Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ewa Oledzka | -- | 4274 | 2024-03-19 17:46:51 | | | |
| 2 | Catherine Yang | Meta information modification | 4274 | 2024-03-20 01:39:15 | | | | |
| 3 | Catherine Yang | Meta information modification | 4274 | 2024-03-22 02:36:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Oledzka, E. Chemical and Biological Properties of Xanthohumol. Encyclopedia. Available online: https://encyclopedia.pub/entry/56409 (accessed on 07 February 2026).
Oledzka E. Chemical and Biological Properties of Xanthohumol. Encyclopedia. Available at: https://encyclopedia.pub/entry/56409. Accessed February 07, 2026.
Oledzka, Ewa. "Chemical and Biological Properties of Xanthohumol" Encyclopedia, https://encyclopedia.pub/entry/56409 (accessed February 07, 2026).
Oledzka, E. (2024, March 19). Chemical and Biological Properties of Xanthohumol. In Encyclopedia. https://encyclopedia.pub/entry/56409
Oledzka, Ewa. "Chemical and Biological Properties of Xanthohumol." Encyclopedia. Web. 19 March, 2024.
Copy Citation
Xanthohumol (Xn), a prenylated chalcone found in Hop (Humulus lupulus L.), has been shown to have potent anti-aging, diabetes, inflammation, microbial infection, and cancer properties.
xanthohumol
Humulus lupulus
chalcone
1. Introduction
Phytochemicals present in plants, herbs, and spices have recently shown promise not just for their antibacterial and antiviral activities, but also for the treatment of cancer and chemoprevention [1]. Hops are gaining importance in this context. Hops (Humulus lupulus L., family Cannabaceae), or more accurately, the female plant’s flowers (cones), are widely used in the brewing industry to provide a characteristic flavour and bitterness to beer. Depicting the traditional use of hop, it has been used for the preservation and flavouring of alcohol-based beverages since 200 A.D., with therapeutic treatments beginning as early as the ninth century [2]. When it was discovered in 79 A.D., it was popular as a vegetable, but it was later utilised as a dye and a culinary flavour ingredient [3][4]. It was also used in the production of coarse textiles and paper. Hop plants were traditionally used as a sedative for treating sleeplessness and restlessness, as well as for relieving ear discomfort, toothache, and loss of appetite [2]. Currently, hop products are often used as dietary supplements because of their hypnotic and anxiolytic characteristics, and they are utilized in the treatment of postmenopausal symptoms in women. Furthermore, they are broadly applied in the cosmetic and pharmaceutical industries, owing to their antibacterial and antiviral properties [5][6]. Hop versatility lies in the fact that it possesses a wide range of physiologically active compounds [7][8]. Terpenes, bitter acids, and chalcones are the three main structural types of chemical substances identified in hop mature cones. Hops are also rich in flavonol glycosides (kaempferol, quercetin, quercitrin, rutin) and catechins (catechin gallate, epicatechin gallate) [3][9]. Hundreds of terpenoid components were found in the volatile oil (0.3–1.0% of hop strobile weight): principally caryophyllene, farnesene, and humulene (sesquiterpenes) and myrcene (monoterpene) [10]. Bitter acids (5–20% of hop strobile weight) are phloroglucinol derivatives categorised as α-acids and β-acids. Hops contain bitter acids in a complex combination with varying compositions and concentrations [3]. Aside from the volatile oil and bitter acids, many prenylflavonoids have been identified in hop cones [9]. The most significant chemical is chalcone xanthohumol (Xn, Figure 1) (up to 1% in dried hop cones), which can be converted to prenylfavanone isoxanthohumol (IX) at a higher pH value and under thermal treatment [9]. As a result, IX is the primary prenylflavonoid found in beer. Other chalcones isomerize to corresponding flavanones at concentrations 10–100-times lower than Xn. A chalcone known as xanthogalenol (XG) has only been found in a few hop varieties. Desmethylxanthohumol (DMX), also known as 2′,4′,6′,4-tetrahydroxy-3′-C-prenylchalcone, has been determined to be the precursor of most flavonoids found in hops. Through chemical isomerization, the major estrogen of hops is produced, identified as the 1:1 racemate (±)-8-prenylnaringenin (8-PN), along with racemic 6-prenylnaringenin (6-PN). In humans, 8-PN has been shown to be derived from IX via activation by the gut microbiota or liver cytochrome P450 enzymes. Thus, estrogen-inactive Xn possesses the estrogenic potential of being converted to IX and then to 8-PN [3][11][12][13].
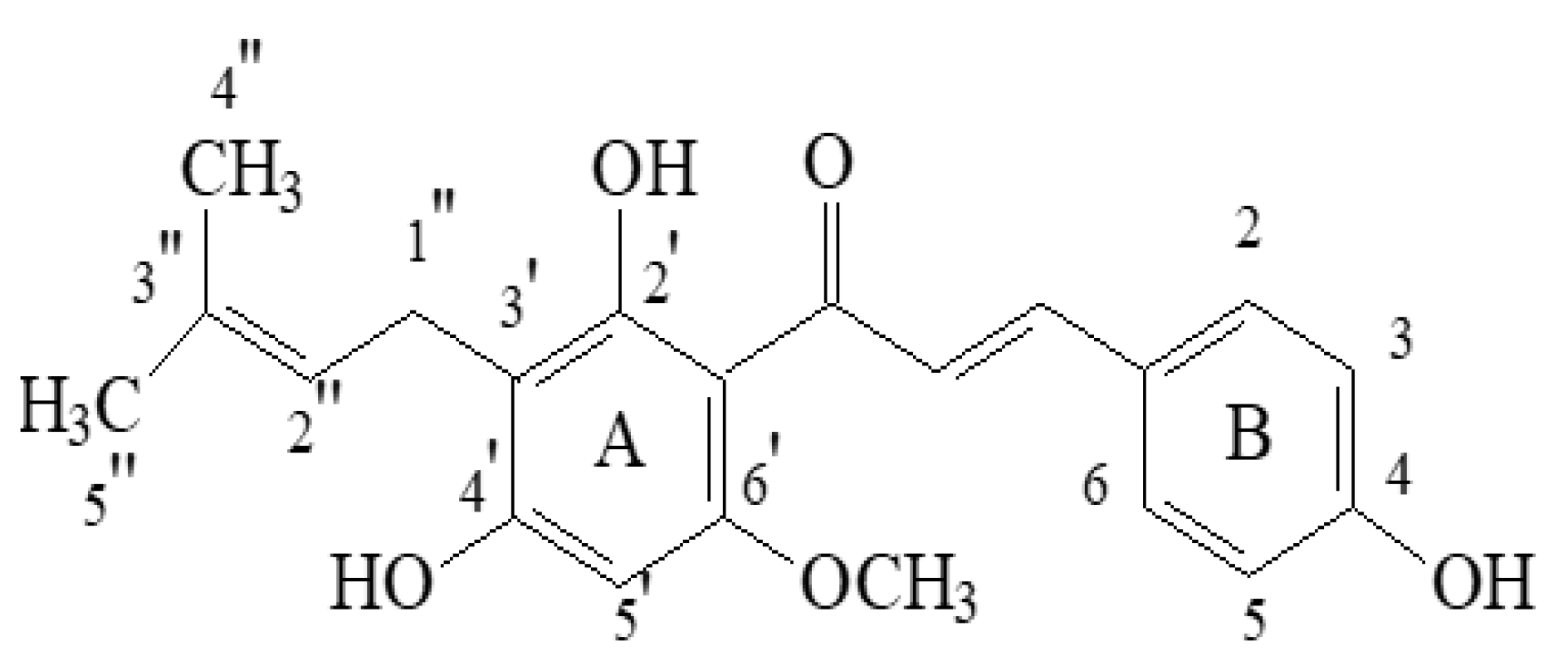
Figure 1. Chemical structure of xanthohumol (Xn).
2. Structure and Chemistry of Xn
Xn (1-(2,4-dihydroxy-6-methoxy-3-[3-methylbut-2-en-1-yl] phenyl)-3-(4 hydroxyphenyl) prop-2-en-1-one) is usually generated as solid yellow crystals with a melting point of 172 °C (Pubchem CID 639665) [6]. It is insoluble in water and in petroleum ether, and it can be crystallized in 50% alcohol, 50% acetone, acetic acid, chloroform, benzene and toluene. It dissolves in strong alkali and in sulphuric acid, and it shows no optical activity. The mean value of its elementary analyses is 72.0% C and 6.18% H (calculated for C2lH22O5: 71.2% C and 6.21% H) [14].
Xn’s structure comprises of flavonoids chain with aromatic rings A and B arranged in the trans-position and substituted with hydroxyl and methoxy groups, one unsaturated double bond α, β, and a prenyl unit (Figure 1). Xn has biological activity due to the existence of α,β-unsaturated ketone group. This compound’s lipophilicity is increased by substituting the A ring with a prenyl unit and the -OCH3 group, resulting in a high affinity for membranes in biological systems [4][6][15].
The spectroscopic and spectrophotometric characteristics of this compound are as follows:
1H NMR (DMSO-d6): 1.61 (3H, s, H-5″), 1.70 (3H, s, H-4″), 3.13 (2H, d, H-1″), 3.87 (3H, s, C6′O-CH3), 5.14 (1H, m, H-2″), 6.08 (1H, s, H-5′), 6.84 (1H, m, H-3 and H-5), 7.58 (1H, m, H-2 and H-6), 7.67 (1H, d, J = 15.6 Hz, H-β), 7.77 (1H, d, H-α), and 14.69 (C2′-OH).
13C NMR (DMSO-d6): 17.7 (C-4″), 21.1 (C-1″), 25.5 (C-5″), 55.8 (C6′O-CH3), 91.0 (C-5′), 104.6 (C-1′), 107.4 (C-3′), 116.0 (C-3 and C-5), 123.1 (C-2″), 123.8 (C-α), 126.1 (C-1), 130.0 (C-3″), 130.5 (C-2 and C-6), 142.6 (C-β), 160.0 (C-4), 160.6 (C-6′), 162.4 (C-4′), 164.7 (C-2′), and 191.7 (C=O). UV (MeOH): λmax = 368.1 nm [16].
FTIR (cm−1): 974 trans -CH=CH-, 1027 substituted benzene, 1145 ν(C-C) aromatic ring, ν(-OH), 1228 δν(-OH), 1292 ν(C-C) aromatic ring, 1346 δ(-OH), 1470 δ(C-C), 1545 trans CH=CH-, 1606 νC=O, 2854 νs(-OCH3), 2967 νas (-OCH3), 3189 ν(-OH), ν(C-H) in aromatic ring [17].
It is also important to highlight the most recent literature investigation, in which scientists examined into the storage stability and degradation mechanism of Xn [18]. The authors discovered that this molecule degraded rapidly when exposed to high temperatures and light. Furthermore, at high temperatures, Xn was susceptible to isomerization, hydration, and ortho-cyclization processes, resulting in the creation of a variety of degradation products. According to the data, Xn degraded according to a first-order kinetic model.
3. Biological Properties of Xn
Xn has a broad variety of pharmacological activities against diabetes, inflammation, viral infection, cardiovascular disease, and cancer [19]. Xn and its related flavone, IX, reduce adipogenesis by blocking preadipocyte differentiation, decreasing lipogenic proteins, and enabling mature adipocytes to undergo mitochondrial apoptosis [20][21]. Furthermore, Xn treatment raised the AMP-activated protein kinase (AMPK) signalling pathway, inhibiting lipogenesis in a type 2 diabetes mellitus (T2DM) mouse model. Its consumption reduced body weight gain and enhanced plasma lipid profile, with substantial improvements in insulin resistance and glucose tolerance. By modulating glucose and lipid pathways, an Xn-enriched diet may be able to alleviate diabetic-associated metabolic abnormalities [22]. The modulating action of Xn on the farnesoid X receptor (FXR) in vitro and in vivo has also been examined by Nozawa et al. [23]. The authors proved that in the transient transfection test, Xn boosted the activity of the human bile salt export pump (BSEP) promoter-driven luciferase in a dose-dependent manner. Xn-fed KK-Ay mice had decreased levels of plasma glucose, plasma, and liver triglyceride. They also had lower water consumption, lower white adipose tissue weights, and higher plasma adiponectin levels, showing that Xn prevented diabetes in KK-Ay mice. Xn also addresses skin aging and pigmentation while promoting photoprotection, thus making it an effective component for the cosmetics industry. In the light of this, the effects of Xn on melanogenesis in MNT-1 human melanoma cells and normal human melanocytes from darkly pigmented skin (HEM-DP) were studied in the publication [24]. Melanosome degradation was also investigated in human keratinocytes (HaCaT). The researchers discovered that Xn reduced the production of melanin in MNT-1 cells while increasing intracellular tyrosinase activity without changing Reactive Oxygen Species (ROS) levels. Xn inhibited cellular tyrosinase activity in HEM-DP cells without affecting synthesis of melanin. Xn also limited melanosome export by reducing dendrite number and length. Further tests in HaCaT cells demonstrated that Xn caused melanosome degradation without cytotoxicity [24]. The authors of the other study [25] explored Xn as an anti-aging substance by favourably regulating the extracellular matrix. They examined how Xn influenced the activities of elastase and matrix metalloproteinases (MMPs, MMPs 1, 2, and 9), as well as the expression of collagen, elastin, and fibrillins in dermal fibroblasts. It was discovered that Xn inhibited elastase and MMP-9 activities at low concentrations while activating MMP-1 and MMP-2 at higher concentrations. The research results were similar to those of ascorbic acid [25]. It is also worth noting the work of Kang and colleagues, who investigated the effects of Xn and related compounds on the production of interleukin (IL)-12, the most important factor driving T helper 1 immune response [26]. Xn had the greatest inhibitory effect on IL-12 production in macrophages stimulated by lipopolysaccharide (LPS) or LPS/interferon-γ. Furthermore, it was determined whether Xn reduced skin inflammation. Chronic allergic contact dermatitis, an experimental model for psoriasis, was used to assess the anti-inflammatory effects of Xn in vivo. It was established that Xn treatment reduced the degree of ear thickening caused by oxazolone [26]. Furthermore, five strains, Propionibacterium acnes, Staphylococcus epidermidis, Staphylococcus aureus, Kocuria rhizophila, and Staphylococcus pyogenes, were chosen for testing the biological activities of Xn extract on acne vulgaris. Xn demonstrated strong inhibitory activity against all strains. Furthermore, it showed moderate to strong anticollagenase inhibitory activity. Antioxidant capacity was also assessed using seven different methods based on various ROS. Xn had the highest activity in both total oxygen radical absorbance capacity and singlet oxygen absorbance capacity [27].
The modern cosmetics industry provides its customers with a wide range of products, many of which are holistic in nature. This is achieved by combining synergistic active ingredients in novel ways or by utilising the multifunctionality of a single component. Xn should be considered a substance that meets both of the above criteria because, in addition to the anti-aging and anti-pigmentation properties mentioned above, as well as photoprotection, it also possesses antibacterial, antiviral, antimalarial, and antifungal properties.
3.1. Antibacterial Activity
Previously, the ability of Xn to inhibit the growth of Gram-positive Staphylococcus aureus, a pathogen commonly found in pneumonia and sepsis, was compared to antibiotic activity against Escherichia coli. This compound was discovered to inhibit Escherichia coli proliferation while being a potent inhibitor of Staphylococcus aureus with a minimal inhibitory concentration (MIC) of 17.7 μM [28]. In addition, Xn was tested against three strains of Streptococcus and its activity was compared to that of some essential oils commonly found in anticaries mouth washes [29]. Xn demonstrated antimicrobial activity against Streptococcus mutans, Streptococcus salivarius, and Streptococcus sanguis in a disc diffusion assay. Xn (140 nmol) produced similar zones of inhibition against all three strains at a dose of 50 μg per disc as thymol (333 nmol). Furthermore, the MIC of Xn from hop extracts ranged from 10 to 50 μg/mL for Clostridium perfringens strains and from 15 to 60 μg/mL for Bacteroides fragilis strains, indicating that this molecule is more effective against these two strains than α- and β-acids [29]. Based on Xn’s potent antibacterial effects on Clostridium difficile, as well as its low toxicity and favourable pharmacokinetics, this molecule was being considered as a promising therapeutic agent for the treatment and prevention of Clostridium difficile infections. The data showed that the MIC and minimum bactericidal concentrations of Xn ranged from 32 to 107 μg/mL and from 40 to 107 μg/mL, respectively [29][30].
Latest in vivo studies on the bioavailability of Xn in rats revealed that this chalcone and its metabolites are primarily excreted in faeces [31]. As a result, it was interesting to evaluate if Xn could influence the intestinal microbiota. Hanske et al. used polymerase chain reaction denaturing gradient gel electrophoresis (PCR-DGGE) to investigate the composition of rat intestinal microbiota. In comparison to untreated controls, daily Xn applications to male and female Sprague Dawley (SD) rats for 4 weeks had no effect on the diversity of the faecal microbial community [32]. Added to that, the purified extracts, which comprise α- and β-acids, as well as Xn, were examined in vivo for their inhibitory action against Clostridioides difficile, a prominent pathogen responsible for nosocomial gastrointestinal infections in humans [33][34]. The researchers developed a rat model for this pathogen attacking the peroral intestinal tract. Both Xn and hop acids were discovered to have substantial antibacterial activity. The Xn application, in particular, showed antibacterial activity as well as a reduction in local inflammatory symptoms in the large intestine [33].
3.2. Antifungal Activity
There have been only a few investigations on the antifungal activity of Xn. However, it was discovered that this compound was a potent antifungal agent against five human pathogenic fungi, namely Trichophyton mentagrophytes, Trichophyton rubrum, Candida albicans, Fusarium oxysporum, and Mucor rouxianus [28]. Xn inhibited the growth of the dermatophytic fungi Trichophyton mentagrophytes and Trichophyton rubrum more effectively than the positive control griseofulvin (MIC: 6.25 μg/mL). Xn also inhibited Mucor rouxianus (MIC = 50 μg/mL). Candida albicans and the problematic human pathogen Fusarium oxysporum, on the other hand, were unresponsive to Xn (MIC > 200 μg/mL).
3.3. Antiviral Activity
In 2004, Buckwold and coworkers tested Xn against a number of DNA and RNA viruses in vitro [35]. As RNA viruses, bovine viral diarrhoea virus (BVDV), a surrogate model for hepatitis C virus, and human rhinovirus (HRV) were included. In addition, the DNA herpesviruses cytomegalovirus (CMV) and herpes simplex virus types 1 and 2 (HSV-1 and -2) were used to test antiviral activity of Xn. The inhibitory effects of this chalcone against BVDV (NADL strain in MDBK cells), HRV (rhinovirus 14 strain in MRC-5 cells), HSV-1 (F strain in Vero cells), and HSV-2 (MS strain in Vero cells) were assessed using cell-based assays designed to assess inhibition of cytopathic effects (CPE). In MRC-5 cells, CMV (strain AD169) was tested in a plaque reduction assay. The authors discovered that Xn potently inhibited the growth of BVDV, CMV, HSV-1, and HSV-2. The half-maximal inhibitory concentrations (IC50) values of Xn to inhibit viral replication ranged from 1.5 to 2.7 μg/mL. Xn, on the other hand, exhibited no antiviral activity against HRV. An Xn-enriched extract was also tested in the same study. The Xn in the extract appeared to account for almost all of the extract’s antiviral activity, as the therapeutic indices TI (TC50/IC50) of this compound against BVDV, HSV-1, and HSV-2 were comparable to those of the Xn-enriched extract [35]. Additionally, Wang and coworkers investigated the ability of Xn to inhibit various steps required for HIV-1 replication [36]. In C8166 lymphocytes infected with HIV-1IIIB, Xn was found to inhibit HIV-1-induced CPE, as well as viral p24 antigen production and reverse transcriptase activity as measures of active retroviral replication, with IC50 values of 2.3, 3.6, and 1.4 μM, respectively. With an IC50 value of 58.5 μM, this chalcone also inhibited HIV-1 replication in peripheral blood mononuclear cells. In this study, Xn had no effect on recombinant HIV-1 reverse transcriptase activity or HIV-1 entry into cells [36]. It is also worth noting that Liu et al. examined the therapeutic effect of Xn against highly pathogenic porcine reproductive and respiratory syndrome viruses (PRRSV) [37]. The authors discovered that Xn had a low IC50 value for inhibiting PRRSV infection in porcine primary alveolar macrophages (PAMs). Furthermore, it reduced the expression of interleukin (IL)-1, IL-6, IL-8, and tumour necrosis factor α in PRRSV-infected or lipopolysaccharide-treated PAMs. Xn effectively alleviated clinical signs, lung pathology, and inflammatory responses in pig lung tissues induced by highly pathogenic PRRSV infection, according to animal challenge experiments [37]
The pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in substantial worldwide morbidity and mortality, with significant financial and social repercussions [38]. Coronavirus’s main protease (Mpro) is crucial for viral replication and transcription, making it an appealing drug target for antiviral drug development. Lin et al. identified Xn to be a potent pan-inhibitor for various coronaviruses by targeting Mpro, including β-coronavirus SARS-CoV-2 (IC50 value of 1.53 μM) and α-coronavirus PEDV (IC50 value of 7.51 μM). In enzymatic assays, Xn inhibited Mpro activities, while pretreatment with this molecule inhibited the SARS-CoV-2 and PEDV replication in Vero-E6 cells [38]. Furthermore, Dabrowski et al. investigated the impact of Xn on the inflammatory response and clinical outcome of COVID-19 patients [39]. As a result, adult patients with acute respiratory failure (PaO2/FiO2 less than 150) were examined. In this study, patients were randomly assigned into two groups: Xn—patients who received adjuvant treatment with Xn at a daily dose of 4.5 mg/kg body weight for 7 days, and C—controls. Observations were carried out at four points: immediately after admission to the intensive care unit (ICU), as well as on the third, fifth, and seventh day of treatment. The inflammatory response was measured using plasma IL-6 levels, neutrophil-to-lymphocyte ratios (NLR), platelet-to-lymphocyte ratios (PLR), C-reactive protein (CRP), and D-dimer levels. The death rate was calculated 28 days after ICU admission. The researchers discovered that 72 patients were able to participate in the study, and 50 were included in the final evaluation. The Xn group had a lower mortality rate and a shorter clinical course than the control group (20% vs. 48%, p < 0.05, and 9 ± 3 days vs. 22 ± 8 days, p < 0.001). Furthermore, Xn treatment significantly reduced plasma IL-6 concentrations (p < 0.01), D-dimer levels (p < 0.05), and NLR (p < 0.01) compared to standard treatment [39].
3.4. Antimalarial Activity
Malaria, caused by protozoan parasites of the genus Plasmodium, constitutes one of the most common infectious diseases and an important problem for public health. Only four Plasmodium parasite species have the ability to infect humans. Plasmodium falciparum and Plasmodium vivax, however, cause the most severe forms of the disease [40]. Xn is one of the structural classes for which antiplasmodial/antimalarial activity has been reported with great interest in the scientific community in the recent times [28]. Herath et al. have previously reported the microbial transformation of Xn with Cunninghamella echinulata NRRL 3655 and the evaluation of these products for cytotoxicity towards mammalian cell lines as well as possible antimicrobial and antimalarial properties. The authors demonstrated that Xn and its microbial transformation products were antimalarial against Plasmodium falciparium D6 (chloroquine sensitive) and W2 (chloroquine resistant) strains. This chalcone was active against D6 and W2 strains, with IC50 values of 3.3 and 4.1 μg/mL, respectively [41]. In addition, the in vitro antiplasmodial activity of Xn and seven natural or semi-synthetic derivatives against two different strains of Plasmodium falciparium (chloroquine-sensitive strain poW and the multiresistant clone Dd2 using a [3H]hypoxanthine-incorporation assay), as well as their interaction with GSH-dependent haemin degradation, were evaluated. Xn was the most active chalcone in this study, with IC50 values of 8.2 ± 0.3 μm (poW) and 24.0 ± 0.8 μm (Dd2).
3.5. Antiplatelet Activity
I would also like to briefly discuss Xn’s antiplatelet activity. It has previously been claimed that Xn is responsible for a decreased risk of cardiovascular disease by reducing the probability of platelet hyperreactivity during thrombosis [42]. As an instance, this chalcone considerably reduced ADP-induced blood platelet aggregation and fibrinogen receptor expression on platelet surfaces in C57BL/6J wild-type male mice [43]. Xn reduced platelet activation in C57/BL6 mice and SD rats by decreasing ROS accumulation and inhibiting the cellular damage factor mtDNA-induced DC-SIGN-dependent pathway, hence avoiding arterial and venous thrombosis without inducing bleeding [44]. Lee et al. found that Xn inhibited the phosphorylation of phospholipase C (PLC)γ2, p38 mitogen-activated protein kinase, extracellular antiregulated kinase 1/2, JNK1, and Akt, leading to decreased thromboxane A2 formation and Ca2+ mobilisation [30][45]. Nonetheless, Xin et al. discovered that Xn has antiplatelet and antithrombotic properties, which may reduce ROS accumulation by upregulating sirtuin1 (SIRT1) expression, followed by inhibition of mitochondrial dysfunction and a reduction in respiratory disorders as well mitochondrial hyperpolarization [44]. Another recent study found that this molecule could be beneficial in arrhythmias [46]. The effects of Xn (5–1000 nM) on Ca2+ signalling pathways were investigated in isolated rat ventricular myocytes incubated with Fluo-4AM using the perforated patch-clamp technique. The authors discovered that 5–50 nM Xn decreased the frequency of spontaneously occurring Ca2+ sparks and Ca2+ waves in control myocytes and cells subjected to Ca2+ overload. It also reduced the Ca2+ content of the Sarcoplasmic Reticulum (SR) and its rate of recirculation. Lastly, multiple investigations evaluating the potential of Xn in cancer treatment have discovered that it is effective in vitro and in vivo against a variety of cancer models: breast and cervical cancers, cholangiocarcinoma, glioblastoma, colon, colorectal, haematological, laryngeal, liver, ovarian, pancreatic, prostate, thyroid, and oesophageal cancers, melanoma, and oral squamous cell carcinoma have all been studied [1][4][6][46][47][48][49][50][51][52][53]. As a result, these findings will not be replicated in this research.
3.6. Safety of the Xn Use
Throughout various activity studies, the impact of Xn on normal cells was additionally examined. In normal cells, such as human lung fibroblast cells (MRC-5), primary human hepatocytes, oligodendroglia-derived cells (OLN-93), and human skin fibroblasts, Xn demonstrated very low or no toxicity [48]. These outcomes indicated that Xn was specifically targeting cancer cells; however, Xn may be a safe and effective agent. Similar results were obtained in vivo. Vanhoecke and coworkers presented the findings of a four-week safety study of Xn in mice. The daily administration of 23 mg/kg body weight (b.w.) showed no signs of toxicity in bone marrow, liver, exocrine pancreas, kidneys, muscles, thyroid, or ovaries. The data indicate that oral administration of Xn to laboratory mice does not affect major organ functions and opens the gate for further safety studies in humans [54]. Furthermore, Hussong et al. explored the sub-chronic toxicity of Xn in female SD rats at daily doses up to 1000 mg/kg j.w. for 4 weeks, which causes mild hepatotoxicity in animals, but has no effect on reproduction or the development of two generations of offspring when given at a daily dose of 100 mg/kg b.w. Furthermore, the authors found that the treatment of male rats prior to mating significantly (p = 0.027) increased the sex ratio of male to female offspring. Overall, lifelong treatment at a daily dose of 100 mg/kg b.w. in a two-generation study did not affect the development of SD rats [55].
3.7. Biotransformation, Pharmacokinetics and Clinical Applications of Xn
The available literature confirms the use of Xn, but there are several barriers between basic research and clinical practice [48]. The primary concern is its low bioavailability. As I mentioned above, Nookandeh and coworkers reported this occurrence during research on female SD rats, in which orally administered Xn was excreted within 48 h via faeces and urine [31]. Furthermore, Avula et al. investigated the resorption and metabolism of Xn in rat plasma, urine, and faeces following its oral or intravenous (i.v.) administration [56]. The authors found that plasma levels of Xn fell rapidly within 60 min after i.v. administration; no Xn was detected in plasma after its oral use. Moreover, Xn and its metabolites were excreted mainly in faeces within 24 h of administration. Meanwhile, Pang et al. determined that facilitated transport was not responsible for Xn uptake; rather, the accumulation in human colorectal adenocarcinoma (Caco-2) cells was presumably caused by specific binding to cytosolic proteins. This is mostly owing to its biotransformation in the gastrointestinal tract by hepatic enzymes and its accumulation in 70% on the apical side of Caco-2 cells. By binding to the cytosolic proteins, about 93% of intracellular Xn was localised in the cytosol. As a result, this agent failed to induce an effective therapeutic response at the intended site [57]. It is also worth noting that research was also conducted to determine the fundamental aspects of Xn absorption, distribution, and metabolism in male jugular vein-cannulated SD rats. As a result, the authors carried out a single-dose pharmacokinetics (PK) study at three oral dose levels and one i.v. dose level to assess bioavailability and dose-dependence of PK parameters. It was discovered that the dose-dependent bioavailability of Xn emphasises the importance of additional investigation into Xn metabolism in order to elucidate and optimise the potential health benefits of Xn and its metabolites [58]. Therefore, two years later, the same authors published the data collected in healthy men and women to determine basic PK parameters for Xn in order to establish dose–concentration relationships and predict dose–effect relationships in humans diagnosed with metabolic syndrome. According to the authors of this study, the Xn PK exhibited a distinct biphasic absorption pattern, with Xn and IX conjugates being the primary circulating metabolites following oral Xn administration in humans [59]. The next study attempted to determine whether, in healthy, normal-weight women, consuming a low dose of Xn, based on concentrations found in 250 mL beer consumed with a light breakfast, influences the LPS-dependent immune response of peripheral blood mononuclear cells (PBMCs) isolated following the intake of the hop compound [60]. It was proposed that acute consumption of low doses of Xn extract may suppress the LPS-dependent immune response of PBMCs in healthy women, and the beneficial effects of the hop compound were related to an inhibition of LPS binding to the cluster of differentiation 14 (CD14). At the end of this chapter, I would like to mention two more articles presenting the protocols for phase I and II triple-masked, placebo-controlled clinical trials on Xn microbiome and signature in healthy adults (the XmaS trial). The major goal of the first of them was to evaluate the clinical safety and tolerability of Xn in healthy and adult patients. The researchers also examined biomarkers reflecting inflammation, gut permeability, bile acid metabolism, and products of this compound metabolism in vivo, as well as the influence of Xn on the composition of gut microbial [61]. The performed preclinical studies have indicated that Xn has multiple therapeutic properties, involving modulating inflammatory pathways through the FXR agonists, inhibiting the nuclear factor-kappa B (NFκB) activation, regulating gut permeability, bile acid metabolism, and activating the nuclear factor erythroid 2 -related factor 2 (NRF2) to regulate antioxidant protein expression. The study demonstrated that Xn might impact the expression of numerous downstream genes in vivo, making it a possible Crohn’s disease (CD) treatment. Furthermore, it was discovered that Xn served as a prebiotic for intestinal microbiota, altering the gut microbiota and its bacterial metabolites [61]. Assuming in the second study, the same authors monitored the safety and tolerability of the same amount of Xn (24 mg daily) in adults with clinically active CD in a placebo-controlled phase II clinical trial, as well as the effect of Xn on inflammatory biomarkers, platelet function, CD clinical activity, and stool microbial composition [62]. The investigators have noticed some limitations since the trial began, but the findings of this study will add to the evidence base for the use of Xn for this disease patient population. This was owing, among other things, to the demographic distribution of Portland, which limited the generalizability of trial results; the fact that the population in the Portland area consumed a high volume of several types of compounds prohibited in this study, as well as the rarity of CD in the general population. However, this phase II trial yielded a considerable amount of data for a straight comparison with an otherwise healthy group in the phase I trial [62].
References
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567.
- Moir, M. Hops—A Millennium Review. J. Am. Soc. Brew. Chem. 2000, 58, 131–146.
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396.
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for cancer prevention and treatment. IUBMB Life 2021, 73, 1016–1044.
- Stompor, M.; Żarowska, B. Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues. Molecules 2016, 21, 608.
- Harish, V.; Haque, E.; Śmiech, M.; Taniguchi, H.; Jamieson, S.; Tewari, D.; Bishayee, A. Xanthohumol for Human Malignancies: Chemistry, Pharmacokinetics and Molecular Targets. Int. J. Mol. Sci. 2021, 22, 4478.
- Olšovská, J.; Boštíková, V.; Dušek, M.; Jandovská, V.; Bogdanová, K.; Čermák, P.; Boštík, P.; Mikyska, A.; Kolář, M. Humulus lupulus L. (Hops)—A valuable source of compounds with bioactive effects for future therapies. Mil. Med. Sci. Lett. 2016, 85, 19–30.
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.S.; Lorenzo, J.M. Humulus lupulus L. as a Natural Source of Functional Biomolecules. Appl. Sci. 2020, 10, 5074.
- Stevens, J.F.; Miranda, C.L.; Buhler, D.R.; Deinzer, M.L. Chemistry and Biology of Hop Flavonoids. J. Am. Soc. Brew. Chem. 1998, 56, 136–145.
- Eri, S.; Khoo, B.K.; Lech, J.; Hartman, T.G. Direct Thermal Desorption−Gas Chromatography and Gas Chromatography−Mass Spectrometry Profiling of Hop (Humulus lupulus L.) Essential Oils in Support of Varietal Characterization. J. Agric. Food Chem. 2000, 48, 1140–1149.
- Stevens, J.F.; Taylor, A.W.; Nickerson, G.B.; Ivancic, M.; Henning, J.; Haunold, A.; Deinzer, M.L. Prenylflavonoid variation in Humulus lupulus: Distribution and taxonomic significance of xanthogalenol and 4′-O-methylxanthohumol. Phytochemistry 2000, 53, 759–775.
- Guo, J.; Nikolic, D.; Chadwick, L.R.; Pauli, G.F.; van Breemen, R.B. Identification of human hepatic cytochrome p450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.). Drug Metab. Dispos. 2006, 34, 1152–1159.
- Chadwick, L.; Pauli, G.; Farnsworth, N. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine 2006, 13, 119–131.
- Verzele, M.; Stockx, J.; Fontijn, F.; Anteunis, M. Xanthohumol, a New Natural Chalkone. Bull. Des Sociétés Chim. Belg. 1957, 66, 452–475.
- Pafiti, K.S.; Vlasiou, M.C. Evaluation of xanthohumol as a potent drug from nature: Synthesis, isolation and anticancer activity. SCIREA J. Chem. 2019, 4, 1–19.
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Rychlicka, M.; Sordon, S.; Filip-Psurska, B.; Milczarek, M.; Wietrzyk, J.; Huszcza, E. Prenylated Flavonoids with Selective Toxicity against Human Cancers. Int. J. Mol. Sci. 2023, 24, 7408.
- Arczewska, M.; Kamiński, D.M.; Górecka, E.; Pociecha, D.; Rój, E.; Sławińska-Brych, A.; Gagoś, M. The molecular organization of prenylated flavonoid xanthohumol in DPPC multibilayers: X-ray diffraction and FTIR spectroscopic studies. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 213–222.
- Luo, J.; Pan, Q.; Chen, Y.; Huang, W.; Chen, Q.; Zhao, T.; Guo, Z.; Liu, Y.; Lu, B. Storage stability and degradation mechanism of xanthohumol in Humulus lupulus L. and beer. Food Chem. 2023, 437, 137778.
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754–779.
- Yang, J.-Y.; Della-Fera, M.A.; Rayalam, S.; Baile, C.A. Effect of xanthohumol and isoxanthohumol on 3T3-L1 cell apoptosis and adipogenesis. Apoptosis 2007, 12, 1953–1963.
- Kiyofuji, A.; Yui, K.; Takahashi, K.; Osada, K. Effects of Xanthohumol-Rich Hop Extract on the Differentiation of Preadipocytes. J. Oleo Sci. 2014, 63, 593–597.
- Costa, R.; Rodrigues, I.; Guardão, L.; Rocha-Rodrigues, S.; Silva, C.; Magalhães, J.; Ferreira-De-Almeida, M.; Negrão, R.; Soares, R. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J. Nutr. Biochem. 2017, 45, 39–47.
- Nozawa, H. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-Ay mice. Biochem. Biophys. Res. Commun. 2005, 336, 754–761.
- Goenka, S.; Simon, S.R. Depigmenting effect of Xanthohumol from hop extract in MNT-1 human melanoma cells and normal human melanocytes. Biochem. Biophys. Rep. 2021, 26, 100955.
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.-J.; Conte, J.; Natrajan, P.; Haas, G.; Gonzalez, S. Abstracts: Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. Int. J. Cosmet. Sci. 2010, 32, 395–396.
- Cho, Y.-C.; You, S.-K.; Kim, H.J.; Cho, C.-W.; Lee, I.-S.; Kang, B.Y. Xanthohumol inhibits IL-12 production and reduces chronic allergic contact dermatitis. Int. Immunopharmacol. 2010, 10, 556–561.
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine 2009, 16, 369–376.
- Gerhäuser, C. Broad spectrum antiinfective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831.
- Cermak, P.; Olsovska, J.; Mikyska, A.; Dusek, M.; Kadleckova, Z.; Vanicek, J.; Nyc, O.; Sigler, K.; Bostikova, V.; Bostik, P. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. APMIS 2017, 125, 1033–1038.
- Lin, M.; Xiang, D.; Chen, X.; Huo, H. Role of Characteristic Components of Humulus lupulus in Promoting Human Health. J. Agric. Food Chem. 2019, 67, 8291–8302.
- Nookandeh, A.; Frank, N.; Steiner, F.; Ellinger, R.; Schneider, B.; Gerhäuser, C.; Becker, H. Xanthohumol metabolites in faeces of rats. Phytochemistry 2004, 65, 561–570.
- Hanske, L.; Hussong, R.; Frank, N.; Gerhäuser, C.; Blaut, M.; Braune, A. Xanthohumol does not affect the composition of rat intestinal microbiota. Mol. Nutr. Food Res. 2005, 49, 868–873.
- Sleha, R.; Radochova, V.; Mikyska, A.; Houska, M.; Bolehovska, R.; Janovska, S.; Pejchal, J.; Muckova, L.; Cermak, P.; Bostik, P. Strong Antimicrobial Effects of Xanthohumol and Beta-Acids from Hops against Clostridioides difficile Infection In Vivo. Antibiotics 2021, 10, 392.
- Awouafack, M.D.; Lee, Y.-E.; Morita, H. Xanthohumol: Recent advances on resources, biosynthesis, bioavailability and pharmacology. In Handbook of Dietary Flavonoids; Xiao, J., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–23. ISBN 978-3-030-94753-8.
- Buckwold, V.; Wilson, R.J.; Nalca, A.; Beer, B.B.; Voss, T.G.; Turpin, J.; Buckheit, R.; Wei, J.; Wenzelmathers, M.; Walton, E.M.; et al. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antivir. Res. 2004, 61, 57–62.
- Wang, Q.; Ding, Z.-H.; Liu, J.-K.; Zheng, Y.-T. Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194.
- Liu, X.; Bai, J.; Jiang, C.; Song, Z.; Zhao, Y.; Nauwynck, H.; Jiang, P. Therapeutic effect of Xanthohumol against highly pathogenic porcine reproductive and respiratory syndrome viruses. Veter Microbiol. 2019, 238, 108431.
- Lin, Y.; Zang, R.; Ma, Y.; Wang, Z.; Li, L.; Ding, S.; Zhang, R.; Wei, Z.; Yang, J.; Wang, X. Xanthohumol Is a Potent Pan-Inhibitor of Coronaviruses Targeting Main Protease. Int. J. Mol. Sci. 2021, 22, 12134.
- Dabrowski, W.; Gagos, M.; Siwicka-Gieroba, D.; Piechota, M.; Siwiec, J.; Bielacz, M.; Kotfis, K.; Stepulak, A.; Grzycka-Kowalczyk, L.; Jaroszynski, A.; et al. Humulus lupus extract rich in xanthohumol improves the clinical course in critically ill COVID-19 patients. Biomed. Pharmacother. 2022, 158, 114082.
- Magalhães, P.J.; Carvalho, D.O.; Cruz, J.M.; Guido, L.F.; Barros, A.A. Fundamentals and Health Benefits of Xanthohumol, a Natural Product Derived from Hops and Beer. Nat. Prod. Commun. 2009, 4, 591–610.
- Herath, W.; Ferreira, D.; Khan, S.I.; Khan, I.A. Identification and biological activity of microbial metabolites of xanthohu-mol. Chem. Pharm. Bull. 2003, 51, 1237–1240.
- Schini-Kerth, V.B.; Étienne-Selloum, N.; Chataigneau, T.; Auger, C. Vascular Protection by Natural Product-Derived Polyphenols:In VitroandIn VivoEvidence. Planta Medica 2011, 77, 1161–1167.
- Luzak, B.; Kassassir, H.; Rój, E.; Stanczyk, L.; Watala, C.; Golanski, J. Xanthohumol from hop cones (Humulus lupulus L.) prevents ADP-induced platelet reactivity. Arch. Physiol. Biochem. 2016, 123, 54–60.
- Xin, G.; Wei, Z.; Ji, C.; Zheng, H.; Gu, J.; Ma, L.; Huang, W.; Morris-Natschke, S.L.; Yeh, J.-L.; Zhang, R.; et al. Xanthohumol isolated from Humulus lupulus prevents thrombosis without increased bleeding risk by inhibiting platelet activation and mtDNA release. Free. Radic. Biol. Med. 2017, 108, 247–257.
- Lee, Y.-M.; Hsieh, K.-H.; Lu, W.-J.; Chou, H.-C.; Chou, D.-S.; Lien, L.-M.; Sheu, J.-R.; Lin, K.-H. Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus), Prevents Platelet Activation in Human Platelets. Evid. Based Complement. Altern. Med. 2012, 2012, 852362.
- Arnaiz-Cot, J.J.; Cleemann, L.; Morad, M. Xanthohumol Modulates Calcium Signaling in Rat Ventricular Myocytes: Possible Antiarrhythmic Properties. J. Pharmacol. Exp. Ther. 2016, 360, 239–248.
- Sikorska, E.; Khmelinskii, I.; Sikorski, M. Fluorescence methods for analysis of beer. In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2008; pp. 963–976. ISBN 9780123738912.
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530.
- Vesaghhamedani, S.; Ebrahimzadeh, F.; Najafi, E.; Shabgah, O.G.; Askari, E.; Shabgah, A.G.; Mohammadi, H.; Jadidi-Niaragh, F.; Navashenaq, J.G. Xanthohumol: An underestimated, while potent and promising chemotherapeutic agent in cancer treatment. Prog. Biophys. Mol. Biol. 2022, 172, 3–14.
- Natural Products for Cancer Chemoprevention: Single Compounds and Combinations; Pezzuto, J.M.; Vang, O. (Eds.) Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-39854-5.
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178.
- Logan, I.E.; Miranda, C.L.; Lowry, M.B.; Maier, C.S.; Stevens, J.F.; Gombart, A.F. Antiproliferative and Cytotoxic Activity of Xanthohumol and Its Non-Estrogenic Derivatives in Colon and Hepatocellular Carcinoma Cell Lines. Int. J. Mol. Sci. 2019, 20, 1203.
- Wyns, C.; van Steendam, K.; Vanhoecke, B.; Deforce, D.; Bracke, M.; Heyerick, A. Prenylated chalcone xanthohumol associates with histones in breast cancer cells–a novel target identified by a monoclonal antibody. Mol. Nutr. Food Res. 2012, 56, 1688–1696.
- Vanhoecke, B.W.; Delporte, F.; Van Braeckel, E.; Heyerick, A.; Depypere, H.T.; Nuytinck, M.; De Keukeleire, D.; Bracke, M.E. A safety study of oral tangeretin and xanthohumol administration to laboratory mice. In Vivo 2005, 19, 103–107.
- Hussong, R.; Frank, N.; Knauft, J.; Ittrich, C.; Owen, R.; Becker, H.; Gerhäuser, C. A safety study of oral xanthohumol administration and its influence on fertility in Sprague Dawley rats. Mol. Nutr. Food Res. 2005, 49, 861–867.
- Avula, B.; Ganzera, M.; Warnick, J.E.; Feltenstein, M.W.; Sufka, K.J.; Khan, I.A. High-performance liquid chromatographic determination of xanthohumol in rat plasma, urine, and fecal samples. J. Chromatogr. Sci. 2004, 42, 378–382.
- Pang, Y.; Nikolic, D.; Zhu, D.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; van Breemen, R.B. Binding of the hop (Humulus lupulus L.) chalcone xanthohumol to cytosolic proteins in Caco-2 intestinal epithelial cells. Mol. Nutr. Food Res. 2007, 51, 872–879.
- Legette, L.; Ma, L.; Reed, R.L.; Miranda, C.L.; Christensen, J.M.; Rodriguez-Proteau, R.; Stevens, J.F. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol. Nutr. Food Res. 2011, 56, 466–474.
- Legette, L.; Karnpracha, C.; Reed, R.L.; Choi, J.; Bobe, G.; Christensen, J.M.; Rodriguez-Proteau, R.; Purnell, J.Q.; Stevens, J.F. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol. Nutr. Food Res. 2013, 58, 248–255.
- Jung, F.; Staltner, R.; Baumann, A.; Burger, K.; Halilbasic, E.; Hellerbrand, C.; Bergheim, I. A Xanthohumol-Rich Hop Extract Diminishes Endotoxin-Induced Activation of TLR4 Signaling in Human Peripheral Blood Mononuclear Cells: A Study in Healthy Women. Int. J. Mol. Sci. 2022, 23, 12702.
- Bradley, R.; Langley, B.O.; Ryan, J.J.; Phipps, J.; Hanes, D.A.; Stack, E.; Jansson, J.K.; Metz, T.O.; Stevens, J.F. Xanthohumol microbiome and signature in healthy adults (the XMaS trial): A phase I triple-masked, placebo-controlled clinical trial. Trials 2020, 21, 835.
- Langley, B.O.; Ryan, J.J.; Phipps, J.; Buttolph, L.; Bray, B.; Aslan, J.E.; Metz, T.O.; Stevens, J.F.; Bradley, R. Xanthohumol microbiome and signature in adults with Crohn’s disease (the XMaS trial): A protocol for a phase II triple-masked, placebo-controlled clinical trial. Trials 2022, 23, 885.
More
Information
Subjects:
Chemistry, Applied
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
738
Revisions:
3 times
(View History)
Update Date:
22 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




