Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | P. A. Bretscher | -- | 3003 | 2024-03-18 15:51:50 | | | |
| 2 | Mona Zou | Meta information modification | 3003 | 2024-03-19 10:25:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bretscher, P.A. How Immune Responses Are Regulated. Encyclopedia. Available online: https://encyclopedia.pub/entry/56385 (accessed on 07 February 2026).
Bretscher PA. How Immune Responses Are Regulated. Encyclopedia. Available at: https://encyclopedia.pub/entry/56385. Accessed February 07, 2026.
Bretscher, Peter Alan. "How Immune Responses Are Regulated" Encyclopedia, https://encyclopedia.pub/entry/56385 (accessed February 07, 2026).
Bretscher, P.A. (2024, March 18). How Immune Responses Are Regulated. In Encyclopedia. https://encyclopedia.pub/entry/56385
Bretscher, Peter Alan. "How Immune Responses Are Regulated." Encyclopedia. Web. 18 March, 2024.
Copy Citation
Most basic studies directed at how immune responses are regulated employ chemically “simple antigens”, usually purified proteins. The target antigens in many clinical situations, such as in autoimmunity, infectious diseases and cancer, are chemically “complex”, consisting of several distinct molecules, and they often are part of a replicating entity.
antibody
B cells
multiple sclerosis
neuroimmunology
neurodegeneration
T cells
treatment
1. Findings from Basic Studies on How Immune Responses Are Regulated
1.1. Variables of Immunization That Affect the Th1/Th2 of the Ensuing Response
One of the earliest studies examining how the dose of a simple antigen and time after antigen impact affect the nature of the ensuing immune response was undertaken by Salvin in the 1950s [1]. His findings are summarized in Figure 1. Low doses of antigen generate, in modern terminology, an exclusive Th1, delayed-type hypersensitivity (DTH) response. Moderate doses lead to a more rapid Th1 DTH response that declines as IgG antibody and Th2 cells are generated. The administration of an even higher dose of antigen results in even more rapid responses, and the Th1 DTH phase may be eclipsed. Salvin’s findings have been found to indicate how responses to diverse antigens are regulated, as I discuss later.
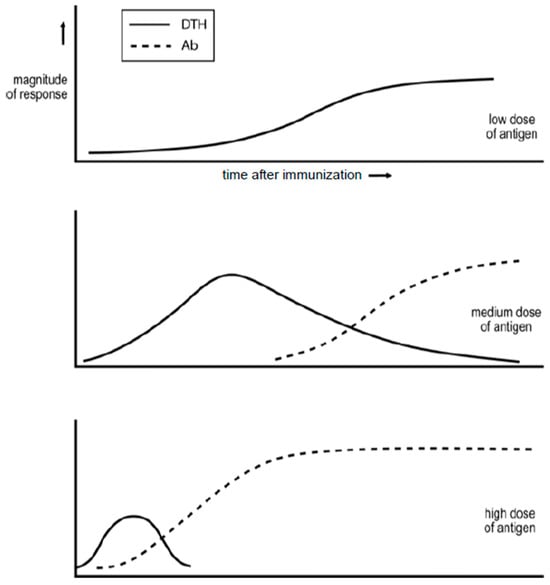
Figure 1. Synopsis of the observations of Salvin (3) on how the dose of a simple antigen and time after antigen impact affect the DTH/IgG antibody nature of the ensuing response.
1.2. Humoral Immune Deviation
Studies by a number of investigators in the 1960s showed that immunization, in a manner that results in the production of IgG antibody, renders the immunized animal refractory for the induction of a DTH response [2]. A DTH response is not generated upon immunization with an antigen challenge that generates such a response in naïve animals. It appears that the immune response is locked into an IgG humoral mode. This situation is referred to as a state of humoral immune deviation. Note that humoral immune deviation is likely pertinent to understanding why Salvin found that, upon immunization with a high dose of antigen, the expression of DTH declines as IgG antibody is produced; see Figure 1.
1.3. Requirements to Establish and Maintain Self-Tolerance
Mitchison undertook an extensive investigation in the 1960s [3] that was well recognized forty to fifty years ago. I conclude from more recent conversations with my younger colleagues that most do not know of these classical studies, and from older colleagues that the significance of this investigation is not evident in terms of contemporary concepts. This investigation and related studies are consequentially largely forgotten. These studies are central to my thinking. I therefore think it helpful if I first trace the ideas inspiring, and summarize the observations that emanated from, these studies.
Burnet and Fenner had proposed in 1949 that tolerance to self-antigens was a consequence of their early presence in development, before birth [4]. Medawar and colleagues [5], as well as Hasek and his colleagues [6], showed that immunocompetent animals that had been exposed during development to a foreign antigen could no longer respond to this foreign antigen. These studies were correctly taken as evidence supporting Burnet and Fenner’s conjecture. It is technically difficult to administer an antigen to developing animals in most species. Immunologists therefore subsequently examined whether it was possible to generate unresponsiveness in neonates. This was possible in a number of cases. For example, Weigle undertook a series of such studies to which 1shall return. It was natural in such circumstances to also pose the question of whether unresponsiveness could also be established in immunocompetent animals. This was particularly interesting once it was realized that tolerance is not uniquely established during development as originally envisaged by Burnet; it is an ongoing process throughout our life. This recognition came about in the following way.
Most of the studies testing Burnet and Fenner’s conjecture were carried out with “foreign” stem cells that resulted in the adult animal being a chimera of self-cells and of cells derived from the foreign stem cells. Mitchison did a similar kind of experiment to Medawar’s but with non-replicating foreign cells [7]. He found that animals exposed during development were unable to respond to the target antigen when very young. However, they could respond at an older age unless the target antigen was periodically administered. This finding showed that tolerance was not uniquely and stably established in the developing animal. Maintaining tolerance was a continual process throughout life. Lederberg correctly recognized that this finding was consistent with the idea that lymphocytes are continually generated in adults [8]. Incidentally, this change in perspective also allowed scope for understanding how autoimmunity could arise in adults, as was known to often occur [9].
It is natural, if maintenance of tolerance is an ongoing process during adult life, to explore whether immunocompetent animals can also be made tolerant. In addition, self-antigens are usually present at a constant level, whereas the “concentration” of foreign antigens dramatically changes as the antigen impacts the immune system. I suspect but do not know that the virtual constancy in the level of self-antigens was a driving force in the design of Mitchison’s study of the mid-1960s. He gave immunocompetent mice a series of injections of the antigen over several weeks, with each mouse receiving the same dose each time but with mice belonging to different groups receiving different doses. The mice, after this regimen, were all given an antigen challenge that generated a robust IgG antibody response in aged-matched but naïve mice. He found that the IgG response to the challenge of the mice pre-exposed to the “priming” antigen fell into four groups, depending on the size of the priming dose, which varied over about a 10,000-fold range [3]. Mice injected only with saline exhibited a robust IgG antibody response. Those repeatedly pre-exposed to low, medium and high doses of antigen respectively generate a reduced, an enhanced and a reduced IgG antibody response to the challenge. Mitchison concluded that a virtually steady state of low and high levels of antigen reduced the IgG antibody response to the challenge by processes he respectively called low- and high-zone paralysis; a medium level of antigen primed the immune system for an antibody response. Mitchison interpreted the physiological significance of low- and high-zone paralysis as contributing to the mechanism of self-tolerance among immunocompetent lymphocytes [3]. I argue below for an alternative explanation.
1.4. Cell-Mediated Immune Deviation
Parish reported in the late 1960s a study of very similar design to Mitchison’s, employing again a simple antigen [10]. He used rats as his experimental animal. The major difference between the two studies was that Parish examined the state of DTH at the time of the antigen challenge. His observations confirmed and extended those of Mitchison. He found that the lower IgG antibody responses associated with “low-” and “high-zone paralysis” were associated with a state of DTH to the antigen, whereas there was no detectable or a lower level of DTH, respectively, in saline-injected rats or rats pre-exposed to medium doses of the antigen. Tolerance to self-antigens is a state of unresponsiveness for all classes of immunity. I suggest that Mitchison’s terms of “low-“ and “high-zone paralysis”, which reflect his idea that these processes reflect mechanisms of self-tolerance, is misleading. I refer to these states as low- and high-zone cell-mediated immune deviation, making clear their relationship with the processes controlling the class of immunity generated. Note the relationship between Salvin’s and Parish’s observations. Immunization once with a low dose of antigen to immunocompetent animals results in an exclusive DTH response, and immunization multiple times with a low dose of antigen results in a state of DTH and of cell-mediated immune deviation.
2. Importance of Quantitative Considerations in Understanding the Regulation of Immune Class
Thus, diverse observations in diverse experimental systems support the importance of antigen dose in affecting the class of immunity generated. This support is even broader than just outlined. It is clearly of basic and practical importance to assess the breadth of the validity of such generalizations. Moreover, as these generalizations are of a quantitative nature, they bring quantitative considerations to the fore. There is another set of observations critical in further assessing the universality/non-universality of these generalizations. In addition, a further experimental generalization will be described that again illustrates the centrality of quantitative considerations.
The researchers injected different strains of mice with different numbers of leishmania parasites and assessed the nature of the ensuing response. The researchers could thereby define for each mouse strain a “transition number”, Nt. Infection with a number of parasites below Nt results in a stable Th1 response, whereas infection with a number above Nt results in a response that in time develops a substantial Th2 component. Infection with a number considerably above Nt rapidly results in a predominant Th2 response. The value of Nt in different mouse strains varied over a 100,000-fold range [11]. This finding provides very strong evidence for the generality of the importance of low doses/numbers of slowly replicating entities in generating an exclusive Th1 response. It also indicates the importance of genetics in influencing the value of Nt.
As already documented, the researchers have been able to generate exclusive Th1 responses by immunization with a low dose of antigen or infection with a low number of slowly replicating entities in diverse systems. Infection with very high doses or very high numbers, on the other hand, does not universally result in Th1 responses; in fact, it rarely leads to such a response. Parish’s high-zone cell-mediated immune deviation does not in practice hold for many antigens. However, the generality of the finding that stimulation with low levels of antigen generates exclusive Th1 responses means we have a general means for controlling whether an antigen generates a Th1 or Th2 response, unless the antigen is part of a rapidly replicating entity. This is important. Such an ability to control the Th1/Th2 phenotype of the response to most antigens is not evident in the context of the most popular frameworks that address how the Th1/Th2 phenotype of a response is determined, as I discuss below.
3. Models to Explain Immune Class Regulation
3.1. PAMP/DAMP-Centric Models
The most popular frameworks for what controls whether an antigen activates or inactivates its naive CD4 T cells is whether or not antigen impingement is associated with a pathogen-associated molecular pattern (PAMP) [12][13][14] or a danger-associated molecular pattern (DAMP) [15][16][17]. These models posit that a PAMP/DAMP-dependent signal is required to activate naïve CD4 T cells, and that an antigen can inactivate the CD4 T cells in the absence of such a signal. The grounds for these models have been well described by their proponents and are broadly known, so they will not be even outlined here. I have discussed extensively elsewhere why I find them implausible [9][18][19]. In addition, it is widely held that the nature of the PAMP/DAMP signal is of signature importance in determining the Th1/Th2 phenotype of the ensuing response [14][16][17]. This, too, I find implausible [18][19]. Although I have described the grounds for my scepticism elsewhere [18], I will briefly outline them here, in synoptic form, as these frameworks are so pertinent to the issues discussed.
The researchers have reviewed above how important quantitative variables of immunization are in determining the Th1/Th2 phenotype of the ensuing response. The dependence on the dose of antigen, or number of slowly replicating entities, is true for foreign vertebrate antigens, such as SRBCs in mice, which are anticipated to be PAMP-free; for transplantable tumors; and for mycobacteria and protozoa, which express very different PAMPs. It seems a PAMP-independent mechanism is required to explain this dose dependence. Moreover, the Th1/Th2 phenotype of the response often evolves, after antigen impact, from an exclusive Th1 mode toward a Th2 mode. This is paradoxical to the PAMP/DAMP view, as the PAMP and DAMP signals do not change with time. Lastly, the alternative I favor, outlined in the next section, predicts that the partial depletion of CD4 T cells at the time of immunization will modulate a response with a substantial Th2 component toward a Th1 mode. This prediction has been tested by us and others in diverse systems, as reviewed in [18]. It is paradoxical to the PAMP/DAMP-centric view, as partial depletion of CD4 T cells is not anticipated to change the PAMP and DAMP signals. These considerations indicate why I think the PAMP/DAMP-centric views are incorrect and so an impediment to progress [18].
3.2. Threshold Hypothesis
This hypothesis was formulated in the early 1970s to describe the events that determined whether the activation of naïve CD4 T cells generates, in contemporary terms, Th1 or Th2 cells [20]. I then regarded, as one of its virtues, its ability to account for how all the known quantitative variables of immunization affect the Th1/Th2 phenotype of the ensuing response. I outline this feature below. I have recently described why I feel the hypothesis has become ever more plausible in view of the multiple tests of its unique predictions in diverse experimental systems [18]. I provide a synoptic account here to provide context.
The two-signal model of lymphocyte activation was proposed as a minimal description of the activation and inactivation of mature lymphocytes that provides an explanation of peripheral tolerance, as outlined elsewhere [9]. This two-signal model provided the context for the formulation of the threshold hypothesis. The activation of CD4 T cells, according to the most recent formulation of the two-signal model, requires an antigen to facilitate the interaction of CD4 T cells mediated by B cells, as the antigen-resenting cells [18][20]. Many observations support this model [18]. The threshold hypothesis postulates that weak and robust CD4 T cell interactions give rise respectively to Th1 and Th2 cells. There are few CD4 T cells specific for minimally foreign antigens, and so, even in the presence of optimal doses of antigen to mediate CD4 T cell cooperation, only Th1 cells are generated. This hypothesis thus accounts for the Pearson and Raffel generalization described above. There are more CD4 T cells specific for more foreign antigens. In the presence of low amounts of antigen, the antigen-mediated CD4 T cell interactions will be weak and so result in the generation of Th1 cells. It is known that, after antigen impacts the immune system, helper T cells multiply, and so, as long as the level of antigen is sufficiently sustained, CD4 T cell cooperation will increase in intensity and the response will develop a Th2 component. Even greater amounts of antigen, more optimal for supporting CD4 T cell cooperation, will result in more rapid responses, and the Th1 phase may even be eclipsed. Thus, the proposed threshold mechanism accounts for the major generalizations of how quantitative variables of immunization affect the Th1/Th2 phenotype of the response [21]. The hypothesis predicts that partial depletion of CD4 T cells will modulate an immune response with a substantial Th2 component toward a Th1 mode. This “CD4 T-cell depletion” prediction has been tested and confirmed in diverse experimental systems [18] and, as discussed above, is difficult to square with the PAMP/DAMP-centric view [18].
3.3. Cytokine Milieu Hypothesis
The plausibility of a framework depends both upon what observations it can explain and whether there are other observations that appear incongruent. One cannot assess the plausibility of the threshold mechanism without addressing the multitude of observations showing the importance of cytokines in affecting the Th1/Th2 phenotype of the response and the further role of cytokines in whether Th cells belonging to other Th subsets are generated. I have recently addressed this issue [19], and so I just indicate here the view I favor.
I suggest there is more than one mechanism contributing to how the Th1/Th2 phenotype is determined. I propose the threshold mechanism is the primary mechanism. The envisaged role of cytokines is best introduced by a generalization about the activity of cytokines that Th cells produce.
Most cytokines produced by Th cells that belong to one Th subset favor the further generation of Th cells belonging to this subset directly, or indirectly by inhibiting the generation of Th cells belonging to opposing subsets [19]. Examples would be the IL-4 made by Th2 cells that stimulates Th2 but not Th1 cells to multiply [22], and another the IFN-γ made by Th1 cells that inhibits the proliferation of Th2 but not of Th1 cells [23]. I argue that such properties of cytokines have interesting consequences; if a particular Th subset predominates in an antigen-specific population of Th cells, there is a tendency for this subset to become ever more dominant; in other words, Th cells of a particular Th subset tend to be self-promoting by virtue of the cytokines they produce [19].
Consider an immune response to a complex antigen. As its different components will in general exist in different amounts, they would, if uncoupled, generally induce immune responses of different Th1/Th2 phenotypes. The phenotype of the response to a prevalent component, p, would usually be different from that of a response to a scarce, other component, o. However, p and o will sometimes be either directly physically linked to one another or indirectly linked through another component of the complex antigen. Thus, an anti-o-specific B cell will not only present o-specific peptides but also other peptides generated by processing other components of the complex antigen to which o is linked. Thus, the strength of the cooperation determining the Th1/Th2 phenotype of an o-specific naïve CD4 T cell, according to the threshold mechanism, will depend not only on the number of other CD4 T cells specific for the nominal antigen o but also on the number of CD4 T cells specific for other components belonging to the complex antigen. In addition, the property of Th cells belonging to a particular subset to be self-promoting by virtue of the cytokines they produce contributes to making the Th response ever more coherent with time [19]. I provide two illustrative observations that support this cytokine implementation hypothesis. The researchers showed that the IL-4 required to support the generation of Th2 cells is made by Th cells themselves [19]. Kelsoe showed that the coherence of the Th1/Th2 phenotype of immune responses increases as they evolve [24].
References
- Salvin, S.B. Occurrence of delayed hypersensitivity during the development of Arthus type hypersensitivity. J. Exp. Med. 1958, 107, 109–124.
- Asherson, G.L.; Stone, S.H. Selective and specific inhibition of 24 hour skin reactions in the guinea-pig. I. Immune deviation: Description of the phenomenon and the effect of splenectomy. Immunology 1965, 9, 205–217.
- Mitchison, N.A. The dosage requirements for immunological paralysis by soluble proteins. Immunology 1968, 15, 509–530.
- Burnet, F.M.; Fenner, F. The Production of Antibodies, 2nd ed.; Macmillan: Melbourne, Australia; London, UK, 1949.
- Billingham, R.E.; Brent, L.; Medawar, P.B. ‘Actively Acquired Tolerance’ of foreign cells. Nature 1953, 172, 603–606.
- Hasek, M.; Hraba, T. Active mechanisms of immunological tolerance. Surv. Immunol. Res. 1984, 3, 253–258.
- Mitchison, N.A. Tolerance of erythrocytes in poultry:induction and specificity. Immunology 1962, 5, 341–358.
- Lederberg, J. Genes and Antibodies: Do antigens bear instructions for antibody specificity or do they select cell lines that arise by mutation? Science 1959, 129, 1649–1653.
- Bretscher, P.A. The historical postulate: Is it the basis, at the level of the system, for self-nonself discrimination? Scand J. Immunol. 2021, 94, e13033.
- Parish, C.R. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972, 13, 35–66.
- Menon, J.N.; Bretscher, P.A. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur. J. Immunol. 1998, 28, 4020–4028.
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54 Pt 1, 1–13.
- Janeway, C.A., Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 1992, 13, 11–16.
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu. Rev. Immunol. 2002, 20, 197–216.
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045.
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305.
- Matzinger, P. Friendly and dangerous signals: Is the tissue in control? Nat. Immunol. 2007, 8, 11–13.
- Bretscher, P. What Determines the Class of Immunity an Antigen Induces? A Foundational Question Whose Rational Consideration Has Been Undermined by the Information Overload. Biology 2023, 12, 1253.
- Bretscher, P.A. The role of cytokines in determining the Th1/Th2 phenotype of an immune response: Coherence of the T cell response and the Cytokine Implementation Hypothesis. Scan. J. Immunol. 2022, 95, e13110.
- Bretscher, P.A. On the control between cell-mediated, IgM and IgG immunity. Cell Immunol. 1974, 13, 171–195.
- Lagrange, P.H.; Mackaness, G.B.; Miller, T.E. Influence of dose and route of antigen injection on the immunological induction of T cells. J. Exp. Med. 1974, 139, 528–542.
- Swain, S.L.; Weinberg, A.D.; English, M.; Huston, G. IL-4 directs the development of Th2-like helper effectors. J. Immunol. 1990, 145, 3796–3806.
- Gajewski, T.F.; Fitch, F.W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 1988, 140, 4245.
- Kelso, A. Th1 and Th2 subsets: Paradigms lost? Immunol. Today 1995, 16, 374–379.
More
Information
Subjects:
Primary Health Care
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
715
Revisions:
2 times
(View History)
Update Date:
19 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




