Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yingdi Liao | -- | 1734 | 2024-03-20 06:12:11 | | | |
| 2 | Camila Xu | Meta information modification | 1734 | 2024-03-21 02:53:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhu, Y.; Chen, D.; Yu, X.; Liu, R.; Liao, Y. Properties of Seashells. Encyclopedia. Available online: https://encyclopedia.pub/entry/56424 (accessed on 05 March 2026).
Zhu Y, Chen D, Yu X, Liu R, Liao Y. Properties of Seashells. Encyclopedia. Available at: https://encyclopedia.pub/entry/56424. Accessed March 05, 2026.
Zhu, Yunpeng, Da Chen, Xiaotong Yu, Ruiwen Liu, Yingdi Liao. "Properties of Seashells" Encyclopedia, https://encyclopedia.pub/entry/56424 (accessed March 05, 2026).
Zhu, Y., Chen, D., Yu, X., Liu, R., & Liao, Y. (2024, March 20). Properties of Seashells. In Encyclopedia. https://encyclopedia.pub/entry/56424
Zhu, Yunpeng, et al. "Properties of Seashells." Encyclopedia. Web. 20 March, 2024.
Copy Citation
Researchers around the world have conducted extensive experiments with waste seashells in the form of seashell aggregates and seashell powder. The physical, mechanical, and durability properties of seashell concrete are largely determined by the properties of the aggregates and powders that make up the shell.
seashell
concrete
durability
mechanical properties
1. Physical Properties
Researchers around the world have conducted extensive experiments with waste seashells in the form of seashell aggregates and seashell powder. The physical, mechanical, and durability properties of seashell concrete are largely determined by the properties of the aggregates and powders that make up the shell. The properties of seashells from the available literature have been presented in Table 1.
Table 1. Physical properties of seashell waste as aggregate.
| Seashell Type | Literature | Size (mm) | Fineness Modulus | Specific Gravity | Water Absorption (%) |
|---|---|---|---|---|---|
| Oyster | Yang et al. [1] | <5 | 2.80 | 2.48 | 2.90 |
| Oyster | Kuo et al. [2] | <4.75 | 2.00 | 2.10 | 7.70 |
| Oyster | Islam et al. [3] | <2 | 2.27 | 2.29 | - |
| Oyster | Eo and Yi [4] | <5 | 1.85 | 2.59 | 1.61 |
| 25 | 7.68 | 2.67 | 0.40 | ||
| Oyster | Chen et al. [5] | <5 | 3.66 | - | 6.84 |
| Oyster | Chen et al. [6] | <5 | 3.72 | - | 8.87 |
| Scallop | Cuadrado-Rica et al. [7] | <5 | 4.40 | 2.64 | 3.65 |
| Mussel | Martínez-García et al. [8] | 0–1 | 1.90 | 2.73 | 4.12 |
| 1–4 | 4.64 | 2.65 | 2.56 | ||
| 4–16 | 5.38 | 2.62 | 2.17 | ||
| Cockle | Khankhaje et al. [9] | 4.75–6.3 | - | 2.64 | 2.50 |
| 6.3–9.5 | - | 2.09 | 1.80 |
The specific gravity of any material is the ratio of the density of the particular material to that of water. In general, crushed seashells were used as fine aggregate with sizes of less than 5 mm. On the other hand, when they were used as coarse aggregate, they were processed with a maximum size of between 16 and 25 mm. However, when incorporated in pervious concrete, Martínez-García et al. [8] and Khankhaje et al. [9] claimed that aggregates between 4 and 9.5 mm in size can also be used as coarse aggregate. The specific gravity of coarse and fine seashell aggregate varies in the range of 2.09–2.67 and 2.10–2.73, respectively. Khankhaje et al. [9] displayed the lowest specific gravity value of 2.09 for coarse aggregate, while Kuo et al. [2] showed the same value of specific gravity for fine aggregate. The highest value of specific gravity for coarse aggregate was reported by Eo and Yi [4] to be 2.67, while Martínez-García et al. [8] displayed the highest specific gravity of 2.73 for fine aggregate. The specific gravity of seashell aggregates is usually lower than that of natural aggregates. The researchers found through testing that the specific gravity of natural coarse and fine aggregates varied in the range of 2.51–2.87 and 2.58–2.83, respectively. Although some of the seashells were outside the ACI limits for normal weight aggregates used in concrete (2.30–2.90), such as some oyster shells and cockle shells, the specific gravity of all seashells was above the ACI recommendations for light aggregates.
A significant variation in the water absorption of seashell aggregates was observed, depending on the presence of an irregular surface and number of internal pores [10], as seen in Table 1 Under normal circumstances, the water absorption of normal aggregates is less than 2% [11], and the maximum water absorption recommended in ACI cannot exceed 8%. Studies showed that the water absorption of coarse aggregate is lower than that of fine aggregate. They did not vary much, usually between 1.88 and 8.87%. But in some studies, the authors gave different results. Eo and Yi [4] found that oyster shell aggregates up to 25 mm had a water absorption of 0.4% and Falade [12] (not listed in Table 1) found that the water absorption of periwinkle shell aggregate was up to 12.99%. The water absorption of aggregates has an influence on the workability and consistency of concrete or mortar. Therefore, it is necessary to specify the amount of water absorption of seashell aggregates required for effective mix design.
In some past studies, waste seashells were also ground into powder to be a replacement for cement. The results showed that the specific gravity of seashell powder was generally lower than that of OPC (3.10), and the particle size depended on the temperature of the calcination and grinding processes. Lertwattanaruk et al. [13] found the specific gravity of clam shell, mussel shell, oyster shell, and cockle shell powder to be 2.71, 2.86, 2.65, and 2.82, respectively. By grinding oyster shells in wet and dry methods, Zhong et al. [14] obtained different median sizes with D50 of 1.61 and 58.53 μm, respectively. Ez-Zaki et al. [15] achieved powder of 6.27 and 10.22 μm by milling the same seashell type. From the study by Lertwattanaruk et al. [13], it can be found that the average particle sizes of Portland cement and the clam, mussel, oyster, and cockle shells were 22.82, 20.80, 29.87, 13.93, and 13.56 μm, respectively, which corresponded to a specific surface area of 3376, 8279, 6186, 14,280, and 8299 cm2/g, respectively. Compared to Portland cement, seashell powder has a greater specific surface area after processing, making it more reactive for the cementitious material to react with other substances to form a binder with appreciable strength.
2. Chemical Composition
The chemical composition of seashells varies depending on the type of shells and where they were collected. Most researchers calcined shells to study their chemical composition. Table 2 lists the chemical composition of the raw shells and the shells after calcination. It is obvious that there is no significant difference in the original chemical composition of oyster shells collected from rivers and the sea, except that the river oyster is slightly higher in calcium carbonate content. The data measured by Abinaya and Venkatesh [16] confirmed this regularity. There is no obvious difference in the chemical composition of different types of shells, all of which are composed of calcium carbonate and a small number of other oxides, and the calcium carbonate content of most shells is more than 95%. Apparently, the shells after calcination contained higher calcium oxide, which suggests that seashells could be an inert material in concrete and mortar, similar to limestone.
Table 2. Chemical composition of seashells (%).
| Seashell Type | Literature | CaCO3/CaO | SiO2 | Al2O3 | MgO | Fe2O3 | Na2O | K2O | SO3 | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw shells | |||||||||||
| Seashell | Abinaya and Venkatesh [16] | 89.56 | 4.04 | 0.42 | 0.65 | - | 0.98 | - | 0.72 | 0.20 | - |
| River shell | 95.99 | 1.28 | 0.40 | 0.68 | - | 0.98 | - | 072 | 0.20 | - | |
| Oyster | Kong et al. [17] | 95.32 | 1.01 | 0.26 | 0.71 | 0.15 | 1.18 | - | 0.66 | - | - |
| Mussel | Figueroa et al. [18] | 96.9 | 1.30 | - | - | 0.50 | - | 0.40 | 0.30 | - | - |
| Cockle | Oh et al. [19] | 97.6 | 0.13 | 0.10 | 0.32 | 0.28 | 1.22 | 0.03 | 0.12 | - | - |
| After calcination | |||||||||||
| Oyster | Yang et al. [1] | 51.06 | 2.00 | 0.50 | 0.51 | 0.20 | 0.58 | 0.06 | 0.60 | 0.18 | 44.16 |
| Oyster | Jung et al. [20] | 53.81 | 0.40 | 0.22 | 0.70 | 0.04 | - | - | - | - | 44.87 |
| Scallop | Varhen et al. [21] | 53.70 | 0.10 | 0.10 | 0.18 | 0.03 | 0.50 | 0.01 | 0.32 | - | 44.4 |
| Mussel | Jung et al. [20] | 53.70 | 0.20 | 0.13 | 0.33 | 0.03 | - | - | - | - | 45.61 |
| Mussel | Felipe-Sese et al. [22] | 87.21 | 0.55 | 0.03 | 0.49 | 0.05 | 0.50 | 0.04 | - | 0.09 | - |
| Cockle | Olivia et al. [23] | 51.56 | 1.60 | 0.92 | 1.43 | - | 0.08 | 0.06 | - | - | 41.84 |
| Cockle | Olivia et al. [24] | 51.91 | 0.38 | 0.65 | - | 0.05 | - | - | - | - | - |
| Clam | Jung et al. [20] | 53.92 | 0.46 | 0.20 | 0.22 | 0.04 | - | - | - | - | 45.16 |
| Clam | Olivia et al. [24] | 67.70 | 0.39 | 0.28 | - | 0.02 | - | - | - | - | - |
| Periwinkle | Etuk et al. [25] | 55.53 | 26.26 | 8.79 | 0.40 | 4.82 | 0.25 | 0.20 | 0.18 | 0.05 | - |
| Periwinkle | Umoh and Ujene [26] | 52.10 | 27.20 | 6.42 | 0.82 | 4.64 | 0.26 | 0.25 | 0.26 | - | - |
| Snail | Zaid and Ghorpade [27] | 51.09 | 0.60 | 0.51 | 0.69 | 0.56 | 1.20 | 0.12 | 0.19 | 0.21 | 40.54 |
| Cardiidae | Soltanzadeh et al. [28] | 52.34 | 3.65 | 1.15 | 0.42 | 0.20 | 0.35 | 0.13 | 0.47 | - | 41.25 |
It is reported that in order to reduce impurities, organic matter, and salt content, especially chloride ions, seashells need to be washed before reusing [8]. Chloride ions and sulfates in seashells prevent the effective bonding of aggregates to cement matrix, thereby affecting the setting properties and ultimate strength of concrete. The percentages of organics and chloride ions in untreated seashell aggregates often exceed the maximum values allowed for conventional concrete [8][21][29]. The excessive chloride content in concrete could accelerate the corrosion of steel reinforcement, while excessive sulfate content could trigger the expansion of hardened concrete.
Differences in calcium oxide content in shells after calcination depend mainly on the type of shells, cleaning method, and the method or temperature of the calcining treatment. Felipe-Sese et al. [22] calcined at 1100 °C to obtain shells with a calcium oxide content as high as 87.21%. For the same type of seashell (mussel shell), Lertwattanaruk et al. [13] obtained only 53.58% calcium oxide in shells at a calcination temperature of 550 °C. Therefore, in general, all types of seashells have similar chemical compositions when similar calcination temperatures are employed.
3. Microstructure
According to the study by Martinez-Garcia et al. [8], the structure of mussel shells can be divided into three parts: the outer layer called periostracum, the middle layer called the prismatic layer, and the inner layer referred to as nacre [8][30][31]. Most of the other species of seashells were also made up of these three parts. The periostracum is unmineralized and consists mainly of proteins. Its morphological characteristics are shown in Figure 1a,b. The central and thicker layer (approximately 400 mm) has an array of parallel prisms with polygonal cross-sections and its main component is calcium carbonate, as shown in Figure 1c. The last layer, about 10 mm wide, consists of layers of aragonite parallel to the surface, as shown in Figure 1d,e.
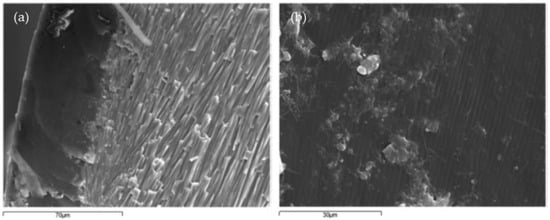
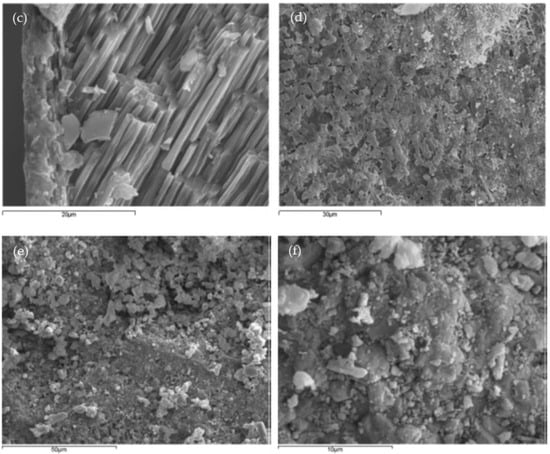
Figure 1. SEM analysis of mussel shell composition by Martínez-García et al. [32]: (a) periostracum (external layer)—prismatic structure layer; (b) periostracum layer front view; (c) prismatic structure layer; (d) nacre layer front view; (e) nacre layer; (f) limestone particle.
When seashells were ground into powder for cement replacement, Wang et al. [10] observed the surface morphology of seashell powder, limestone powder, and cement powder particles by SEM. From Figure 2a,b, it can be seen that the surface textures of limestone powder and cement powder are relatively smooth, while the surface of seashell powder particles has many tiny protrusions, irregularities, and walls. This explains why seashell powder has a larger surface area compared to limestone powder and cement powder. This positively affects the rheological properties, hydration development, and mechanical strength.

Figure 2. Particle surface morphology of (a) limestone powder; (b) Portland cement powder; (c) seashell powder [10].
Martinez Garcia et al. [8] found that when seashells were added to concrete in the form of aggregates, it reduced the bonding of seashell aggregates, which is especially enhanced with coarse aggregates. Cracks and pores were found in the interfacial transition zone with the periostracum in the scanning electron microscope (SEM) image (Figure 3), while the interfacial transition zone (ITZ) with the nacre layer showed a complete lack of bonding and very high porosity. Similarly, some researchers [2][33] also observed through SEM images that the use of seashells as aggregate resulted in poor cement paste-aggregate bonding and the creation of a large number of pores. In addition, Martínez-García et al. [33] observed a large number of cracks in the mortar at 28 days (Figure 4). The researchers found that the use of seashells as a mixing material in concrete did not produce unusual chemical reactions or new substances [1][4].
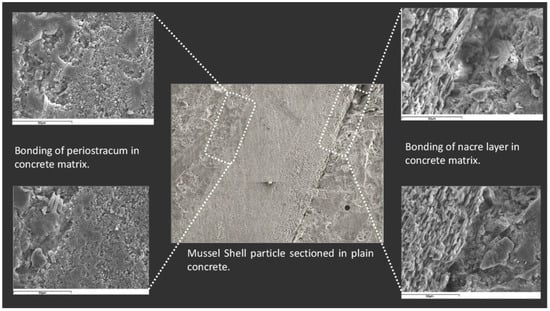
Figure 3. SEM observation of microstructure of seashell concrete [8].
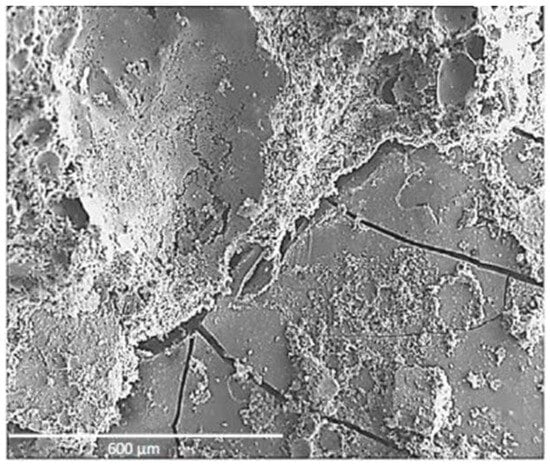
Figure 4. Cracks in seashell particles and cement paste [33].
As can be seen in Figure 5a, the most common hydration products in 100% ordinary Portland cement typically consist of C-S-H, Ca(OH)2 and ettringite. However, in blended cement mixtures containing seashell powder, ettringite-, and calcium carboaluminate-like phases appear near the seashell powder. And it increases with the increase in amount of shellac in the mixture [10].
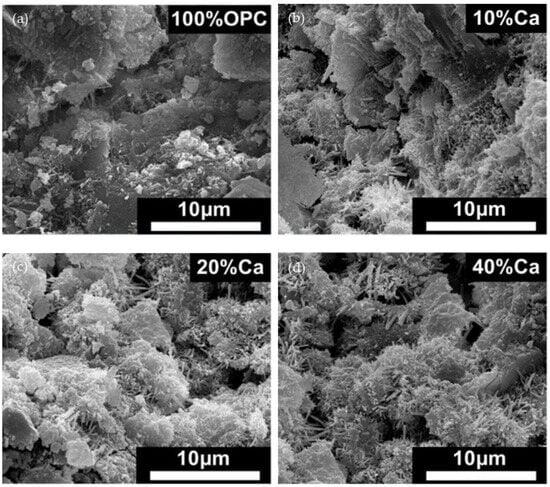
Figure 5. SEM of hydrated cement matrix produced in seashell cement mixtures [10]: (a) 100% OPC; (b) 10%Ca mixture; (c) 20%Ca mixture; (d) 40%Ca mixture.
References
- Yang, E.; Yi, S.; Leem, Y. Effect of oyster shell substituted for fine aggregate on concrete characteristics: Part I. Fundamental properties. Cem. Concr. Res. 2005, 35, 2175–2182.
- Kuo, W.; Wang, H.; Shu, C.; Su, D. Engineering properties of controlled low-strength materials containing waste oyster shells. Constr. Build. Mater. 2013, 46, 128–133.
- Islam, M.R.; Satoru, I.; Masayoshi, Y. Surface heat reduction of asphalt concrete by top filling with mortar prepared by oyster shell aggregate and ground granulated blast furnace slag and their strength in different curing condition. IDRE J. 2015, 296, 1–7.
- Eo, S.; Yi, S. Effect of oyster shell as an aggregate replacement on the characteristics of concrete. Mag. Concr. Res. 2015, 67, 833–842.
- Chen, D.; Zhang, P.; Pan, T.; Liao, Y.; Zhao, H. Evaluation of the eco-friendly crushed waste oyster shell mortars containing supplementary cementitious materials. J. Clean. Prod. 2019, 237, 117811.
- Chen, D.; Pan, T.; Yu, X.; Liao, Y.; Zhao, H. Properties of Hardened Mortars Containing Crushed Waste Oyster Shells. Environ. Eng. Sci. 2019, 36, 1079–1088.
- Cuadrado-Rica, H.; Sebaibi, N.; Boutouil, M.; Boudart, B. Properties of ordinary concretes incorporating crushed queen scallop shells. Mater. Struct. 2016, 49, 1805–1816.
- Martínez-García, C.; González-Fonteboa, B.; Martínez-Abella, F.; Carro-López, D. Performance of mussel shell as aggregate in plain concrete. Constr. Build. Mater. 2017, 139, 570–583.
- Khankhaje, E.; Salim, M.R.; Mirza, J.; Salmiati Hussin, M.W.; Khan, R.; Rafieizonooz, M. Properties of quiet pervious concrete containing oil palm kernel shell and cockleshell. Appl. Acoust. 2017, 122, 113–120.
- Wang, J.; Liu, E.; Li, L. Characterization on the recycling of waste seashells with Portland cement towards sustainable cementitious materials. J. Clean. Prod. 2019, 220, 235–252.
- Neville, A.M.; Brooks, J.J. Concrete Technology, 2nd ed.; Pearson Education: Essex, UK, 2010.
- Falade, F. An investigation of periwinkle shells as coarse aggregate in concrete. Build. Environ. 1995, 30, 573–577.
- Lertwattanaruk, P.; Makul, N.; Siripattarapravat, C. Utilization of ground waste seashells in cement mortars for masonry and plastering. J. Environ. Manag. 2012, 111, 133–141.
- Zhong, B.; Zhou, Q.; Chan, C.; Yu, Y. Structure and property characterization of oyster shell cementing material. Chin. J. Struct. Chem. 2012, 31, 85–92.
- Ez-zaki, H.; Diouri, A.; Kamali-Bernard, S.; Sassi, O. Composite cement mortars based on marine sediments and oyster shell powder. Mater. Constr. 2016, 66, e080.
- Abinaya, S.; Venkatesh, S.P. An effect on oyster shell powder’s mechanical properties in self compacting concrete. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 11785–11789.
- Kong, J.; Cong, G.; Ni, S.; Sun, J.; Guo, C.; Chen, M.; Quan, H. Recycling of waste oyster shell and recycled aggregate in the porous ecological concrete used for artificial reefs. Constr. Build. Mater. 2022, 323, 126447.
- Figueroa, J.; Fuentealba, M.; Ponce, R.; Zúñiga, R. Effects on the Compressive Strength and Thermal Conductivity of Mass Concrete by the Replacement of Fine Aggregate by Mussel Shell Particulate. IOP Conf. Ser. Earth Environ. Sci. 2020, 503, 012070.
- Oh, S.E.; Chung, S.Y.; Kim, K.; Han, S.H. Comparative analysis of the effects of waste shell aggregates on the material properties of cement mortars. Constr. Build. Mater. 2024, 412, 134887.
- Jung, J.; Shon, B.; Yoo, K.; Oh, K. Physicochemical characteristics of waste sea shells for acid gas cleaning absorbent. Korean J. Chem. Eng. 2000, 17, 585–592.
- Varhen, C.; Carrillo, S.; Ruiz, G. Experimental investigation of Peruvian scallop used as fine aggregate in concrete. Constr. Build. Mater. 2017, 136, 533–540.
- Felipe-Sese, M.; Eliche-Quesada, D.; Corpas-Iglesias, F.A. The use of solid residues derived from different industrial activities to obtain calcium silicates for use as insulating construction materials. Ceram. Int. 2011, 37, 3019–3028.
- Olivia, M.; Mifshella, A.A.; Darmayanti, L. Mechanical properties of seashell concrete. Proc. Eng. 2015, 125, 760–764.
- Olivia, M.; Oktaviani, R.; Ismeddiyanto. Properties of concrete containing ground waste cockle and clam seashells. Proc. Eng. 2017, 171, 658–663.
- Etuk, B.R.; Etuk, I.F.; Asuquo, L.O. Feasibility of using sea shells ash as admixtures for concrete. J. Environ. Sci. Eng. 2012, A1, 121–127.
- Umoh, A.A.; Ujene, A.O. Improving the strength performance of high volume periwinkle shell ash blended cement concrete with sodium nitrate as accelerator. J. Civ. Eng. Sci. Technol. 2015, 6, 18–22.
- Zaid, S.T.; Ghorpade, V.G. Experimental investigation of snail shell ash (SSA) as partial repalacement of ordinary portland cement in concrete. Int. J. Eng. Res. Technol. 2014, 3, 1049–1053.
- Soltanzadeh, F.; Emam-Jomeh, M.; Edalat-Behbahani, A.; Soltan-Zadeh, Z. Development and characterization of blended cements containing seashell powder. Constr. Build. Mater. 2018, 161, 292–304.
- Nguyen, D.H.; Boutouil, M.; Sebaibi, N.; Baraud, F.; Leleyter, L. Durability of pervious concrete using crushed seashells. Constr. Build. Mater. 2017, 135, 137–150.
- Martínez-García, C.; González-Fonteboa, B.; Carro-López, D.; Martínez-Abella, F. Effects of mussel shell aggregates on hygric behavior of air lime mortar at different ages. Constr. Build. Mater. 2020, 252, 119113.
- Yao, Z.; Xia, M.; Li, H.; Chen, T.; Ye, Y.; Zheng, H. Bivalve shell: Not an abundant useless waste but a functional and versatile biomaterial. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2502–2530.
- Martínez-García, C.; González-Fonteboa, B.; Carro-López, D.; Martínez-Abella, F. Impact of mussel shell aggregates on air lime mortars. Pore structure and carbonation. J. Clean. Prod. 2019, 215, 650–668.
- Martínez-García, C.; González-Fonteboa, B.; Carro-López, D.; Martínez-Abella, F. Design and properties of cement coating with mussel shell fine aggregate. Constr. Build. Mater. 2019, 215, 494–507.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.2K
Revisions:
2 times
(View History)
Update Date:
21 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





