Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yingwei Li | -- | 3865 | 2024-03-20 08:26:11 | | | |
| 2 | Mona Zou | -27 word(s) | 3838 | 2024-03-21 08:35:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ma, S.; Ding, P.; Zhou, Z.; Jin, H.; Li, X.; Li, Y. Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties. Encyclopedia. Available online: https://encyclopedia.pub/entry/56427 (accessed on 07 February 2026).
Ma S, Ding P, Zhou Z, Jin H, Li X, Li Y. Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties. Encyclopedia. Available at: https://encyclopedia.pub/entry/56427. Accessed February 07, 2026.
Ma, Shaoqing, Peng Ding, Zhengxuan Zhou, Huilong Jin, Xiaoli Li, Yingwei Li. "Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties" Encyclopedia, https://encyclopedia.pub/entry/56427 (accessed February 07, 2026).
Ma, S., Ding, P., Zhou, Z., Jin, H., Li, X., & Li, Y. (2024, March 20). Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties. In Encyclopedia. https://encyclopedia.pub/entry/56427
Ma, Shaoqing, et al. "Terahertz Radiation Modulates Neuronal Morphology and Dynamics Properties." Encyclopedia. Web. 20 March, 2024.
Copy Citation
Terahertz radiation falls within the spectrum of hydrogen bonding, molecular rotation, and vibration, as well as van der Waals forces, indicating that many biological macromolecules exhibit a strong absorption and resonance in this frequency band. Research has shown that the terahertz radiation of specific frequencies and energies can mediate changes in cellular morphology and function by exciting nonlinear resonance effects in proteins. However, current studies have mainly focused on the cellular level and lack systematic studies on multiple levels. Moreover, the mechanism and law of interaction between terahertz radiation and neurons are still unclear.
terahertz radiation
neurobiological effects
neurons
morphology
dynamics properties
1. The Mechanisms of Terahertz Radiation Modulation on Neurons
While the energy of individual terahertz photons is as low as a milli-electronvolt and lacks direct ionizing damage similar to X-rays [1], increasing the intensity of terahertz radiation leads to a series of biological effects on neurons. From a physics perspective, the biological effects of terahertz radiation on neurons fundamentally originate from the thermal and non-thermal effects of terahertz radiation [2]. The thermal effect results from the strong absorption of terahertz radiation by a large number of water molecules in neurons or the neuronal environment. In contrast, the non-thermal effect primarily arises from the nonlinear resonance effects induced by terahertz radiation on biological macromolecules in neurons.
1.1. Thermal Effect
The generation of terahertz radiation’s thermal effects is primarily due to the absorption of terahertz radiation by neurons and its conversion into thermal energy. The neuronal absorption of terahertz radiation is mainly related to water molecules and biological macromolecules. Since the water content in neurons is much higher than that of biological macromolecules, it serves as the major chromophore in the terahertz frequency range, resulting in the dominant contribution of water to the thermal effect [3]. Water possesses several unique properties, including the ability to form hydrogen bonds with adjacent water molecules, creating a dynamic hydrogen bond network [4]. The stretching and bending vibrational modes between the molecules within this network occur within the terahertz frequency range (with the most prominent resonant frequencies at 5.6 and 1.5 THz) [5][6][7]. When neurons are exposed to terahertz waves, the vibrational modes of the hydrogen bonds are excited, causing resonance. This disrupts the dynamic equilibrium of the water molecule’s network structure, resulting in the strong absorption of terahertz radiation by water. The absorbed terahertz radiation energy is transformed into the kinetic energy of the random motion of water molecules, increasing the frequency of collisions between water molecules, thus generating thermal energy [2]. In the absence of photochemical processes and phase changes, the continuous accumulation of heat will directly lead to an increase in neuronal temperature [3].
The thermal effects of terahertz radiation on neurons can lead to changes in neuronal morphology and function. These changes have a dual nature and are primarily associated with the extent of neuronal temperature increase and the duration of elevated temperatures. The prolonged exposure of neurons to high-power terahertz radiation, leading to a significant and sustained increase in temperature, can result in disrupted neuronal growth, dehydration effects, neuronal morphological damage, neuronal stress responses, and, in severe cases, structural protein denaturation and neuronal cell death [3][8][9]. However, when the duration of elevated temperature within neurons is on the millisecond scale, it can affect neuronal calcium homeostasis, induce the generation of action potentials in neurons, impact neuronal synaptic transmission, and affect firing rates [10], and these effects are reversible.
1.2. Non-Thermal Effect
Some studies have indicated that certain changes in gene expression and individual behavior observed in biological systems exposed to terahertz radiation cannot be explained by thermal effects (without significant temperature elevation or changes in heat shock protein expression) [11][12]. For these effects observed in terahertz radiation biological systems, they are generally attributed to non-thermal effects. The theory of the non-thermal effects of terahertz radiation was initially proposed by Frohlich and others in 1971, and more recent research results suggest that many biological macromolecules have energy levels associated with rotation, oscillation, and torsion in the terahertz frequency range. Specific frequencies and energy levels of terahertz waves can be directly coupled with proteins, inducing coherent excitations and resulting in non-thermal effects [13][14].
Although the energy of terahertz radiation is insufficient to break chemical bonds between molecules, both theoretical and experimental evidence suggests that terahertz radiation can interact with hydrogen bonds in proteins, inducing low-frequency molecular vibrations, consequently altering the conformation and functional characteristics of proteins. Additionally, radiation can induce non-thermal structural changes in protein crystals [15][16][17]. Research by Aleksandrov and others, using computer simulations, revealed that terahertz radiation can disrupt the natural dynamics of a local strand separation in double-stranded DNA, thereby affecting DNA function [18]. Furthermore, studies have pointed out that terahertz radiation can precisely control proton transfer processes in base pair hydrogen bonds, thereby regulating DNA demethylation [19][20]. These studies indicate that terahertz radiation can mediate changes in cellular morphology and function by exciting nonlinear resonance effects in proteins and DNA. Based on this mechanism, terahertz radiation of specific frequencies and energy levels can impact the morphology and function of neurons.
2. Methods for Terahertz Radiation Regulation of Neurons
2.1. Radiation System for Terahertz Regulation of Neurons
The research on the terahertz radiation regulation of neuron morphology and function is also influenced by the radiation system. When investigating the regulatory patterns of the terahertz radiation on the nervous system, it is essential to understand how the nervous system responds to different terahertz radiation parameters. Therefore, it is necessary to adjust the output frequency and power of the terahertz radiation system to generate various radiation protocols. Additionally, the terahertz radiation system needs to be compatible with the biological experimental platform. However, current technology cannot always meet these requirements. Consequently, in research, different types of terahertz radiation systems are usually employed to achieve the adjustable power and frequency demands. For example, researchers frequently use both broadband and narrowband terahertz radiation systems. Broadband terahertz radiation systems cover a broader frequency range, making it easier to induce resonance in biological macromolecules. In contrast, when the controlling resonance peaks of biological macromolecules are identified, narrowband terahertz radiation systems are often chosen, which operate near the resonance peak.
Broadband terahertz systems generally use pulsed terahertz sources, which emit pulsed signals with durations on the order of picoseconds in the time domain and exhibit broad spectra in the frequency domain [14]. Pulsed broad-spectrum terahertz sources generated through optical methods typically employ femtosecond lasers as the pump light source [14]. These lasers excite different materials to produce terahertz waves using methods like photoconductive antennas, optical rectification, and gas plasma [21]. Among these techniques, the terahertz waves generated through photoconductive antennas primarily cover the lower frequency range and have relatively lower power levels. Optical rectification is currently an important method for producing high-energy single-pulse terahertz radiation. It uses femtosecond laser-pumped DSTMS organic crystals to generate terahertz radiation with single-pulse energies of up to 0.9 mJ [14][22]. Additionally, the spectrum of terahertz radiation produced through optical rectification is much broader than that of photoconductive antennas, spanning from 0.1 to 100 THz, covering the entire terahertz range [14]. Gas plasma terahertz sources are also common for generating pulsed broad-spectrum terahertz radiation. Compared to the methods mentioned above for generating terahertz waves through photoconductive and optical rectification, gas plasma terahertz sources can achieve ultra-broad terahertz spectra ranging from 0.1 to 30 THz [14][23]. However, since the medium used to generate terahertz radiation in gas plasma sources is unstable, the intensity of the produced terahertz radiation can also exhibit certain fluctuations [14], which may affect research on terahertz neurobiological effects.
When studying the effects of terahertz radiation on neuron morphology and function, the choice of terahertz radiation frequencies typically falls within the range of 0.1–5 THz because many energy levels of various biomolecules (proteins, DNA, RNA) are within this frequency range [24]. Furthermore, higher terahertz radiation frequencies can result in neuronal heating and induce thermal effects [8]. Therefore, the terahertz radiation power levels used in research are usually kept below 10 mW. Additionally, considering the compatibility of terahertz radiation systems with biological experimental platforms, it is desirable for these systems to have as compact a form factor as possible. Currently, the systems used for researching the impact of terahertz radiation on neuron morphology and function predominantly involve broadband terahertz radiation systems generated using photoconductive antennas. For instance, in the study investigating the effects of broadband terahertz radiation in minute quantities on neuron growth and development, the terahertz radiation system used had a frequency range of 0.3–3 THz, a maximum radiation power of up to 100 μW, and a repetition rate of 100 MHz. To ensure that an adequate amount of terahertz radiation reached the samples, the experimental process also included real-time measurements of terahertz radiation intensity through the culture dishes [25]. Additionally, M.V. Tsurkan and colleagues employed a broadband terahertz radiation system with a frequency range of 0.05–2 THz and a repetition rate of 50 MHz to study the impact of radiation on chicken embryonic neurons. They obtained terahertz waves with power levels of 11.1 and 1.07 mW through different terahertz filters [26]. Furthermore, M.I. Sulatsky and others used a broadband terahertz radiation system (0.1–2 THz) to investigate the impact of radiation power on chicken embryonic spinal cord ganglia [27].
When determining the resonance peaks of biological macromolecules in the controlled neurons, narrowband terahertz radiation systems are typically chosen around these resonance peaks. These narrowband terahertz radiation systems generally allow for the continuous emission of terahertz radiation and are produced using various methods, including free-electron lasers, gas lasers, quantum cascade lasers, backward wave oscillators, and avalanche diodes [14]. Terahertz sources based on free-electron lasers exhibit an output power that surpasses that of the photoconductive antenna method by more than six orders of magnitude, but they tend to have a larger footprint and higher production costs [14][28]. Terahertz gas lasers can also generate terahertz waves with a relatively high average power. By altering the type of gas and gas pressure, these lasers can produce terahertz radiation at different frequencies (approximately 0.9–7 THz). However, their conversion efficiency is low, and they tend to be bulky [29]. Quantum cascade lasers, based on semiconductor technology, can achieve output frequencies as low as 1.19 THz. They offer the advantage of being compact and suitable for integration but are not operable at room temperature [30]. Backward wave oscillators can adjust their output frequency by varying the acceleration voltage. Typically, their operating frequency is below 1.5 THz, and they provide an average output power in the milliwatt range [14][31]. Terahertz sources based on avalanche diodes are widely used due to their relatively high linear output power and compact design. They generally operate at frequencies below 1 THz and can achieve output powers of up to 100 mW [32].
When studying the effects of terahertz radiation on brain tissue slices or in vivo neuronal discharge characteristics, it becomes crucial to enhance the output power of terahertz sources. This is due to the significant absorption of terahertz radiation by cerebrospinal fluid, skin, and cranial bones. Additionally, to ensure compatibility with biological experimental platforms, terahertz radiation systems should be as compact as possible and operate at room temperature. Currently, the most widely used terahertz radiation source at the cellular, tissue, and in vivo levels is the avalanche diode-based terahertz source. For example, Zhang et al. utilized a terahertz radiation system operating at 0.1 THz with a power density of 2.65 mW/cm2 to confirm that terahertz radiation can modulate neuronal discharge characteristics by affecting ion concentrations inside neurons [33]. Furthermore, this study found that terahertz radiation at 0.138 THz and 2 mW promotes synaptic transmission in the hippocampal CA1 region. At the in vivo level, Miao et al. increased terahertz radiation power to 90 mW and observed that 0.14 THz radiation reduces anxiety and depression symptoms in mice while enhancing their social behavior [34]. Some studies have also used backward wave oscillators and quantum cascade lasers as terahertz sources, but these investigations are often limited to the cellular level. In such studies, due to the size and operating temperature of terahertz radiation sources, they are usually placed at specific locations in the experiments, and the terahertz radiation is transmitted to the samples using lenses and mirrors [35][36].
2.2. Radiation Protocol for Terahertz Radiation Regulation of Neurons
Previous research has indicated that terahertz radiation can impact the morphology and functionality of neurons, and these effects are related to factors such as terahertz frequency, duration, and power [33][34][35]. Therefore, to further select appropriate terahertz radiation protocols, it is essential to analyze the distribution of research subjects and terahertz radiation protocols in the relevant literature. In retrieving the related literature, both Chinese and English keywords were initially set, including terahertz, neurons, nervous system, proteins, growth and development, action potentials, membrane potentials, receptors, etc. Subsequently, articles in both Chinese and English containing at least “terahertz” and one other keyword were searched in databases such as Web of Science, Google, PubMed, and China National Knowledge Infrastructure. Finally, parameters such as publication year, radiation frequency, power density, and exposure duration were summarized, and the results are shown in Figure 1.
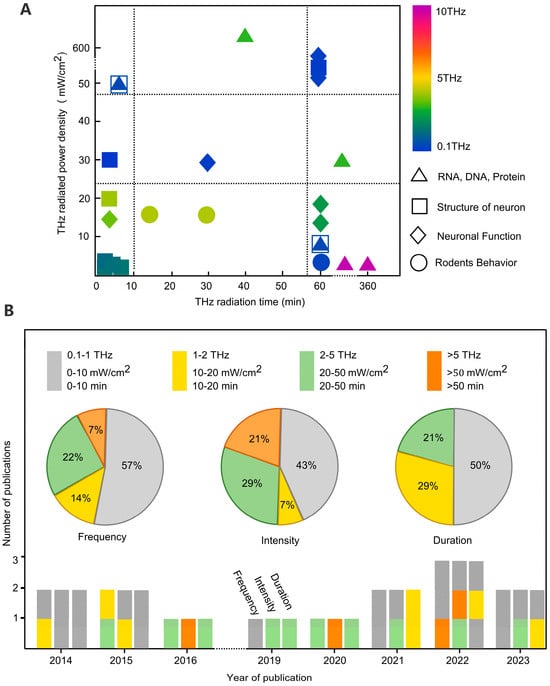
Figure 1. Analysis of the literature on terahertz neurobiological effects. (A) Heat map of the relationship between study objects and terahertz radiation protocols in the literature. (B) Analysis of terahertz radiation parameters in the literature related to terahertz neurobiological effects in the last decade.
Currently, most research aims to uncover terahertz neurobiological effects at the cellular level, with the majority of studies focusing on frequencies below 5 THz, particularly emphasizing the impact of low power densities on the nervous system. Over the past decade, 57% of the studies on the effects of terahertz radiation on neuronal morphology and functionality were conducted in the 0.1–1 THz range, and 93% in the 0.1–5 THz range. This concentration in the lower frequency range may be related to the vibrational rotation frequencies of biological macromolecules and is also influenced by the frequency of terahertz radiation sources (most commercial terahertz radiation sources are concentrated in the lower frequency range). Regarding terahertz radiation power, 43% of the studies had radiation power densities in the range of 0–10 mW/cm2, while 21% had power densities greater than 50 mW/cm2.
Through the analysis above, it is evident that research primarily focuses on two extremes: low- and high-power terahertz radiation. A higher radiation power often leads to neuronal heating, causing thermal effects [8]. On the other hand, a lower radiation power typically induces non-thermal effects on neurons, thereby influencing neuronal morphology and functionality [35][36]. Furthermore, some studies indicate that a lower power terahertz radiation can have positive impacts on neurons, such as promoting growth and enhancing synaptic transmission [33][36], while a higher power terahertz radiation can result in damage to neuronal morphology and function, leading to growth disturbances, dehydration, and cell death, among other effects [8][37]. In terms of terahertz radiation duration, studies generally involve single exposures lasting less than 50 min, with 50% of the research falling within the 0–10 min range. Most studies aim to mitigate the thermal effects of terahertz radiation on neurons by reducing exposure duration.
3. The Impact of Terahertz Radiation on Neuronal Morphology and Dynamic Properties
Recently, an increasing number of scholars have begun to focus on the neurobiological effects of terahertz radiation, as depicted in Figure 2. Some studies have shown that specific frequencies and energies of terahertz radiation can mediate changes in neuronal morphology and dynamic properties by exciting nonlinear resonances within proteins and DNA [15][16][17]. This section provides a detailed analysis of the impact of terahertz radiation on neuronal growth and development, the structure and composition of neuronal receptors, and neuronal action potential characteristics. And it discusses the correlation of these phenomena with the parameters of terahertz radiation (frequency, time, power).
Figure 2. Research content related to terahertz neurobiological effects.
3.1. The Influence of Terahertz Radiation on the Growth and Development of Neurons
The cell body and processes of a neuron are fundamental structures that underpin the transmission and reception of information by neurons. At the tip of the neurite, there is an extension structure known as the growth cone [38][39], which serves as the driving organ of neuronal growth. The growth cone is capable of sensing signals in the environment and modifying the development process of the neuron, a process that also involves the polymerization and dissolution of cytoskeletal proteins [38][39]. Studies have shown that continuous terahertz radiation (0.094 THz, 1.86 W/cm2) for 30 min can disrupt the structure of actin [40], and that increasing the radiation power to 3.1 W/cm2 results in even more significant disintegration [40]. Subsequently, a reduction in both the power and duration of terahertz radiation was investigated, with the finding that after radiation at a power of 310 mW/cm2 for 3 min, the growth rate of neurons increased from 13.0 ± 0.9μm/min to 30.8 ± 3.9 μm/min, although the neuron temperature increased by more than 7 °C [41]. The relationship between temperature and microtubule assembly was analyzed, and it was determined that this phenomenon is associated with the increased temperature of the neuron [40][41]. Additionally, higher radiation powers (0.12 THz, 10 mW; 0.157 THz, 50 mW) have been shown to induce neuronal apoptosis, along with mitochondrial damage and increased lysosomes [42].
The above studies have shown that high-power terahertz radiation disrupts actin structural disassembly and causes neuronal death, mitochondrial damage, and increased lysosomes and that there is a significant increase in neuronal temperature [40][41][42]. It is evident that high-power terahertz radiation causes thermal effects on neurons and can negatively affect them [43][44]. Ma et al. constructed a thermal effect model for the interaction between terahertz and neurons and found that the temperature of neurons increases rapidly during terahertz radiation and stabilizes after a period of time. And, with the increase in the terahertz radiation power, the peak temperature in the neuron also increases [45]. Thus, terahertz radiation power and time are important factors that affect neurons.
To mitigate the effects of thermal radiation, some studies have chosen to reduce the power and duration of terahertz radiation exposure. For instance, Sulatsky et al. exposed chick embryo spinal cord neurons to terahertz radiation (0.1–2 THz) for 3 and 5 min. The results indicated that neuronal growth was inhibited at a power density of 928 mW/cm2; however, when the power density was reduced to 78 mW/cm2, terahertz radiation was found to promote neuronal growth. Furthermore, when the terahertz power density decreased to 19 mW/cm2, the growth of neurons in the irradiated group was 118.76 ± 11.3% greater than that in the control group [27]. To further reduce the thermal effects of terahertz radiation on neurons, a study employed terahertz radiation with a power density of 1.1 μW/cm2 to irradiate chick embryo sensory neurons for 10–12 days. The results showed that the growth of neurons in the irradiated group was 147 ± 22% greater than that in the control group [46]. These findings suggest that terahertz radiation power is a critical parameter affecting neuronal growth and development. Tsurkan et al. further confirmed the correlation between terahertz radiation power and neuronal growth and development. In their study, broad-spectrum pulsed terahertz radiation (0.05–2 THz) was used to irradiate chick embryo neurons for 3 min. They found no significant impact on neuronal growth and development when the terahertz radiation power density was 5 μW/cm2 and 50 μW/cm2 [26]. However, when the terahertz power density was reduced to 0.5 μW/cm2, terahertz radiation significantly promoted neuronal growth [26].
The frequency of terahertz radiation has also been found to influence the growth and development of neuronal processes. Some studies have observed the reflection spectrum of neurons exposed to terahertz radiation and found that the impact of terahertz radiation on the growth and development of neuronal processes is highly correlated with the absorption characteristics of neurons towards terahertz radiation [27]. The research conducted by Zhao and colleagues further supports this conclusion, as they demonstrated that exposure to 3.1 THz radiation can promote neuronal growth and synaptic formation by altering the dynamics of gene expression associated with neuronal development [36]. In summary, the frequency of terahertz radiation is also an important factor affecting neurons by the mechanism of absorption and resonance of terahertz radiation by neurons. Since both the neuron size and the terahertz wavelength are on the order of micrometers, they are equipped to interact [47][48][49]. In addition, the resonance peaks between biological macromolecules are different, so there are differences in the effects of different frequencies of terahertz radiation on neurons.
3.2. The Impact of Terahertz Radiation on Neuronal Membrane Permeability and Integrity
The neuronal membrane has a dual significance in neurons: firstly, as a barrier, it maintains the integrity of the cell and its internal compartments; secondly, as a medium for communication between the organelle and the external environment, it has a significant role in neuronal resting potential maintenance and electrical signal transduction [50][51]. Neuronal excitability depends not only on the difference in ion concentrations in-side and outside the cell but also on the neuronal membrane’s precise control and selective permeation [52][53].
Several studies have explored the effects of terahertz radiation at wavelengths of 130 μm and 150 μm on neuronal cell membrane integrity and permeability using a fluorescent dye that does not penetrate intact cell membranes (BCECF-AM) and a live cell assay [54]. It was found that some neurons were exposed to terahertz waves with a wavelength of 130 μm, and the permeability of the neuronal membrane was changed and successfully stained by the dye, with the proportion of stained cells proportional to the terahertz radiation power. The membrane potential of unstained neurons is about −60 mV, while the membrane potential of stained neurons is usually greater than −60 mV or close to 0 mV. But similar results do not appear for terahertz waves with a wavelength of 150 μm. Subsequently, researchers found that the terahertz effect at a wavelength of 130 μm caused reversible damage to the barrier properties of neuronal membranes due to reactive oxygen metabolites [54].
However, it has also been shown that terahertz waves have no significant effects on neuronal membranes [35]. In this study, seven different types of neurons were selected and exposed to different frequencies (0.16 THz, 0.17 THz), powers (10 mW, 50 mW), and irradiation times (10 min, 60 min) of terahertz radiation, and the results showed that terahertz radiation had no significant effects on the roughness of neuronal membranes.
3.3. The Impact of Terahertz Radiation on Neuronal Dynamical Properties
Neurons possess the capacity to perceive stimuli and transmit excitation, with the propagation of neuronal excitation being achieved through the selective permeation of charged ions such as Ca2+, K+, and Na+ via ion channels [55]. Factors such as electric fields and magnetic fields often influence the opening and closing of ion channels, thereby affecting the neuronal membrane potential and action potentials. Guo and colleagues utilized Brownian Dynamics (BD) simulations to solve mathematical–physical models and investigated the spontaneous radiation produced by Ca2+ movement within calcium channels, as well as the impact of terahertz radiation on Ca2+ transport [56]. The study found that Ca2+ can emit terahertz radiation during its movement within calcium channels. Additionally, external terahertz radiation can accelerate Ca2+ transit through ion channels, and the acceleration effect is related to the frequency and amplitude of the terahertz radiation [56].
Sun and colleagues further experimentally demonstrated the correlation between terahertz radiation power and intraneuronal Ca2+ concentration. They found that terahertz radiation (0.094 THz) can regulate intraneuronal Ca2+ concentration by adjusting the secretion of ATP [57]. When the radiation power was 30 mW, the secretion of ATP increased by a factor of 5, and the peak Ca2+ concentration in neurons also increased; however, when the terahertz radiation power was increased to 60 mW, the secretion of ATP increased by a factor of 10, but the change in Ca2+ concentration in neurons was slow [57]. Furthermore, Titushkin and others also discovered that exposing neurons to terahertz radiation (0.094 THz, 18.6 kW/m2) led to a significant increase in the number of Ca2+ spikes in neurons [40].
References
- Li, W.; Hu, X.; Wu, J.; Fan, K.; Chen, B.; Zhang, C.; Hu, W.; Cao, X.; Jin, B.; Lu, Y.; et al. Dual-Color Terahertz Spatial Light Modulator for Single-Pixel Imaging. Light Sci. Appl. 2022, 11, 191.
- Peng, X.; Zhou, H. Biological Effects of Terahertz Waves. Acta Phys. Sin. 2021, 70, 240701.
- Zhang, H.; Liu, R.; Li, B.; Liu, D.; Xu, D. Progress of Terahertz Radiation and Its Biological Effects. Prog. Biochem. Biophys. 2021, 48, 1471–1482.
- Fan, S.; Ma, Y.; Shu, G.; Qian, Z. The interaction of terahertz with water molecules: Mechanism, applications, and new trends. J. Shenzhen Univ. (Sci. Eng.) 2019, 36, 200–206.
- Ladanyi, B.; Skaf, M. Computer Simulation of Hydrogen-Bonding Liquids. Annu. Rev. Phys. Chem. 1993, 44, 335–368.
- Russo, D.; Hura, G.; Head-Gordon, T. Hydration dynamics near a model protein surface. Biophys. J. 2004, 86, 1852–1862.
- Yada, H.; Nagai, M.; Tanaka, K. Origin of the fast relaxation component of water and heavy water revealed by terahertz time-domain attenuated total reflection spectroscopy. Chem. Phys. Lett. 2008, 464, 166–170.
- Ol’shevskaia, I.S.; Kozlov, A.; Petrov, A.; Zapara, T.; Ratushniak, A. Influence of Terahertz (Submillimeter) Laser Radiation on Neurons in vitro. Zhurnal Vyss. Nervn. Deyatelnosti Im. I P Pavlov. 2009, 59, 353–359.
- Chen, C.; Ma, Q.; Tao, J.; Lu, Y.; Lin, M.; Gao, P.; Deng, P.; He, M.; Pi, H.; Zhang, L.; et al. Effects of terahertz exposure on skin injury in mouse model. J. Third Mil. Med. Univ. 2020, 42, 2282–2289.
- Xia, Q. Photothermal Effect of Near-Infrared Laser on the Electrical Activity of Cortical Neurons in Rats. Ph.D. Thesis, Chongqing University, Chongqing, China, 2019; pp. 10–42.
- Alexandrov, B.; Rasmussen, K.; Bishop, A.; Usheva, A.; Alexandrov, L.; Chong, S.; Dagon, Y.; Booshehri, L.; Mielke, C.; Phipps, M.; et al. Non-thermal effects of terahertz radiation on gene expression in mouse stem cells. Biomed. Opt. Express 2011, 2, 2679–2689.
- Kirichuck, V.F.; Ivanov, A.N.; Antipova, O.N.; Krenickiy, A.P.; Mayborodin, A.V.; Tupikin, V.D. Sex-specific differences in changes of disturbed functional activity of platelets in albino rats under the effect of terahertz electromagnetic radiation at nitric oxide frequencies. Bull. Exp. Biol. Med. 2008, 145, 75–77.
- Fröhlich, H. The extraordinary dielectric properties of biological materials and the action of enzymes. Proc. Natl. Acad. Sci. USA 1975, 72, 4211–4215.
- Fu, W.; Luo, Y. Terahertz Technology and Its Biological Applications; People’s Medical Publishing House: Beijing, China, 2017; pp. 150–200.
- Fischer, B.; Walther, M.; Uhd Jepsen, P. Far-infrared vibrational modes of DNA components studied by terahertz time-domain spectroscopy. Phys. Med. Biol. 2002, 47, 3807–3814.
- Cherkasova, O.; Fedorov, V.; Nemova, E.; Pogodin, A. Influence of terahertz laser radiation on the spectral characteristics and functional properties of albumin. Opt. Spectrosc. 2009, 107, 534–537.
- Lundholm, I.; Rodilla, H.; Wahlgren, W.; Duelli, A.; Bourenkov, G.; Vukusic, J.; Friedman, R.; Stake, J.; Schneider, T.; Katona, G. Terahertz radiation induces non-thermal structural changes associated with Fröhlich condensation in a protein crystal. Struct. Dyn. 2015, 2, 054702.
- Alexandrov, B.; Gelev, V.; Bishop, A.; Usheva, A.; Rasmussen, K. DNA Breathing Dynamics in the Presence of a Terahertz Field. Phys. Lett. A 2010, 374, 1214–1217.
- Cheon, H.; Paik, J.; Choi, M.; Yang, H.; Son, J. Detection and manipulation of methylation in blood cancer DNA using terahertz radiation. Sci. Rep. 2019, 9, 6413.
- Cheon, H.; Yang, H.J.; Choi, M.; Son, J.H. Effective demethylation of melanoma cells using terahertz radiation. Biomed. Opt. Express 2019, 10, 4931–4941.
- Ye, Q.; Yang, C. Recent progress in THz sources based on photonics methods. Chin. Opt. 2012, 5, 1–11.
- Vicario, C.; Jazbinsek, M.; Ovchinnikov, A.; Chefonov, O.; Ashitkov, S.; Agranat, M.; Hauri, C. High efficiency THz generation in DSTMS, DAST and OH1 pumped by Cr: Forsterite laser. Opt. Express 2015, 23, 4573–4580.
- Kim, K.; Taylor, A.; Glownia, J.; Rodriguez, G. Coherent control of terahertz supercontinuum generation in ultrafast laser–gas interactions. Nat. Photonics 2008, 2, 605–609.
- Wilmink, G.; Grundt, J. Invited Review Article: Current State of Research on Biological Effects of Terahertz Radiation. J. Infrared Millim. Terahertz Waves 2011, 32, 1074–1122.
- Ma, S.; Gong, S.; Zhang, W.; Lu, C.; Li, X.; Li, Y. Neuronal growth and development promoted by low-intensity roadband terahertz radiation. Acta Phys. Sin. 2022, 71, 208701.
- Tsurkan, M.; Smolyanskaya, O.; Bespalov, V.; Penniyainen, V.; Kipenko, A.; Lopatina, E.; Krylov, B. Changing Growth of Neurites of Sensory Ganglion by Terahertz Radiation. Proc. SPIE 2012, 8261, 82610.
- Sulatsky, M.; Duka, M.; Smolyanskaya, O. Stimulation of neurite growth under broadband pulsed THz radiation. Phys. Wave Phenom. 2014, 22, 197–201.
- Tan, P.; Huang, J.; Liu, K.; Xiong, Y.; Fan, M. Terahertz radiation sources based on free electron lasers and their applications. Sci. China-Inf. Sci. 2011, 55, 1–15.
- Votintsev, A.; Borisov, A.; Makashev, D.; Stoyanova, M.; Kistenev, Y. Widely tunable compact terahertz gas lasers. Science 2019, 366, 856–860.
- Köhler, R.; Tredicucci, A.; Beltram, F.; Beere, H.; Linfield, E.; Davies, A.; Ritchie, D.; Iotti, R.; Rossi, F. Terahertz semiconductor-heterostructure laser. Nature 2002, 417, 156–159.
- Xi, H.; Wang, P.; Bao, C.; Liu, Y. The Research on Backward Wave Oscillator with Wide Tunable Bandwidth and High Power. Infocommun. Radio Technol. 2022, 5, 101–107.
- Castellano, F.; Li, L.; Linfield, E.; Davies, A.; Vitiello, M. Frequency and amplitude modulation of ultra-compact terahertz quantum cascade lasers using an integrated avalanche diode oscillator. Sci. Rep. 2016, 6, 23053.
- Zhang, X.; He, M.; Zhao, J.; Chen, X.; Liu, L.; Lu, X.; Tian, T.; Chen, M.; Wang, P. Effect of 0.1 THz Radiation on Excitability of Hippocampal Neurons in Sprague Dawley Rats. Chin. J. Lasers 2020, 47, 295–301.
- Qi, M.; Liu, R.; Li, B.; Wang, S.; Fan, R.; Zhao, X.; Xu, D. Behavioral Effect of Terahertz Waves in C57BL/6 Mice. Biosensors 2022, 12, 79.
- Tan, S.; Tan, P.; Luo, L.; Chi, Y.; Yang, Z.; Zhao, X.; Zhao, L.; Dong, J.; Zhang, J.; Yao, B.; et al. Exposure Effects of Terahertz Waves on Primary Neurons and Neuron-like Cells Under Nonthermal Conditions. Biomed. Environ. Sci. 2019, 32, 739–754.
- Zhao, X.; Zhang, M.; Liu, Y.; Liu, H.; Ren, K.; Xue, Q.; Zhang, H.; Zhi, N.; Wang, W.; Wu, S. Terahertz exposure enhances neuronal synaptic transmission and oligodendrocyte differentiation in vitro. iScience 2021, 24, 103485.
- Deghoyan, A.; Heqimyan, A.; Nikoghosyan, A.; Dadasyan, E.; Ayrapetyan, S. Cell bathing medium as a target for non thermal effect of millimeter waves. Electromagn. Biol. Med. 2012, 31, 132–142.
- Akiyama, H.; Kamiguchi, H. Second Messenger Networks for Accurate Growth Cone Guidance. Dev. Neurobiol. 2013, 75, 411–422.
- Goodhill, G.; Faville, R.; Sutherland, D.; Bicknell, B.; Thompson, A.; Pujic, Z.; Sun, B.; Kita, E.; Scott, E. The Dynamics of Growth Cone Morphology. BMC Biol. 2015, 13, 10.
- Titushkin, I.; Rao, V.; Pickard, W.; Moros, E.; Shafirstein, G.; Cho, M. Altered Calcium Dynamics Mediates P19-Derived Neuron-Like Cell Responses to Millimeter-Wave Radiation. Radiat. Res. 2009, 172, 725–736.
- Samsonov, A.; Popov, S. The Effect of a 94 GHz Electromagnetic Field on Neuronal Microtubules. Bioelectromagnetics 2012, 34, 133–144.
- Zhao, L.; Yi, R.; Liu, S.; Chi, Y.; Tan, S.; Dong, J.; Wang, H.; Zhang, J.; Wang, H.; Xu, X.; et al. Biological Responses to Terahertz Radiation with Different Power Density in Primary Hippocampal Neurons. PLoS ONE 2023, 18, e0267064.
- Wilmink, G.; Rivest, B.; Roth, C.; Ibey, B.; Payne, J.; Cundin, L.; Grundt, J.; Peralta, X.; Mixon, D.; Roach, W. In vitro investigation of the biological effects associated with human dermal fibroblasts exposed to 2.52 THz radiation. Laser Surg. Med. 2011, 43, 152–163.
- Pennes, H. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. J. Appl. Physiol. 1948, 1, 93–122.
- Ma, S.; Li, Z.; Gong, S.; Lu, C.; Li, X.; Li, Y. The laws and effects of terahertz wave interactions with neurons. Front. Bioeng. Biotechnol. 2023, 11, 1147684.
- Duka, M.; Dvoretskaya, L.; Babelkin, N.; Khodzitskii, M.; Chivilikhin, S.; Smolyanskaya, O. Numerical and Experimental Studies of Mechanisms Underlying the Effect of Pulsed Broadband Terahertz Radiation on Nerve Cells. Quantum Electron. 2014, 44, 707–712.
- Guo, L.; Wang, S.; Yang, L.; Wang, K.; Ma, J.; Zhou, J.; Gong, Y. Weak resonance effects of THz wave transimission in nerve cell. Acta Phys. Sin. 2021, 70, 340301.
- Liu, W.; Lu, Y.; She, R.; Wei, G.; Jiao, G.; Lv, J.; Li, G. Thermal Analysis of Cornea Heated with Terahertz Radiation. Appl. Sci. 2019, 9, 917.
- Generalov, V.; Safatov, A.; Kruchinina, M.; Gromov, A.; Buryak, G.; Generalov, K.; Kruchinin, V. Dielectric properties of the human red blood cell Izmer. Meas. Tech. 2020, 63, 580–586.
- Winkle, C.C.; Gupton, S.L. Membrane Trafficking in Neuronal Development: Ins and Outs of Neural Connectivity. Int. Rev. Cell Mol. Biol. 2016, 322, 247–280.
- Ramachandran, K.V.; Fu, J.B.; Schaffer, T.; Na, C.H.; Delannoy, M.; Margolis, S.A. Activity-Dependent Degradation of the Nascentome by the Neuronal Membrane Proteasome. Mol. Cell 2018, 71, 169–177.e6.
- Lemire, S.; Jeromin, A.; Boisselier, É. Membrane Binding of Neuronal Calcium Sensor-1 (NCS1). Colloids Surf. B Biointerfaces 2016, 139, 138–147.
- Karasmanis, E.P.; Phan, C.-T.; Angelis, D.; Kesisova, I.A.; Hoogenraad, C.C.; McKenney, R.J.; Spiliotis, E.T. Polarity of Neuronal Membrane Traffic Requires Sorting of Kinesin Motor Cargo during Entry into Dendrites by a Microtubule-Associated Septin. Dev. Cell 2018, 46, 204–218.e7.
- Zapara, T.A.; Treskova, S.I.; Ratushniak, A.S. Effect of Antioxidants on the Interaction of Terahertz (Submillimeter) Laser Radiation and Neuronal Membrane. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2015, 9, 869–871.
- Paulsen, B.; Velasco, S.; Kedaigle, A.; Pigoni, M.; Quadrato, G.; Deo, A.; Adiconis, X.; Uzquiano, A.; Sartore, R.; Yang, S.; et al. Autism Genes Converge on Asynchronous Development of Shared Neuron Classes. Nature 2022, 602, 268–273.
- Guo, L.; Bo, W.; Wang, K.; Wang, S.; Gong, Y. Theoretical Investigation on the Effect of Terahertz Wave on Ca2+ Transport in the Calcium Channel. iScience 2021, 25, 103561.
- Sun, S.; Titushkin, I.; Varner, J.; Cho, M. Millimeter Wave-Induced Modulation of Calcium Dynamics in an Engineered Skin Co-culture Model: Role of Secreted ATP on Calcium Spiking. J. Radiat. Res. 2012, 53, 159–167.
More
Information
Subjects:
Biophysics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
692
Revisions:
2 times
(View History)
Update Date:
21 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




