Olivera, A. Research Advances in Mast Cell Biology and Their Translation into Novel Therapies for Anaphylaxis. J. Allergy Clin. Immunol. Pract. 2023, 11, 2032–2042.
- Jutel, M.; Agache, I.; Zemelka-Wiacek, M.; Akdis, M.; Chivato, T.; del Giacco, S.; Gajdanowicz, P.; Gracia, I.E.; Klimek, L.; Lauerma, A.; et al. Nomenclature of allergic diseases and hypersensitivity reactions: Adapted to modern needs: An EAACI position paper. Allergy 2023, 78, 2851–2874.
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328.
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241.
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol. 2018, 9, 3027.
- St John, A.L.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463.
- Ogasawara, H.; Noguchi, M. Therapeutic Potential of MRGPRX2 Inhibitors on Mast Cells. Cells 2021, 10, 2906.
- Roy, S.; Chompunud Na Ayudhya, C.; Thapaliya, M.; Deepak, V.; Ali, H. Multifaceted MRGPRX2: New insight into the role of mast cells in health and disease. J. Allergy Clin. Immunol. 2021, 148, 293–308.
- Kumar, M.; Duraisamy, K.; Chow, B.K. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells 2021, 10, 1033.
- Baldo, B.A. MRGPRX2, drug pseudoallergies, inflammatory diseases, mechanisms and distinguishing MRGPRX2- and IgE/FcεRI-mediated events. Br. J. Clin. Pharmacol. 2023, 89, 3232–3246.
- Quan, P.L.; Sabaté-Brescó, M.; Guo, Y.; Martín, M.; Gastaminza, G. The Multifaceted Mas-Related G Protein-Coupled Receptor Member X2 in Allergic Diseases and Beyond. Int. J. Mol. Sci. 2021, 22, 4421.
- West, P.W.; Bulfone-Paus, S. Mast cell tissue heterogeneity and specificity of immune cell recruitment. Front. Immunol. 2022, 13, 932090.
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365.
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494.
- Gordon, J.R.; Galli, S.J. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J. Exp. Med. 1991, 174, 103–107.
- Molderings, G.J.; Afrin, L.B. A survey of the currently known mast cell mediators with potential relevance for therapy of mast cell-induced symptoms. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2881–2891.
- Boyce, J.A. Mast cells and eicosanoid mediators: A system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 2007, 217, 168–185.
- Vliagoftis, H.; Befus, A.D. Rapidly changing perspectives about mast cells at mucosal surfaces. Immunol. Rev. 2005, 206, 190–203.
- Wasiuk, A.; de Vries, V.C.; Hartmann, K.; Roers, A.; Noelle, R.J. Mast cells as regulators of adaptive immunity to tumours. Clin. Exp. Immunol. 2009, 155, 140–146.
- Bischoff, S.C. Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat. Rev. Immunol. 2007, 7, 93–104.
- Oskeritzian, C.A.; Zhao, W.; Min, H.K.; Xia, H.Z.; Pozez, A.; Kiev, J.; Schwartz, L.B. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J. Allergy Clin. Immunol. 2005, 115, 1162–1168.
- Krishnaswamy, G.; Ajitawi, O.; Chi, D.S. The human mast cell: An overview. Methods Mol. Biol. 2006, 315, 13–34.
- Tauber, M.; Basso, L.; Martin, J.; Bostan, L.; Pinto, M.M.; Thierry, G.R.; Houmadi, R.; Serhan, N.; Loste, A.; Blériot, C.; et al. Landscape of mast cell populations across organs in mice and humans. J. Exp. Med. 2023, 220, e20230570, Erratum in J. Exp. Med. 2024, 221, e2023057001172024c.
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622–633.e9.
- Pyatilova, P.; Ashry, T.; Luo, Y.; He, J.; Bonnekoh, H.; Jiao, Q.; Moñino-Romero, S.; Hu, M.; Scheffel, J.; Frischbutter, S.; et al. The Number of MRGPRX2-Expressing Cells Is Increased in Skin Lesions of Patients with Indolent Systemic Mastocytosis, but Is Not Linked to Symptom Severity. Front. Immunol. 2022, 13, 930945.
- Manorak, W.; Idahosa, C.; Gupta, K.; Roy, S.; Panettieri, R., Jr.; Ali, H. Upregulation of Mas-related G Protein coupled receptor X2 in asthmatic lung mast cells and its activation by the novel neuropeptide hemokinin-1. Respir. Res. 2018, 19, 1.
- Ray, P.; Torck, A.; Quigley, L.; Wangzhou, A.; Neiman, M.; Rao, C.; Lam, T.; Kim, J.Y.; Kim, T.H.; Zhang, M.Q.; et al. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: An RNA-seq-based resource for pain and sensory neuroscience research. Pain 2018, 159, 1325–1345.
- Kühn, H.; Kolkhir, P.; Babina, M.; Düll, M.; Frischbutter, S.; Fok, J.S.; Jiao, Q.; Metz, M.; Scheffel, J.; Wolf, K.; et al. Mas-related G protein-coupled receptor X2 and its activators in dermatologic allergies. J. Allergy Clin. Immunol. 2021, 147, 456–469.
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216.
- Seldeslachts, A.; Peigneur, S.; Mebs, D.; Tytgat, J. Unraveling the venom chemistry with evidence for histamine as key regulator in the envenomation by caterpillar Automeris zaruma. Front. Immunol. 2022, 13, 972442.
- Wang, L.; Hu, G.Z.; Lu, Y.; Jiang, S.J.; Qi, J.; Su, H. Anti-pseudo-allergic components in licorice extract inhibit mast cell degranulation and calcium influx. Chin. J. Nat. Med. 2022, 20, 421–431.
- Lei, P.; Liu, Y.; Ding, Y.; Su, X.; Liang, J.; Chen, H.; Ma, W. Thebaine induces anaphylactic reactions via the MRGPRX2 receptor pathway on mast cells. Cell. Immunol. 2022, 375, 104514.
- Wang, J.; Zhang, Y.; Che, D.; Zeng, Y.; Wu, Y.; Qin, Q.; Wang, N. Baicalin induces Mrgprb2-dependent pseudo-allergy in mice. Immunol. Lett. 2020, 226, 55–61.
- Callahan, B.N.; Kammala, A.K.; Syed, M.; Yang, C.; Occhiuto, C.J.; Nellutla, R.; Chumanevich, A.P.; Oskeritzian, C.A.; Das, R.; Subramanian, H. Osthole, a Natural Plant Derivative Inhibits MRGPRX2 Induced Mast Cell Responses. Front. Immunol. 2020, 11, 703.
- Yang, L.; Zeng, Y.; Wang, J.; Zhang, Y.; Hou, Y.; Qin, Q.; Ma, W.; Wang, N. Discovery and analysis the anti-pseudo-allergic components from Perilla frutescens leaves by overexpressed MRGPRX2 cell membrane chromatography coupled with HPLC-ESI-IT-TOF system. J. Pharm. Pharmacol. 2020, 72, 852–862.
- Qiao, C.; Hu, S.; Che, D.; Wang, J.; Gao, J.; Ma, R.; Jiang, W.; Zhang, T.; Liu, R. The anti-anaphylactoid effects of Piperine through regulating MAS-related G protein-coupled receptor X2 activation. Phytother. Res. 2020, 34, 1409–1420.
- Lin, Y.; Wang, C.; Hou, Y.; Sun, W.; Che, D.; Yang, L.; Zhang, T.; Sun, M.; He, H.; He, L. Simultaneous identification of three pseudoallergic components in Danshen injection by using high-expression Mas-related G protein coupled receptor X2 cell membrane chromatography coupled online to HPLC-ESI-MS/MS. J. Sep. Sci. 2018, 41, 2488–2497.
- Han, S.; Lv, Y.; Kong, L.; Sun, Y.; Fu, J.; Li, L.; He, L. Simultaneous identification of the anaphylactoid components from traditional Chinese medicine injections using rat basophilic leukemia 2H3 and laboratory of allergic disease 2 dual-mixed/cell membrane chromatography model. Electrophoresis 2018, 39, 1181–1189.
- Lansu, K.; Karpiak, J.; Liu, J.; Huang, X.P.; McCorvy, J.D.; Kroeze, W.K.; Che, T.; Nagase, H.; Carroll, F.I.; Jin, J.; et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat. Chem. Biol. 2017, 13, 529–536.
- Adhikari, N.; Shim, W.S. Caffeic acid phenethyl ester inhibits pseudo-allergic reactions via inhibition of MRGPRX2/MrgprB2-dependent mast cell degranulation. Arch. Pharm. Res. 2022, 45, 644–657.
- Wang, L.; Huang, C.; Li, Z.; Hu, G.; Qi, J.; Fan, Z. Liquiritin inhibits MRGPRX2-mediated pseudo-allergy through the PI3K/AKT and PLCγ signaling pathways. Heliyon 2023, 9, e13290.
- Zhang, Y.; Huang, Y.; Dang, B.; Hu, S.; Zhao, C.; Wang, Y.; Yuan, Y.; Liu, R. Fisetin alleviates chronic urticaria by inhibiting mast cell activation via MRGPRX2. J. Pharm. Pharmacol. 2023, 75, 1310–1321.
- Jia, Q.; Fu, J.; Gao, C.; Wang, H.; Wang, S.; Liang, P.; Han, S.; Lv, Y.; He, L. MrgX2-SNAP-tag/cell membrane chromatography model coupled with liquid chromatography-mass spectrometry for anti-pseudo-allergic compound screening in Arnebiae Radix. Anal. Bioanal. Chem. 2022, 414, 5741–5753.
- Liu, R.; Hu, S.; Ding, Y.; Wang, J.; Wang, Y.; Gao, J.; He, L. Dictamnine is an effective anti-anaphylactoid compound acting via the MrgX2 receptor located on mast cells. Phytother. Res. 2021, 35, 3181–3193.
- Sun, W.; Wang, S.; Liang, P.; Zhou, H.; Zhang, L.; Jia, Q.; Fu, J.; Lv, Y.; Han, S. Pseudo-allergic compounds screened from Shengmai injection by using high-expression Mas-related G protein-coupled receptor X2 cell membrane chromatography online coupled with liquid chromatography and mass spectrometry. J. Sep. Sci. 2021, 44, 1421–1429.
- Wang, N.; Wang, J.; Zhang, Y.; Zeng, Y.; Hu, S.; Bai, H.; Hou, Y.; Wang, C.; He, H.; He, L. Imperatorin ameliorates mast cell-mediated allergic airway inflammation by inhibiting MRGPRX2 and CamKII/ERK signaling pathway. Biochem. Pharmacol. 2021, 184, 114401.
- Lin, Y.; Xu, J.; Jia, Q.; Sun, W.; Fu, J.; Lv, Y.; Han, S. Cell membrane chromatography coupled online with LC-MS to screen anti-anaphylactoid components from Magnolia biondii Pamp. targeting on Mas-related G protein-coupled receptor X2. J. Sep. Sci. 2020, 43, 2571–2578.
- Wang, J.; Zhang, Y.; Wang, J.; Liu, R.; Zhang, G.; Dong, K.; Zhang, T. Paeoniflorin inhibits MRGPRX2-mediated pseudo-allergic reaction via calcium signaling pathway. Phytother. Res. 2020, 34, 401–408.
- Jia, Q.; Sun, W.; Zhang, L.; Fu, J.; Lv, Y.; Lin, Y.; Han, S. Screening the anti-allergic components in Saposhnikoviae Radix using high-expression Mas-related G protein-coupled receptor X2 cell membrane chromatography online coupled with liquid chromatography and mass spectrometry. J. Sep. Sci. 2019, 42, 2351–2359.
- Ding, Y.; Che, D.; Li, C.; Cao, J.; Wang, J.; Ma, P.; Zhao, T.; An, H.; Zhang, T. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+ fluctuations. Int. Immunopharmacol. 2019, 66, 185–197.
- Lin, Y.; Lv, Y.; Fu, J.; Jia, Q.; Han, S. A high expression Mas-related G protein coupled receptor X2 cell membrane chromatography coupled with liquid chromatography and mass spectrometry method for screening potential anaphylactoid components in kudiezi injection. J. Pharm. Biomed. Anal. 2018, 159, 483–489.
- Hou, Y.; Che, D.; Ma, P.; Zhao, T.; Zeng, Y.; Wang, N. Anti-pseudo-allergy effect of isoliquiritigenin is MRGPRX2-dependent. Immunol. Lett. 2018, 198, 52–59.
- Wang, N.; Che, D.; Zhang, T.; Liu, R.; Cao, J.; Wang, J.; Zhao, T.; Ma, P.; Dong, X.; He, L. Saikosaponin A inhibits compound 48/80-induced pseudo-allergy via the Mrgprx2 pathway in vitro and in vivo. Biochem. Pharmacol. 2018, 148, 147–154.
- Johnson, T.; Siegel, D. Complanadine A, a selective agonist for the Mas-related G protein-coupled receptor X2. Bioorg. Med. Chem. Lett. 2014, 24, 3512–3515.
- Yao, C.; Ye, W.; Chen, M. Inhibition of Mast Cell Degranulation in Atopic Dermatitis by Celastrol through Suppressing MRGPRX2. Dis. Markers 2023, 2023, 9049256.
- Ye, F.; Jiang, Y.; Zhang, J.; Zong, Y.; Yu, M.; Chen, C.; Zhu, C.; Yang, Y.; Jia, K.; Chen, G.; et al. Water Extract of Senecio scandens Buch.Ham Ameliorates Pruritus by Inhibiting MrgprB2 Receptor. J. Inflamm. Res. 2022, 15, 5989–5998.
- Jiang, Y.; Zong, Y.; Du, Y.; Zhang, M.; Ye, F.; Zhang, J.; Yang, Y.; Zhu, C.; Tang, Z. Curcumin inhibits the pruritus in mice through mast cell MrgprB2 receptor. Inflamm. Res. 2023, 72, 933–945.
- Cao, J.; Wang, Y.; Hu, S.; Ding, Y.; Jia, Q.; Zhu, J.; An, H. Kaempferol ameliorates secretagogue-induced pseudo-allergic reactions via inhibiting intracellular calcium fluctuation. J. Pharm. Pharmacol. 2020, 72, 1221–1231.
- Liu, R.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Lv, Y.; Ma, P.; Ding, Y.; Wang, N.; Wang, X.; et al. The anti-anaphylactoid effects of hydroxysafflor yellow A on the suppression of mast cell Ca2+ influx and degranulation. Phytomedicine 2018, 48, 43–50.
- Cao, C.; Roth, B.L. The structure, function, and pharmacology of MRGPRs. Trends Pharmacol. Sci. 2023, 44, 237–251.
- Gupta, K.; Subramanian, H.; Klos, A.; Ali, H. Phosphorylation of C3a receptor at multiple sites mediates desensitization, β-arrestin-2 recruitment and inhibition of NF-κB activity in mast cells. PLoS ONE 2012, 7, e46369.
- Cahill, T.J., 3rd; Thomsen, A.R.; Tarrasch, J.T.; Plouffe, B.; Nguyen, A.H.; Yang, F.; Huang, L.Y.; Kahsai, A.W.; Bassoni, D.L.; Gavino, B.J.; et al. Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc. Natl. Acad. Sci. USA 2017, 114, 2562–2567.
- Mi, Y.N.; Ping, N.N.; Cao, Y.X. Ligands and Signaling of Mas-Related G Protein-Coupled Receptor-X2 in Mast Cell Activation. Rev. Physiol. Biochem. Pharmacol. 2021, 179, 139–188.
- Chompunud Na Ayudhya, C.; Roy, S.; Alkanfari, I.; Ganguly, A.; Ali, H. Identification of Gain and Loss of Function Missense Variants in MRGPRX2’s Transmembrane and Intracellular Domains for Mast Cell Activation by Substance P. Int. J. Mol. Sci. 2019, 20, 5247.
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Heydenreich, F.M.; Flock, T.; Miljus, T.; Balaji, S.; Bouvier, M.; Veprintsev, D.B.; Tate, C.G.; et al. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature 2016, 536, 484–487.
- Chompunud Na Ayudhya, C.; Amponnawarat, A.; Ali, H. Substance P Serves as a Balanced Agonist for MRGPRX2 and a Single Tyrosine Residue Is Required for β-Arrestin Recruitment and Receptor Internalization. Int. J. Mol. Sci. 2021, 22, 5318.
- Murakami, T.; Suzuki, K.; Niyonsaba, F.; Tada, H.; Reich, J.; Tamura, H.; Nagaoka, I. MrgX2-mediated internalization of LL-37 and degranulation of human LAD2 mast cells. Mol. Med. Rep. 2018, 18, 4951–4959.
- Song, J.; Xian, D.; Yang, L.; Xiong, X.; Lai, R.; Zhong, J. Pruritus: Progress toward Pathogenesis and Treatment. Biomed. Res. Int. 2018, 2018, 9625936.
- Cao, C.; Kang, H.J.; Singh, I.; Chen, H.; Zhang, C.; Ye, W.; Hayes, B.W.; Liu, J.; Gumpper, R.H.; Bender, B.J.; et al. Structure, function and pharmacology of human itch GPCRs. Nature 2021, 600, 170–175.
- Meixiong, J.; Anderson, M.; Limjunyawong, N.; Sabbagh, M.F.; Hu, E.; Mack, M.R.; Oetjen, L.K.; Wang, F.; Kim, B.S.; Dong, X. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity 2019, 50, 1163–1171.e5.
- Kolkhir, P.; Pyatilova, P.; Ashry, T.; Jiao, Q.; Abad-Perez, A.T.; Altrichter, S.; Vera Ayala, C.E.; Church, M.K.; He, J.; Lohse, K.; et al. Mast cells, cortistatin, and its receptor, MRGPRX2, are linked to the pathogenesis of chronic prurigo. J. Allergy Clin. Immunol. 2022, 149, 1998–2009.e5.
- Ding, Y.; Ma, T.; Zhang, Y.; Zhao, C.; Wang, C.; Wang, Z. Rosmarinic acid ameliorates skin inflammation and pruritus in allergic contact dermatitis by inhibiting mast cell-mediated MRGPRX2/PLCγ1 signaling pathway. Int. Immunopharmacol. 2023, 117, 110003.
- Kabashima, K.; Irie, H. Interleukin-31 as a Clinical Target for Pruritus Treatment. Front. Med. 2021, 8, 638325.
- Zheng, T.; Oh, M.H.; Oh, S.Y.; Schroeder, J.T.; Glick, A.B.; Zhu, Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J. Investig. Dermatol. 2009, 129, 742–751.
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The translational revolution in atopic dermatitis: The paradigm shift from pathogenesis to treatment. Cell. Mol. Immunol. 2023, 20, 448–474.
- Nassau, S.; Fonacier, L. Allergic Contact Dermatitis. Med. Clin. N. Am. 2020, 104, 61–76.
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766.
- Greiwe, J.; Bernstein, J.A. Therapy of antihistamine-resistant chronic spontaneous urticaria. Expert. Rev. Clin. Immunol. 2017, 13, 311–318.
- Li, L.; Liu, R.; Peng, C.; Chen, X.; Li, J. Pharmacogenomics for the efficacy and side effects of antihistamines. Exp. Dermatol. 2022, 31, 993–1004.
More
Information
Subjects:
Allergy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
532
Revisions:
2 times
(View History)
Update Date:
27 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




