Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Toshikazu Yoshikawa | -- | 2611 | 2024-03-19 06:01:45 | | | |
| 2 | Mona Zou | Meta information modification | 2611 | 2024-03-21 08:27:50 | | | | |
| 3 | Mona Zou | Meta information modification | 2611 | 2024-03-27 09:40:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Encyclopedia. Available online: https://encyclopedia.pub/entry/56397 (accessed on 07 February 2026).
Yoshikawa T, You F. Oxidative Stress and Bio-Regulation. Encyclopedia. Available at: https://encyclopedia.pub/entry/56397. Accessed February 07, 2026.
Yoshikawa, Toshikazu, Fukka You. "Oxidative Stress and Bio-Regulation" Encyclopedia, https://encyclopedia.pub/entry/56397 (accessed February 07, 2026).
Yoshikawa, T., & You, F. (2024, March 19). Oxidative Stress and Bio-Regulation. In Encyclopedia. https://encyclopedia.pub/entry/56397
Yoshikawa, Toshikazu and Fukka You. "Oxidative Stress and Bio-Regulation." Encyclopedia. Web. 19 March, 2024.
Copy Citation
Reactive oxygen species (ROS) and free radicals work to maintain homeostasis in the body, but their excessive production causes damage to the organism. The human body is composed of a variety of cells totaling over 60 trillion cells. Each cell performs different functions and has a unique lifespan. The lifespan of cells is preprogrammed in their genes, and the death of cells that have reached the end of their lifespan is called apoptosis. This is contrary to necrosis, which is the premature death of cells brought about by physical or scientific forces. Each species has its own unique lifespan, which in humans is estimated to be up to 120 years. Elucidating the mechanism of the death of a single cell will lead to a better understanding of human death, and, conversely, the death of a single cell will lead to exploring the mechanisms of life. In this sense, research on active oxygen and free radicals, which are implicated in biological disorders and homeostasis, requires an understanding of both the physicochemical as well as the biochemical aspects. Based on the discussion above, it is clear to see that active oxygen and free radicals have dual functions of both injuring and facilitating homeostasis in living organisms.
oxidative stress
anti-oxidant
free radicals
biochemistry
bio-regulation
reactive oxygen species
1. Earth’s History and the Evolution of Living Organisms
The Earth’s history and the evolution of living organisms are intricately linked to the development of oxygen and its impact on biological systems. The Earth was formed approximately 4.6 billion years ago. The earliest microorganisms emerged about 3.9 billion years ago, followed by the birth of anaerobic bacteria. Subsequently, organisms capable of utilizing light for survival appeared, and approximately 2.5 billion years ago, oxygen, produced by photosynthetic bacteria, was released on Earth [1][2]. However, the emergence of oxygen led to the extinction of most anaerobic bacteria (Figure 1).
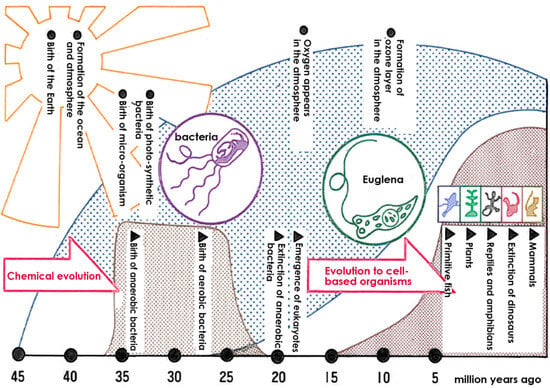
Figure 1. The Earth’s atmosphere and the evolution of living things. Anaerobic bacteria were the first to emerge, but the advent of oxygen caused the extinction of most anaerobic bacteria, and aerobic bacteria adapted to oxygen and cell-based organisms flourished.
In contrast, aerobic bacteria evolved, and they survived by developing mechanisms to protect themselves from the harmful effects of oxygen-induced damage. Developing the ability to resist oxygen-related toxicity [3] resulted in the beginning of the history of aerobic organisms.
Following the generation of oxygen on Earth, the ozone layer was formed in the atmosphere, and this protected living organisms from powerful ultraviolet rays from space [4]. As a result, organisms were shielded from intense ultraviolet radiation, allowing for evolution on the Earth’s surface and the emergence of human beings.
It can be said, therefore, that the current diversity of life on Earth, as well as the survival of the human species, would not be possible without the ozone layer blocking the powerful ultraviolet rays from space. Therefore, the formation of the ozone layer has enabled the survival of humankind. Consequently, the potential consequences of the ozone layer being depleted by fluorocarbon gases [5], which is a global concern, poses a significant threat of extinction to various forms of life on Earth due to the damage caused by reactive oxygen species (ROS) and free radicals resulting from ultraviolet radiation. Protecting living organisms from this intense radiation in outer space is a crucial challenge in space exploration. As such, research on ROS and free radicals is indispensable for space travel.
On the other hand, organisms in tropical seas are exposed to intense ultraviolet radiation but protect themselves from this hazard with powerful reactive active oxygen and free radical scavenging mechanisms. For example, corals and tropical fish, like Ocellaris clownfish (Figure 2), exhibit bright red and yellow colors. Ocellaris clownfish are well known for their body coloration, which is enhanced by the intake of the red carotenoid astaxanthin, a widely recognized antioxidant, in their diet. While the vivid body colors obtained with such pigments are beautiful, they are primarily responsible for the free radical scavenging effect. These organisms in effect use a variety of natural pigments to protect themselves from free radical damage [6].

Figure 2. Highly pigmented tropical fish. The colorful natural pigments of tropical fish serve to scavenge free radicals and protect the fish from the ultraviolet irradiation (Photo by Shin-ichi Ohama, Diving shop Marine Mate, Ishigaki Island, Okinawa, Japan).
2. Active Oxygen and Free Radicals
The discovery of SOD by McCord and Fridovich in 1969 marked a significant milestone in understanding the scavenging of superoxide (O·2−), a type of active oxygen and free radical [7]. This discovery paved the way for identifying various previously unknown chemical, biological, and biochemical reactions associated with O·2−, and other ROS and free radicals, as well as their biological damage mechanism and their implications for different diseases [8][9] (Figure 3 and Figure 4).
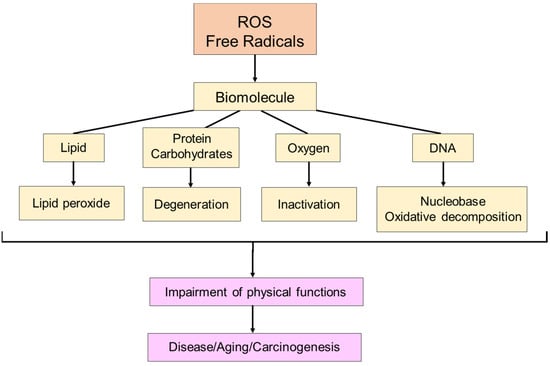
Figure 3. The effect of free radicals on living organisms. Free radicals damage biomolecules, such as lipids, proteins, carbohydrates, oxygen, and DNA, contributing to disease, aging, and cancer in living organisms.
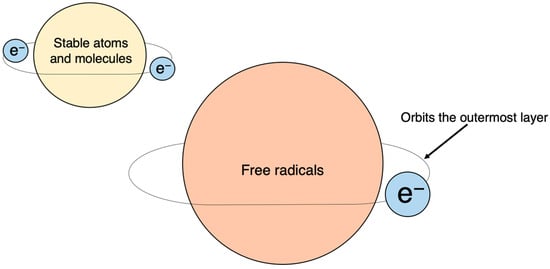
Figure 4. Free radicals: atoms or molecules with unpaired electrons. Electrons normally exist in pairs, with two in each orbital; unpaired electrons, with only one electron in each orbital, are chemically very unstable.
3. Definition of ROS
Oxygen is the most well-known free radical. Atmospheric oxygen possesses two unpaired electrons and is relatively stable on its own. Living organisms, however, convert this oxygen into more reactive forms, known as ROS. These are utilized in substrate oxidation reactions and oxygen addition reactions. Reactive oxygen, a derivative of atmospheric oxygen, encompasses several ROS, including O·2−, H2O2, hydroxyl radical (HO·), and singlet oxygen(1O2). Among these four, O·2− and HO· are free radicals, while the other two are not (Figure 5). These species, on their own, or by cross-reacting with other species, can induce toxicity by attacking different cellular components, such as proteins, lipids, and nucleic acids [10][11], leading to various toxic effects.
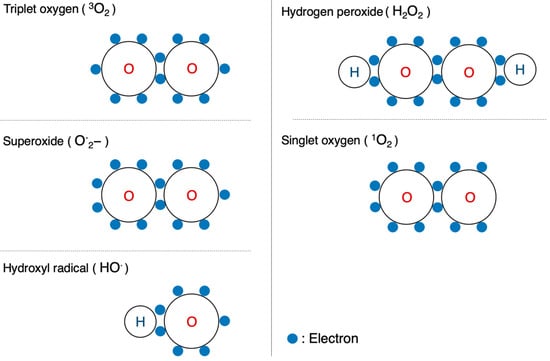
Figure 5. Configuration of oxygen and ROS. Schematic diagrams of the electron configurations of typical molecules are shown.
4. Definition of Free Radicals
Atoms and molecules have a stable configuration when their outermost shell contains a paired set of electrons. However, when an electron is unpaired, the atom or molecule becomes a free radical. Unpaired electrons are unstable and highly reactive, as they constantly seek to form pairs, which makes free radicals highly reactive. Chemical bonds consist of two electrons that can be broken in two ways. The first involves one electron from each pair separating and moving to one side (A-B→A+ + :B−), resulting in each carrying a respective charge called a cation or anion. The second way occurs when the two electrons in a bond split symmetrically, leading to the creation of a free radical (AB→A· +·B).
5. Generation of Active Oxygen and Free Radicals
ROS and free radicals are generated in our immediate environment. For instance, air pollution in the form of NO· and NO2 is constantly present. Tobacco smoke, another pollutant, contains numerous free radicals [12][13]. Additionally, commonly used herbicides, including the well-knowns paraquat, produce free radicals to eliminate weeds and occasionally cause poisoning when ingested by humans [14].
Furthermore, ultraviolet and ionizing radiation also generate free radicals. Both types are utilized in cancer radiation therapy as well as in sterilization lamps. Anti-cancer drugs, such as adriamycin, bleomycin, mitomycin C, cisplatin, and neocarzinostatin generate free radicals to kill cancer; however, the free radicals they produce also cause side effects [15][16][17][18][19][20][21][22][23][24].
Radiation is also used to sterilize imported food, and, while it may not be common knowledge, drying laundry outdoors is another example of benefiting from the effects of free radicals. Hypochlorous acid, which is often used as a sterilizing bleach to disinfect medical operating rooms and toilets, transforms into free radicals and possesses a bactericidal effect. Viewed from this perspective, humans cannot avoid the effects of free radicals.
In addition to the external environment, free radicals are also generated in living organisms. Free radicals are mainly produced by phagocytes, so called “eating cells”, centered on neutrophils. Phagocytes engulf foreign substances, such as bacteria, and generate ROS to eliminate them from the body [25][26]. Moreover, physiological processes, such as ischemia-reperfusion and arachidonic acid metabolism, also lead to the production of a large amount of ROS [27][28][29]. They are produced during the metabolism of arachidonic acid. Physiologically, free radicals are constantly generated within the mitochondria; however, in healthy mitochondria, they tend not to leak out (Figure 6).
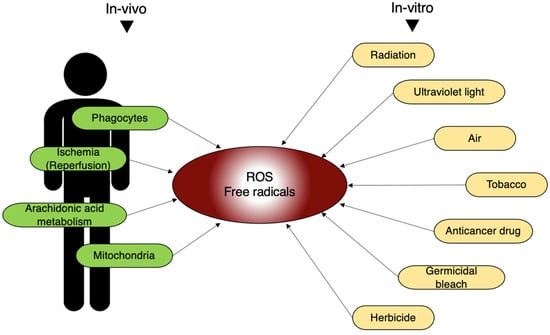
Figure 6. In vivo and in vitro sources of free radicals. In living organisms, ROS and free radicals are generated by various internal and external stimuli mentioned here.
6. Physiological Role of ROS and Free Radicals
Nitric oxide (NO·), a free radical, plays a crucial role in dilating blood vessels and maintaining blood flow as an endothelial-derived vaso-relaxing factor (EDRF). However, the radical nature of NO· leads to its rapid interaction with other radicals, especially O·2−, resulting in the production of highly toxic peroxy-nitrite (ONOO−).
NO· + O·2− → ONOO−
In this way, when the NO· effect is lost due to O·2−, highly toxic ONOO− is simultaneously produced. This indicates that in addition to its own effects, O·2− also regulates the action of NO·. This interplay between NO· and O·2− underscores the regulatory effects of free radicals on physiological processes, and the generation of free radicals and their physiological implications highlight the intricate balance between their beneficial and detrimental effects in various biological systems (Figure 7).
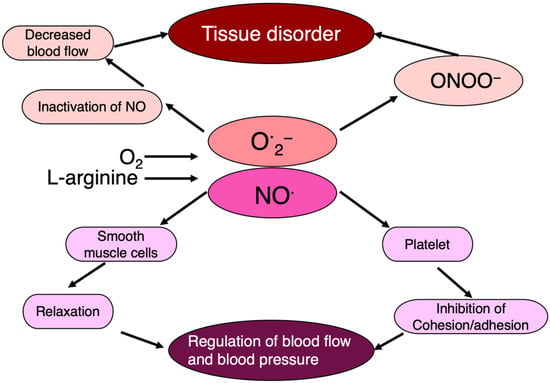
Figure 7. NO· regulates blood flow, blood pressure, and tissue damage. NO· regulates endothelial cell function as an endothelium-derived relaxing factor, in which O·2− is intimately involved.
SOD regulates the tension state of vascular smooth muscle by relaxing blood vessels. In addition, NO· is involved in the dilation and movement of the stomach and intestines. Hence, NO· and its regulatory factor, active oxygen, such as O·2−, may control a variety of physiological reactions.
7. Utilization of Active Oxygen and Free Radicals
Living organisms harness the toxicity of ROS and free radicals to combat various threats. Phagocytic cells, such as neutrophils, employ these species as potent weapons to eliminate not only bacteria but also cancer, a significant threat to the body. During bacterial infections, activated neutrophils release large amounts of ROS to enhance bacterial eradication. Furthermore, the toxicity of these species is leveraged in cancer hyperthermia therapy, where elevated temperatures of 42–43 °C shrink tumors [30][31]. However, reducing neutrophils and scavenging ROS diminishes this anti-tumor effect. Thus, organisms utilize the toxicity of free radicals as a mechanism to eliminate foreign substances from the body (Figure 8).
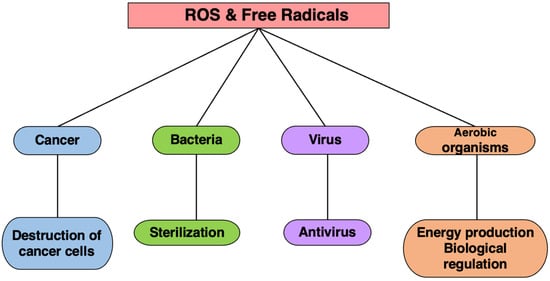
Figure 8. The effects of ROS and free radicals. Living organisms utilize the toxicity of ROS and free radicals to protect themselves from cancer, microorganisms, viruses, etc.
8. Neutralization of Active Oxygen and Free Radicals
Aerobic organisms, such as humans, possess antioxidant functions in various internal and external compartments of their tissues and cells, to safeguard themselves from oxidative damage induced by free radicals and ROS [7]. This defense mechanism comprises three tiers: (1) preventive antioxidants that suppress the formation of free radicals [32], (2) radical-scavenging antioxidants that rapidly eliminate generated free radicals [30], and (3) reparative/generative mechanism for repairing and regenerating DNA, lipids, and proteins damaged by free radicals. Table 1 shows primary antioxidant mechanisms.
Table 1. In vivo mechanism of antioxidants.
| Preventive mechanism |
Hydroperoxide reduction of H2O2 |
Glutathione peroxidase, catalase, glutathione -S- transferase, peroxidase |
| Metal ions chelation |
Transferrin, ferritin, ceruloplasmin, lactoferrin, albumin | |
| Singlet oxygen quenching | Beta-carotene, bilirubin | |
| Radical scavenging mechanism |
Captures radicals in lipid layer | Vitamin E |
| Captures radicals in the water layer | Vitamin C, uric acid, bilirubin | |
| Captures O·2− | SOD | |
| Repair and regeneration mechanism |
Phospholipase A2 Protease AMP-activated protein kinase Endonuclease |
|
While these antioxidant mechanisms are effective, they are not infallible. When excessive free radicals are generated due to OS or weakened antioxidant mechanisms, the body suffers oxidative damage from free radicals, leading to various pathological conditions. While there are other antioxidant systems, the primary ones are the O·2− scavenging, the H2O2, and the lipid peroxide scavenging systems.
9. Understanding Oxidative Stress
What is the real meaning of OS in terms of how it negatively affects living organisms? Stress, originally a term rooted in physics, refers to the “internal distortion that occurs within an object when subjected to external stimuli”. This concept was adapted to biological reactions by Canadian endocrinologist Hans Selye in the 1950s, who defined stress as an internal state in an organism that responds to external factors, leading to a disturbance of the organism’s homeostasis, and used the term “stressors” for the external factors that instigate stress. Due to the discovery of ROS and other factors, the term “oxidative stress” is currently used to describe the phenomenon that creates oxidative environments in living organisms due to external factors. In the context of living organisms, OS arises from the imbalance between free radicals and antioxidants, due to various external factors including physical, scientific, or a complex combination of both as depicted in Table 2.
Table 2. Categories and causes of physiological and psychological stress.
| Physiological stress | Physical stress | Temperature, humidity, light, sound, ultraviolet rays, radiation, atmospheric pressure |
| Chemical stress | Oxygen, pH, osmotic pressure, metal ions, ethanol, environmental pollutants (nitrogen oxides, sulfur oxides, etc.), hazardous chemicals | |
| Combined stress | Starvation, aging, inflammation, infection, allergy, trauma, ischemia | |
| Daily life stress | Family illness and death, divorce, moving, debt, childbirth, entrance exams | |
| Psychological stress | Occupational stress | Human relations, office transfer, reassignment, promotion |
| Other stress | War, natural disaster |
Reduction indicates a reaction in which a molecule receives electrons, while oxidation involves a reaction where a molecule releases electrons, and this often occurs when oxygen (O 2) binds to a molecule.
While animals require oxygen to produce energy, ROS, a byproduct of this energy metabolism, are produced during the conversion of oxygen. Under normal conditions, this reactive oxygen is efficiently removed by antioxidant enzymes and antioxidants. However, when the antioxidant mechanism malfunctions, ROS accumulates in the body, becoming a stressor and leading to a state of OS. In other words, OS may be considered as a state in which the mechanism for maintaining oxidative and reductive states within the body has failed.
This state is widely believed to contribute to the progression of various diseases, including Alzheimer’s disease [33][34], Parkinson’s disease [34][35][36], diabetic complications [37][38], and neurodegeneration caused by motor neuron disease [39]. However, in many cases, it is not clear whether oxidants instigated the disease or whether they are generated secondarily from tissue damage stemming from the onset of the disease. While the exact role of oxidants in disease onset is not always clear, OS has garnered significant attention as a factor in human diseases and has been the focus of extensive research.
Antioxidants are being studied and utilized as both medicinal and nutritional supplements for disease prevention and health maintenance. For example, several research has focused on the treatment of stroke and ischemia-reperfusion injury [40][41]. On the other hand, many compounds have been commercialized as nutritional supplements, and antioxidants are widely used to support health or to prevent malignant tumors and heart disease.
10. Evolution of Antioxidants
The term “antioxidant” was first coined in the late 19th century, initially referring to chemical species that inhibited oxygen consumption. Subsequently, antioxidants found widespread application in various chemical industries to prevent metal corrosion, control rubber crosslinking reactions, and inhibit fuel deterioration due to oxidative polymerization in internal combustion engines [42].
The specific biochemical roles of antioxidants became clearer in the mid-20th century as understanding of in vivo biochemistry and molecular biochemistry advanced. Many biological substances, whose importance became apparent through subsequent research progress, were rediscovered as antioxidants. For instance, the discovery of α-tocopherol (vitamin E) as a critical factor for healthy pregnancy, as evidenced by infertility in mice fed a diet deficient in α-tocopherol, highlighted its role as an antioxidant [43][44].
Such biochemical discoveries have significantly influenced nutrition and food chemistry, leading to the development and use of a plethora of naturally derived antioxidants as nutritional supplements to prevent food deterioration and enhance mineral absorption. Notably, some antioxidants, such as vitamin C and E, are consumed as food additives rather than as treatments for vitamin deficiencies [43][44].
References
- Cavalier-Smith, T. Cell evolution and Earth history: Stasis and revolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 969–1006.
- Goldblatt, C.; Lenton, T.M.; Watson, A.J. Bistability of atmospheric oxygen and the Great Oxidation. Nature 2006, 443, 683–686.
- Stadie, W.C.; Haugaard, N. Oxygen poisoning; the effect of high oxygen pressure upon enzymes; succinicdehydrogenase and cytochrome oxidase. J. Biol. Chem. 1945, 161, 153–174.
- Bais, A.F.; McKenzie, R.L.; Bernhard, G.; Aucamp, P.J.; Ilyas, M.; Madronich, S.; Tourpali, K. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2015, 14, 19–52.
- Andersen, S.O.; Halberstadt, M.L.; Borgford-Parnell, N. Stratospheric ozone, global warming, and the principle of unintended consequences--an ongoing science and policy success story. J. Air Waste Manag. Assoc. 2013, 63, 607–647.
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96.
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055.
- D’Alessandro, A.; Zolla, L. The SODyssey: Superoxide dismutases from biochemistry, through proteomics, to oxidative stress, aging and nutraceuticals. Expert Rev. Proteom. 2011, 8, 405–421.
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic. Biol. Med. 2016, 97, 398–407.
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214.
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.P.; Ravanat, J.L.; Sauvaigo, S. Hydroxyl radicals and DNA base damage. Mutat. Res. 1999, 424, 9–21.
- Zi, Y.; Wang, X.; Zi, Y.; Yu, H.; Lan, Y.; Fan, Y.; Ren, C.; Liao, K.; Chen, H. Cigarette smoke induces the ROS accumulation and iNOS activation through deactivation of Nrf-2/SIRT3 axis to mediate the human bronchial epithelium ferroptosis. Free Radic. Biol. Med. 2023, 200, 73–86.
- Zimmermann, R.; Hertz-Schünemann, R.; Ehlert, S.; Liu, C.; McAdam, K.; Baker, R.; Streibel, T. Highly time-resolved imaging of combustion and pyrolysis product concentrations in solid fuel combustion: NO formation in a burning cigarette. Anal. Chem. 2015, 87, 1711–1717.
- Bus, J.S.; Aust, S.D.; Gibson, J.E. Superoxide- and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem. Biophys. Res. Commun. 1974, 58, 749–755.
- Mimnaugh, E.G.; Trush, M.A.; Bhatnagar, M.; Gram, T.E. Enhancement of reactive oxygen-dependent mitochondrial membrane lipid peroxidation by the anticancer drug adriamycin. Biochem. Pharmacol. 1985, 34, 847–856.
- Rajagopalan, S.; Politi, P.M.; Sinha, B.K.; Myers, C.E. Adriamycin-induced free radical formation in the perfused rat heart. Implications for cardiotoxicity. Caner Res. 1988, 48, 4766–4769.
- Allawzi, A.; Elajaili, H.; Redente, E.F.; Npzik-Grayck, E. Oxidative toxicology of bleomycin: Role of the extracellular redox environment. Curr. Opin. Toxicol. 2019, 13, 68–73.
- Sato, K.; Tashiro, Y.; Chibana, S.; Yamashita, A.; Karakawa, T.; Kohrogi, H. Role of lipid-derived free radical in bleomycin-Induced lung Injury in mice: Availability for ESR spin trap method with organic phase extraction. Biol. Pharm. Bull. 2008, 31, 1855–1859.
- Pritsos, C.A.; Sartorelli, A.C. Generation of reactive oxygen radicals through bioactivation of mitomycin antibiotics. Cancer Res. 1986, 46, 3528–3532.
- Nissenkorn, I.; Herrod, H.; Soloway, M.S. Side effects associated with intravesical mitomycin C. J. Urol. 1981, 126, 596–597.
- Wozniak, K.; Czechowska, A.; Blasiak, J. Cisplatin-evoked DNA fragmentation in normal and cancer cells and its modulation by free radical scavengers and the tyrosine kinase inhibitor STI571. Chem. Biol. Interact. 2004, 147, 309–318.
- Kersten, L.; Bräunlich, H.; Keppler, B.K.; Gliesing, C.; Wendelin, M.; Westphal, J. Comperative nephrotoxicity of some antitumor-active platinum and ruthenium complexes in rats. J. Appl. Toxicol. 1998, 18, 93–101.
- Sato, K.; Akaike, T.; Suga, M.; Ando, M.; Maeda, H. Generation of free radicals from neocarzinostatin mediated by NADPH/Cytochrom P-450 reductase via activation of enediyne chromophore. Biochem. Biophys. Res. Commun. 1994, 205, 1716–1723.
- Ikeda, K.; Saitoh, S.; Kobayashi, M.; Suzuki, Y.; Suzuki, F.; Tsubota, A.; Arase, Y.; Chayama, K.; Murashima, N.; Kumada, H. Hepatic vascular side effects of styrene maleic acid neocarzinostatin in the treatment of hepatocellular carcinoma. J. Gastroenterol. 2000, 35, 353–360.
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96.
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95.
- Zulueta, J.J.; Sawhney, R.; Yu, F.S.; Cote, C.C.; Hassoun, P.M. Intracellular generation of reactive oxygen species in endothelial cells exposed to anoxia-reoxygenation. Am. J. Physiol. 1997, 272, L897–L902.
- Zu, G.; Guo, J.; Che, N.; Zhou, T.; Zhang, X.; Wang, G.; Ji, A.; Tian, X. Protective effects of ginsenoside Rg1 on intestinal ischemia/reperfusion injury-induced oxidative stress and apoptosis via activation of the Wnt/β-catenin pathway. Sci. Rep. 2016, 6, 38480.
- Zweier, J.L.; Broderick, R.; Kuppusamy, P.; Thompson-Gorman, S.; Lutty, G.A. Determination of the mechanism of free radical generation in human aortic endothelial cells exposed to anoxia and reoxygenation. J. Biol. Chem. 1994, 269, 24156–24162.
- Yoshikawa, T.; Kokura, S.; Tainaka, K.; Itani, K.; Oyamada, H.; Kaneko, T.; Naito, Y.; Kondo, M. The role of active oxygen species and lipid peroxidation in the antitumor effect of hyperthermia. Cancer Res. 1993, 53, 2326–2329.
- Yoshikawa, T.; Kokura, S.; Oyamada, H.; Iinuma, S.; Nishimura, S.; Kaneko, T.; Naito, Y.; Kondo, M. Antitumor effect of ischemia-reperfusion injury induced by transient embolization. Cancer Res. 1994, 54, 5033–5035.
- William, A.P. Free Radicals; McGrew-Hill Book Company: New York, NY, USA, 1966; Volume 1, pp. 1–53.
- Abate, G.; Vezzoli, M.; Sandri, M.; Rungratanawanich, W.; Memo, M.; Uberti, D. Mitochondria and cellular redox state on the route from ageing to Alzheimer’s disease. Mech. Ageing Dev. 2020, 192, 111385.
- Abdelhamid, R.F.; Nagano, S. Crosstalk between Oxidative Stress and Aging in Neurodegeneration Disorders. Cells 2023, 12, 753.
- Aaseth, J.; Dusek, P.; Roos, P.M. Prevention of progression in Parkinson’s disease. Biometals 2018, 31, 737–747.
- Abdel-Kawy, M.A.; Aboulhoda, B.E.; Michel, C.G.; Sedeek, M.S.; Kirollos, F.N.; Masoud, M.A. Ameliorating effect of Citrus trifoliata L. fruits extract on motor incoordination, neurodegeneration and oxidative stress in Parkinson’s disease model. Nutr. Neurosci. 2023, 2, 1–13.
- Abbasihormozi, S.H.; Babapour, V.; Kouhkan, A.; Naslji, N.A.; Afraz, K.; Zolfaghary, Z.; Shahverdi, A.H. Stress Hormone and Oxidative Stress Biomarkers Link Obesity and Diabetes with Reduced Fertility Potential. Cell J. 2019, 21, 307–313.
- Abdel-Moneim, A.; Abdel-Reheim, E.S.; Semmler, M.; Addaleel, W. The Impact of Glycemic Status and Metformin Administration on Red Blood Cell Indices and Oxidative Stress in Type 2 Diabetic Patients. Malays. J. Med. Sci. 2019, 26, 47–60.
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010.
- Liu, X.; Yamashita, T.; Shang, J.; Shi, X.; Morihara, R.; Huang, Y.; Sato, K.; Takemoto, M.; Hishikawa, N.; Ohta, Y.; et al. Clinical and Pathological Benefit of Twendee X in Alzheimer’s Disease Transgenic Mice with Chronic Cerebral Hypoperfusion. J. Stroke Cerebrovasc. Dis. 2019, 28, 1993–2002.
- Liu, X.; Yamashita, T.; Shang, J.; Shi, X.; Morihara, R.; Huang, Y.; Sato, K.; Takemoto, M.; Hishikawa, N.; Ohta, Y.; et al. Twendee X Ameliorates Phosphorylated Tau, α-Synuclein and Neurovascular Dysfunction in Alzheimer’s Disease Transgenic Mice with Chronic Cerebral Hypoperfusion. J. Stroke Cerebrovasc. Dis. 2019, 28, 104310.
- Mattill, H.A. Antioxidants. Annu. Rev. Biochem. 1947, 16, 177–192.
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651.
- Papas, A.M. The Vitamin E Factor, 1st ed.; HarperCollins: New York, NY, USA, 1999; pp. 11–29.
More
Information
Subjects:
Biochemistry & Molecular Biology; Biology; Physiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
576
Revisions:
3 times
(View History)
Update Date:
27 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




