
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sam A Masih | -- | 1686 | 2024-03-16 05:42:05 | | | |
| 2 | Wendy Huang | Meta information modification | 1686 | 2024-03-19 08:12:05 | | |
Video Upload Options
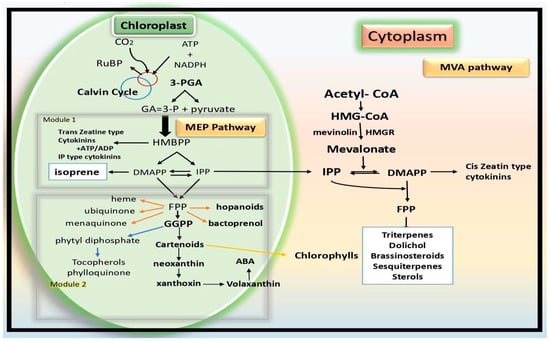
Isoprene, a lipophilic and unstable compound with the chemical formula C5H8, is transported to plant chloroplasts via the 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway, which relies on photosynthesis. Although only about 20% of terrestrial plants can synthesize isoprene, those that emit it are more adaptable to oxidative and thermal stresses. Plants use volatile organic compounds (VOCs) to communicate with other living things. Isoprene, monoterpenes and sesquiterpenes make up the largest class of volatile organic compounds (VOCs) emitted by plants terpenes. In plant–plant interactions, mono- and sesquiterpenes are well-known communication molecules. On the other hand, isoprene, the smallest and most often released terpene, is instead given a role in fighting abiotic stressors.
1. Introduction
2. Brassinosteroids
3. Abscisic Acid
4. Cytokinins
5. Strigolactones
References
- Shinozaki, K.; Uemura, M.; Bailey-Serres, J.; Bray, E.A.; Weretilnyk, E. Responses to abiotic stress. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; Willey: Hoboken, NJ, USA, 2015; pp. 1051–1100.
- Caparrotta, S.; Boni, S.; Taiti, C.; Palm, E.; Mancuso, S.; Pandolfi, C. Induction of priming by salt stress in neighbouring plants. Environ. Exp. Bot. 2018, 147, 261–270.
- Saijo, Y.; Loo, E.P.I. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104.
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2021, 73, 449–462.
- Dani, K.G.S.; Torzillo, G.; Michelozzi, M.; Baraldi, R.; Loreto, F. Isoprene emission in darkness by a facultative heterotrophic green alga. Front. Plant Sci. 2020, 11, 598786.
- Caño-Delgado, A.; Yin, Y.; Yu, C.; Vafeados, D.; Mora-García, S.; Cheng, J.C.; Nam, K.H.; Li, J.; Chory, J. BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular diferentiation in Arabidopsis. Development 2004, 131, 5341–5351.
- Bajguz, A.; Chmur, M.; Gruszka, D. Comprehensive overview of the brassinosteroids biosynthesis pathways: Substrates, prod- ucts, inhibitors, and connections. Front. Plant Sci. 2020, 11, 1034.
- Joo, S.H.; Kim, T.W.; Son, S.H.; Lee, W.S.; Yokota, T.; Kim, S.K. Biosynthesis of a cholesterol-derived brassinosteroid, 28-norcastasterone, in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 1823–1833.
- Joo, S.H.; Jang, M.S.; Kim, M.K.; Lee, J.E.; Kim, S.K. Bio-synthetic relationship between C28-brassinosteroids and C29-brassinosteroids in rice (Oryza sativa) seedlings. Phytochemistry 2015, 111, 84–90.
- North, H.M.; de Almeida, A.; Boutin, J.P.; Frey, A.; To, A.; Botran, L.; Sotta, B.; Marion-Poll, A. The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J. 2007, 50, 810–824.
- Neuman, H.; Galpaz, N.; Cunningham, F.X.; Zamir, D.; Hirschberg, J. The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufcient precursor for abscisic acid biosynthesis. Plant J. 2014, 78, 80–93.
- Dall’Osto, L.; Cazzaniga, S.; North, H.; Marion-Poll, A.; Bassi, R. The Arabidopsis aba4-1 mutant reveals a specifc function for neoxanthin in protection against photooxidative stress. Plant Cell 2007, 19, 1048–1064.
- Qin, X.; Yang, S.H.; Kepsel, A.C.; Schwartz, S.H.; Zeevaart, J.A. Evidence for abscisic acid biosynthesis in Cuscuta reflexa, a parasitic plant lacking neoxanthin. Plant Physiol. 2008, 147, 816–822.
- Frébort, I.; Kowalska, M.; Hluska, T.; Frébortová, J.; Galuszka, P. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 2011, 62, 2431–2452.
- Takei, K.; Yamaya, T.; Sakakibara, H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J. Biol. Chem. 2004, 279, 41866–41872.
- Hecht, S.; Eisenreich, W.; Adam, P.; Amslinger, S.; Kis, K.; Bacher, A.; Arigoni, D.; Rohdich, F. Studies on the nonmevalonate pathway to terpenes: The role of the GcpE (IspG) protein. Proc. Natl. Acad. Sci. USA 2001, 98, 4837–14842.
- Kakimoto, T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases. Plant Cell Physiol. 2001, 42, 677–685.
- Krall, L.; Raschke, M.; Zenk, M.H.; Baron, C. The Tzs protein from Agrobacterium tumefaciens C58 produces zeatin riboside 5′- phosphate from 4-hydroxy-3-methyl-2-(E)-butenyl diphosphate and AMP. FEBS Lett. 2002, 527, 315–318.
- Sakakibara, H.; Kasahara, H.; Ueda, N.; Kojima, M.; Takei, K.; Hishiyama, S.; Asami, T.; Okada, K.; Kamiya, Y.; Yamaya, T.; et al. Agrobacterium tumefaciens increases cytokinin production in plastids by modifying the biosynthetic pathway in the host plant. Proc. Natl. Acad. Sci. USA 2005, 102, 9972–9977.
- Alvi, A.F.; Sehar, Z.; Fatma, M.; Masood, A.; Khan, N.A. Strigolactone: An emerging growth regulator for developing resilience in plants. Plants 2022, 11, 2604.
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194.
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200.
- Ueda, H.; Kusaba, M. Strigolactone regulates leaf senescence in concert with ethylene in Arabidopsis. Plant Physiol. 2015, 169, 138–147.
- Brewer, P.B.; Koltai, H.; Bereridge, C.A. Diverse roles of strigolactones in plant development. Mol. Plant. 2013, 6, 18–28.
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186.
- Arimura, G.I.; Ozawa, R.; Nishioka, T.; Boland, W.; Koch, T.; Kühnemann, F.; Takabayashi, J. Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J. 2002, 29, 87–98.




