Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Grzegorz Porebski and Version 2 by Fanny Huang.
Mast cells (MCs) are immune cells that reside in tissues; particularly in the skin, and in the gastrointestinal and respiratory tracts. In recent years, there has been considerable interest in the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2), which is present on the surface of MCs and can be targeted by multiple exogenous and endogenous ligands. It is potentially implicated in non-IgE-mediated pseudoallergic reactions and inflammatory conditions such as asthma or atopic dermatitis.
- xenobiotic
- Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2)
- mast cell
1. Introduction
Mast cells (MCs) are, among a number of other functions, the primary initiators of allergic and allergic-like symptoms; they swiftly release numerous mediators upon activation. Allergic MC activation occurs via an IgE-dependent pathway, in which the allergen is matched to a specific IgE that binds to a high-affinity IgE receptor (FcεRI) on the cell surface [1]. However, there are also clinical reactions that resemble allergy and develop after exposure to a variety of xenobiotic compounds for which IgE-mediated mechanisms have not been demonstrated and are therefore termed pseudoallergic or anaphylactoid [2]. After a period of uncertainty regarding the responsible pathway, the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2) was proposed to be one of the possible IgE-independent MC activation pathways [3]. McNeil et al. demonstrated that MRGPRX2 can be activated by xenobiotics, including fluoroquinolones, neuromuscular blocking agents, and peptidergic therapeutics (e.g., icatibant, leuprolide), in addition to previously known endogenous ligands such as neuropeptides and substance P (SP) [4]. Since the publication of McNeil’s seminal paper in 2015, the number of publications addressing xenobiotic triggering of MRGPRX2 has increased rapidly. The hypothesis that drug hypersensitivity reactions are induced via an MRGPRX2-dependent pathway, mainly by drugs from the muscle relaxant and flouroquinolone antibiotic groups, has attracted much attention from the scientific community [5]. However, many studies have also been devoted to other xenobiotics—including those found in medicinal plants—and these analyse their association with the MRGPRX2 receptor.
2. Pathophysiological Basis
2.1. Mast Cell Characteristics
MCs are immune cells that are present in almost all tissues of the body but are particularly abundant in those tissues directly exposed to the external environment [6]. While MCs are primarily associated with allergic reactions, they also play a significant role in various physiological and pathological processes [7][8][9][10][11][7,8,9,10,11].
All MCs contain intracellular granules and express the high-affinity IgE receptor FcεRI on their surface [12]. The cross-linking of FcεRI receptors upon antigen-IgE binding is the most recognized pathway of MC activation, playing a crucial role in potentially fatal reactions such as anaphylaxis [1]. MC stimulation leads to degranulation and the release of granule contents, which is a primary cause of hypersensitivity manifestations [1]. The granules store a wide range of preformed mediators, including histamine [13], proteases such as tryptases and chymases [13][14][13,14], and also some cytokines; mainly tumor necrosis factor alpha (TNF-α) [15]. These substances cause various biological effects, such as increasing vascular permeability, smooth muscle contraction and activation of immune cells, which are associated with symptoms of allergic inflammation [16]. In addition to the immediate release of preformed mediators, MCs also secrete de novo synthesized compounds that are produced after MC stimulation [13]. These include lipid mediators—such as prostaglandin D2 (PGD2), which are rapidly produced and released [17]—and cytokines, which are produced and secreted over a longer period of time (hours rather than minutes) [18][19][20][18,19,20].
In humans, MCs are generally categorized into one of three subtypes, based on the content of specific proteases. MCs that contain only tryptase (MCT) are found in the mucosa of the small intestine and in the alveolar septa [21]. MCs that contain only chymase (MCC) are commonly found in synovial tissue. MCs, which contain both tryptase and chymase (MCTC), are predominantly found in the skin, submucosal layers of the small intestine, and tonsils [22]. However, at the transcriptional level, the protease content displays more tissue-specific variability, which is evident both between and within tissues [12]. Cutting-edge advancements in single-cell profiling technologies have opened new avenues to unravel the complexity and diversity of MCs. These breakthroughs shed light on previously unseen heterogeneity among MCs across various tissues, which is distinct from other cell types. In humans, transcriptomic analysis unveiled the existence of seven distinct MC subsets (MC1–7) distributed across 12 organs, each with unique transcriptomic core signatures [23].
All MCs express FcεRI, but there is controversy regarding whether MCT and MCC express MRGPRX2, despite the known expression of MRGPRX2 in skin MCTC [24][25][26][24,25,26]. Furthermore, even among skin MCTC, only a small percentage of cells exhibit MRGPRX2 expression under steady-state conditions [24][25][24,25].
2.2. Structure and Regulation of MRGPRX2 Function
MRGPRX2 is a G protein-coupled receptor (GPCR) that was first reported to be expressed mainly on MCs and sensory neurons [3][27][3,27]. The receptor has low affinity and low selectivity with respect to ligand binding. MRGPRX2 has been shown to be activated by a wide range of endogenous and exogenous compounds, primarily by small cationic molecules and peptides that have amphipathic properties, or share a motif of tetrahydroisoquinoline (THIQ) or a similar motif [4][5][4,5]. Endogenous ligands of MRGPRX2 include neuropeptides such as SP, PAMP-12, and cortistatin-14 (CST-14), as well as antimicrobial host defense peptides such as cathelicidin LL-37, hBD2, and eosinophil granule proteins (e.g., MBP). Exogenous ligands of MRGPRX2 include the cationic polymer compound 48/80 (C48/80), which is commonly used in receptor functional assays, and a variety of drugs approved by the Food and Drug Administration (FDA), such as fluoroquinolones (e.g., ciprofloxacin), neuromuscular blocking agents (e.g., rocuronium, atracurium), opioids (e.g., morphine), and many others [4][9][28][4,9,28]. MRGPRX2 can also be activated or inhibited by other exogenous agents, such as bacterial quorum sensing proteins, insect venoms [3][29][30][3,29,30], or many different plant xenobiotics (Figure 1) [31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59].
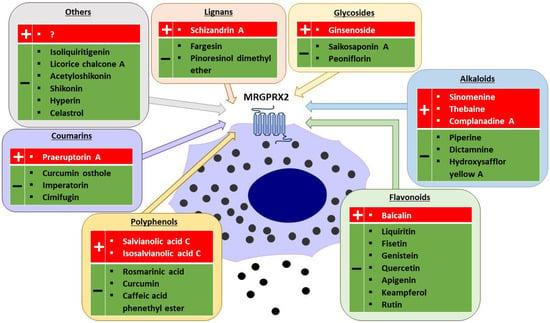
Figure 1.
Plant-derived agonists (+) and inhibitors (−) of MRGPRX2 (created with Motifolio, Motifolio Inc., Elliocott City, MD, USA).
As a GPCR, MRGPRX2 shares the structure of seven transmembrane (TM) α-helices connected by three extracellular loops (ECLs) and three intracellular loops (ICLs) [60]. The ECL region contains the N-terminus responsible for ligand binding, whereas the ICL region contains the C-terminus involved in G protein coupling, β-arrestin recruitment, and downstream signalling [61][62][63][64][61,62,63,64]. The extracellular binding of ligands to MRGPRX2 promotes the conformational changes in the transmembrane domains, resulting in structural changes on the cytoplasmic side of the membrane and activation of G proteins, and subsequent MC degranulation [65]. Conversely, some ligands can induce intracellular β-arrestin recruitment, leading to receptor desensitization and internalization [62]. The downstream signalling pathways of MRGPRX2 involve the activation of the phospholipase C pathway (PLC-PKC-IP3R), which result in intracellular Ca2+ influx and MC degranulation. Additionally, the MAP kinase (ERK-P38-JNK), PI3K-AKT, and NF-κB pathways are activated, leading to cytokines and PGD2 synthesis in MCs [7].
2.3. Role of MRGPRX2 in MC-Driven Skin Diseases
To date, the exact role of MRGPRX2 in MCs has not been fully understood [9]. Numerous in vivo and in vitro studies have been conducted on the receptor (and its mouse ortholog, MrgprB2 [4]), indicating its potential involvement in various physiological and pathological processes. With its ability to bind to a diverse range of ligands, MRGPRX2 has been implicated in drug pseudoallergic reactions, neurogenic inflammation, and a wide array of inflammatory diseases such as allergic contact dermatitis (ACD), chronic urticaria (CU), rosacea, rheumatoid arthritis, atopic dermatitis (AD), mastocytosis, ulcerative colitis, and allergic asthma [8][9][10][11][8,9,10,11]. However, conclusive evidence regarding MRGPRX2′s involvement in these conditions in humans is still lacking.
Endogenous peptides considered to be MRGPRX2 ligand play an important role in the development of inflammatory skin diseases. The neuropeptide SP and the host defense peptide cathelicidin LL-37 are key players in the pathogenesis of rosacea and AD, and are upregulated in the skin of patients [8][10][8,10]. Both peptides in vitro were shown to activate MCs via MRGPRX2, leading to MC degranulation and release of pro-inflammatory mediators, including histamine and cytokines (i.e., TNFα) [66][67][66,67]. It was proposed that the released mediators can subsequently act on sensory neurons and vascular endothelial cells to promote neurogenic inflammation, resulting in itching, erythema, swelling, and pain that exacerbate disease symptoms [8][10][8,10]. In addition, MC-derived mediators recruit immune cells into the inflamed tissue and stimulate both neurons and immune cells (such as neutrophils) to secrete more SP and LL-37, which then could again activate MCs [8][10][11][8,10,11]. Similar mechanisms involving SP and MCs are also present in ACD and CU [8][10][68][8,10,68]. Another neuropeptide ligand of MRGPRX2 involved in the development of neurogenic skin inflammation, such as the non-histaminergic pruritus associated with ACD, is CST-14 [8][10][69][70][71][8,10,69,70,71]. The skin conditions are also characterized by elevated levels of proinflammatory cytokines such as IL-13 and IL-31 [72][73][74][72,73,74]. It is noteworthy that in all these diseases, except CU, an increased number of MCs has been reported in the skin of patients compared to healthy controls [8][10][8,10]. Additionally, the expression of MRGPRX on cutaneous MCs is higher in patients with CU [24]. Therefore, the involvement of MRGPRX2 in inflammatory skin diseases is suggested [8][10][11][8,10,11].
In several of these diseases, the usual treatment with antihistamines and other first-line drugs has been reported to be ineffective [75][76][77][75,76,77]. With the current generation of H1-antihistamines, sedation has become a minor concern, as the use up to fourfold normal doses are minimally or non-sedating [77][78][79][77,78,79]. However, due to incomplete efficacy in all patients, the search for other medications remains a priority.
