
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zoltan Herold | -- | 3396 | 2024-03-14 09:58:23 | | | |
| 2 | Lindsay Dong | Meta information modification | 3396 | 2024-03-15 07:45:33 | | |
Video Upload Options
Ten percent of patients with breast cancer, and probably somewhat more in patients with ovarian cancer, have inherited germline DNA mutations in the breast and ovarian cancer genes BRCA1 and BRCA2. In the remaining cases, the disease is caused by acquired somatic genetic and epigenetic alterations. Targeted therapeutic agents, such as poly ADP-ribose polymerases (PARP) inhibitors (PARPi), have emerged in treating cancers associated with germline BRCA mutations since 2014. The first PARPi was FDA-approved initially for ovarian cancer patients with germline BRCA mutations. Deleterious variants in the BRCA1/BRCA2 genes and homologous recombination deficiency status have been strong predictors of response to PARPi in a few solid tumors since then.
1. Introduction
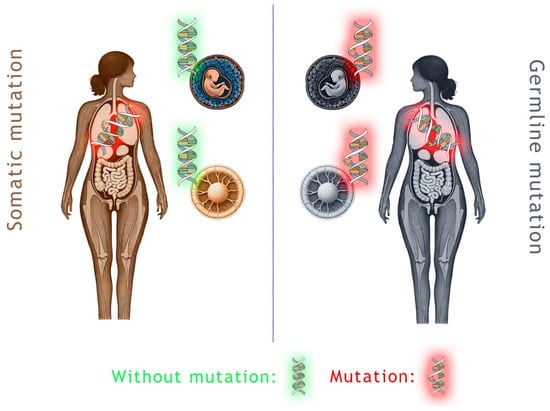
2. Clinical Differences of sBRCA and gBRCA
3. Ovarian Cancer
4. Breast Cancer
5. Pancreatic Cancer
6. Prostate Cancer
References
- Mersch, J.; Jackson, M.A.; Park, M.; Nebgen, D.; Peterson, S.K.; Singletary, C.; Arun, B.K.; Litton, J.K. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015, 121, 269–275.
- Stoppa-Lyonnet, D. The biological effects and clinical implications of BRCA mutations: Where do we go from here? Eur. J. Hum. Genet. 2016, 24 (Suppl. S1), S3–S9.
- Olah, E. A BRCA1 és BRCA2 gének. Magy. Tud. 2005, 8, 989–1000.
- Venkitaraman, A.R. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 2002, 108, 171–182.
- Li, X.Y.; Chen, J.Q.; Aisa, A.; Ding, Y.W.; Zhang, D.; Yuan, Y. Targeting BRCA-mutant biliary tract cancer: Current evidence and future perspectives. J. Dig. Dis. 2023, 24, 85–97.
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111.
- Gumaste, P.V.; Penn, L.A.; Cymerman, R.M.; Kirchhoff, T.; Polsky, D.; McLellan, B. Skin cancer risk in BRCA1/2 mutation carriers. Br. J. Dermatol. 2015, 172, 1498–1506.
- Sweis, R.F.; Heiss, B.; Segal, J.; Ritterhouse, L.; Kadri, S.; Churpek, J.E.; Allen, K.; Conway, D.; Marinier, C.; Smith, N.D.; et al. Clinical Activity of Olaparib in Urothelial Bladder Cancer with DNA Damage Response Gene Mutations. JCO Precis. Oncol. 2018, 2, 1–7.
- Remon, J.; Besse, B.; Leary, A.; Bieche, I.; Job, B.; Lacroix, L.; Auguste, A.; Mauduit, M.; Audigier-Valette, C.; Raimbourg, J.; et al. Somatic and Germline BRCA 1 and 2 Mutations in Advanced NSCLC from the SAFIR02-Lung Trial. JTO Clin. Res. Rep. 2020, 1, 100068.
- Ueki, A.; Yoshida, R.; Kosaka, T.; Matsubayashi, H. Clinical risk management of breast, ovarian, pancreatic, and prostatic cancers for BRCA1/2 variant carriers in Japan. J. Hum. Genet. 2023, 68, 517–526.
- Sorscher, S. Helicobacter pylori and gastric cancer risk in BRCA 1/2 pathogenic germline variant carriers. J. Hum. Genet. 2023, 68, 725.
- Buckley, K.H.; Niccum, B.A.; Maxwell, K.N.; Katona, B.W. Gastric Cancer Risk and Pathogenesis in BRCA1 and BRCA2 Carriers. Cancers 2022, 14, 5953.
- Maccaroni, E.; Giampieri, R.; Lenci, E.; Scortichini, L.; Bianchi, F.; Belvederesi, L.; Brugiati, C.; Pagliaretta, S.; Ambrosini, E.; Berardi, R. BRCA mutations and gastrointestinal cancers: When to expect the unexpected? World J. Clin. Oncol. 2021, 12, 565–580.
- Casaubon, J.T.; Kashyap, S.; Regan, J.P. BRCA1 and BRCA2 Mutations. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024.
- Csokay, B.; Udvarhelyi, N.; Sulyok, Z.; Besznyak, I.; Ramus, S.; Ponder, B.; Olah, E. High frequency of germ-line BRCA2 mutations among Hungarian male breast cancer patients without family history. Cancer Res. 1999, 59, 995–998.
- Van Der Looij, M.; Szabo, C.; Besznyak, I.; Liszka, G.; Csokay, B.; Pulay, T.; Toth, J.; Devilee, P.; King, M.C.; Olah, E. Prevalence of founder BRCA1 and BRCA2 mutations among breast and ovarian cancer patients in Hungary. Int. J. Cancer 2000, 86, 737–740.
- Remenyi Kissne, D.; Gede, N.; Szakacs, Z.; Kiss, I. Breast cancer screening knowledge among Hungarian women: A cross-sectional study. BMC Womens’ Health 2021, 21, 69.
- Janavicius, R. Founder BRCA1/2 mutations in the Europe: Implications for hereditary breast-ovarian cancer prevention and control. EPMA J. 2010, 1, 397–412.
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78.
- Peixoto, A.; Pinto, P.; Guerra, J.; Pinheiro, M.; Santos, C.; Pinto, C.; Santos, R.; Escudeiro, C.; Bartosch, C.; Canario, R.; et al. Tumor Testing for Somatic and Germline BRCA1/BRCA2 Variants in Ovarian Cancer Patients in the Context of Strong Founder Effects. Front. Oncol. 2020, 10, 1318.
- Shi, Y.; Zhou, F.; Jiang, F.; Lu, H.; Wang, J.; Cheng, C. PARP inhibitor reduces proliferation and increases apoptosis in breast cancer cells. Chin. J. Cancer Res. 2014, 26, 142–147.
- Konstantinopoulos, P.A.; Lacchetti, C.; Annunziata, C.M. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline Summary. JCO Oncol. Pract. 2020, 16, e835–e838.
- Tuffaha, H.; Edmunds, K.; Fairbairn, D.; Roberts, M.J.; Chambers, S.; Smith, D.P.; Horvath, L.; Arora, S.; Scuffham, P. Guidelines for genetic testing in prostate cancer: A scoping review. Prostate Cancer Prostatic Dis. 2023, in press.
- Daly, M.B.; Pal, T.; Maxwell, K.N.; Churpek, J.; Kohlmann, W.; AlHilli, Z.; Arun, B.; Buys, S.S.; Cheng, H.; Domchek, S.M.; et al. NCCN Guidelines(R) Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2024. J. Natl. Compr. Cancer Netw. 2023, 21, 1000–1010.
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J. Natl. Compr. Cancer Netw. 2023, 21, 594–608.
- Toss, A.; Piombino, C.; Tenedini, E.; Bologna, A.; Gasparini, E.; Tarantino, V.; Filieri, M.E.; Cottafavi, L.; Giovanardi, F.; Madrigali, S.; et al. The Prognostic and Predictive Role of Somatic BRCA Mutations in Ovarian Cancer: Results from a Multicenter Cohort Study. Diagnostics 2021, 11, 565.
- Tricarico, D.; Convertino, A.S.; Mehmeti, I.; Ranieri, G.; Leonetti, F.; Laface, C.; Zizzo, N. Inflammatory Related Reactions in Humans and in Canine Breast Cancers, A Spontaneous Animal Model of Disease. Front. Pharmacol. 2022, 13, 752098.
- Wang, L.; Wang, D.; Sonzogni, O.; Ke, S.; Wang, Q.; Thavamani, A.; Batalini, F.; Stopka, S.A.; Regan, M.S.; Vandal, S.; et al. PARP-inhibition reprograms macrophages toward an anti-tumor phenotype. Cell Rep. 2022, 41, 111462.
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533.
- Chen, S.; Parmigiani, G. Meta-analysis of BRCA1 and BRCA2 penetrance. J. Clin. Oncol. 2007, 25, 1329–1333.
- Mavaddat, N.; Peock, S.; Frost, D.; Ellis, S.; Platte, R.; Fineberg, E.; Evans, D.G.; Izatt, L.; Eeles, R.A.; Adlard, J.; et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 2013, 105, 812–822.
- Burgess, M.; Puhalla, S. BRCA 1/2-Mutation Related and Sporadic Breast and Ovarian Cancers: More Alike than Different. Front. Oncol. 2014, 4, 19.
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmana, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015, 33, 244–250.
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505.
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Perol, D.; Gonzalez-Martin, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Maenpaa, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428.
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1721–1731.
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Overall Survival with Maintenance Olaparib at a 7-Year Follow-Up in Patients with Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. 2023, 41, 609–617.
- Ray-Coquard, I.; Leary, A.; Pignata, S.; Cropet, C.; Gonzalez-Martin, A.; Marth, C.; Nagao, S.; Vergote, I.; Colombo, N.; Maenpaa, J.; et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: Final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann. Oncol. 2023, 34, 681–692.
- Ragupathi, A.; Singh, M.; Perez, A.M.; Zhang, D. Targeting the BRCA1/2 deficient cancer with PARP inhibitors: Clinical outcomes and mechanistic insights. Front. Cell Dev. Biol. 2023, 11, 1133472.
- Pignata, S.; Oza, A.; Hall, G.; Pardo, B.; Madry, R.; Cibula, D.; Klat, J.; Montes, A.; Glasspool, R.; Colombo, N.; et al. Maintenance olaparib in patients with platinum-sensitive relapsed ovarian cancer: Outcomes by somatic and germline BRCA and other homologous recombination repair gene mutation status in the ORZORA trial. Gynecol. Oncol. 2023, 172, 121–129.
- Redondo, A.; Romero, I. Olaparib after Response to Trabectedin-Pegylated Liposomal Doxorubicin in Recurrent Ovarian Carcinoma. Available online: https://clinicaltrials.gov/study/NCT03470805 (accessed on 15 February 2024).
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961.
- Monk, B.J.; Parkinson, C.; Lim, M.C.; O’Malley, D.M.; Oaknin, A.; Wilson, M.K.; Coleman, R.L.; Lorusso, D.; Bessette, P.; Ghamande, S.; et al. A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients with Newly Diagnosed Ovarian Cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J. Clin. Oncol. 2022, 40, 3952–3964.
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164.
- Del Campo, J.M.; Matulonis, U.A.; Malander, S.; Provencher, D.; Mahner, S.; Follana, P.; Waters, J.; Berek, J.S.; Woie, K.; Oza, A.M.; et al. Niraparib Maintenance Therapy in Patients with Recurrent Ovarian Cancer after a Partial Response to the Last Platinum-Based Chemotherapy in the ENGOT-OV16/NOVA Trial. J. Clin. Oncol. 2019, 37, 2968–2973.
- Rizzolo, P.; Silvestri, V.; Falchetti, M.; Ottini, L. Inherited and acquired alterations in development of breast cancer. Appl. Clin. Genet. 2011, 4, 145–158.
- Cortesi, L.; Rugo, H.S.; Jackisch, C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target Oncol. 2021, 16, 255–282.
- Couch, F.J.; Nathanson, K.L.; Offit, K. Two decades after BRCA: Setting paradigms in personalized cancer care and prevention. Science 2014, 343, 1466–1470.
- Toss, A.; Molinaro, E.; Venturelli, M.; Domati, F.; Marcheselli, L.; Piana, S.; Barbieri, E.; Grandi, G.; Piombino, C.; Marchi, I.; et al. BRCA Detection Rate in an Italian Cohort of Luminal Early-Onset and Triple-Negative Breast Cancer Patients without Family History: When Biology Overcomes Genealogy. Cancers 2020, 12, 1252.
- Baretta, Z.; Mocellin, S.; Goldin, E.; Olopade, O.I.; Huo, D. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine 2016, 95, e4975.
- Vidula, N.; Dubash, T.; Lawrence, M.S.; Simoneau, A.; Niemierko, A.; Blouch, E.; Nagy, B.; Roh, W.; Chirn, B.; Reeves, B.A.; et al. Identification of Somatically Acquired BRCA1/2 Mutations by cfDNA Analysis in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2020, 26, 4852–4862.
- De La Haba, J.; Anton, A.; Quiroga, V.; Guerrero, A.; Andrés, R.; Mele, M.; Gonzalez-Santiago, S.; Perez-Fidalgo, J.A.; Ramon y Cajal, T.; Escudero, M.J.; et al. Olaparib (O) in advanced triple negative breast cancer (aTNBC) patients (pts) with BRCA1/2 promoter methylation: GEICAM/2015-06 study (COMETA-Breast). J. Clin. Oncol. 2023, 41, 1093.
- Balmana, J.; Fasching, P.A.; Couch, F.J.; Delaloge, S.; Labidi-Galy, I.; O’Shaughnessy, J.; Park, Y.H.; Eisen, A.F.; You, B.; Bourgeois, H.; et al. Clinical effectiveness and safety of olaparib in BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: Final analysis of LUCY. Breast Cancer Res. Treat. 2023. Online Ahead of Print.
- Walsh, E.M.; Mangini, N.; Fetting, J.; Armstrong, D.; Chan, I.S.; Connolly, R.M.; Fiallos, K.; Lehman, J.; Nunes, R.; Petry, D.; et al. Olaparib Use in Patients with Metastatic Breast Cancer Harboring Somatic BRCA1/2 Mutations or Mutations in Non-BRCA1/2, DNA Damage Repair Genes. Clin. Breast Cancer 2022, 22, 319–325.
- Rosen, M.N.; Goodwin, R.A.; Vickers, M.M. BRCA mutated pancreatic cancer: A change is coming. World J. Gastroenterol. 2021, 27, 1943–1958.
- Wong, W.; Raufi, A.G.; Safyan, R.A.; Bates, S.E.; Manji, G.A. BRCA Mutations in Pancreas Cancer: Spectrum, Current Management, Challenges and Future Prospects. Cancer Manag. Res. 2020, 12, 2731–2742.
- Klatte, D.C.F.; Wallace, M.B.; Lohr, M.; Bruno, M.J.; van Leerdam, M.E. Hereditary pancreatic cancer. Best Pract. Res. Clin. Gastroenterol. 2022, 58–59, 101783.
- Reiss, K.A.; Mick, R.; O’Hara, M.H.; Teitelbaum, U.; Karasic, T.B.; Schneider, C.; Cowden, S.; Southwell, T.; Romeo, J.; Izgur, N.; et al. Phase II Study of Maintenance Rucaparib in Patients with Platinum-Sensitive Advanced Pancreatic Cancer and a Pathogenic Germline or Somatic Variant in BRCA1, BRCA2, or PALB2. J. Clin. Oncol. 2021, 39, 2497–2505.
- Fizazi, K.; Gillessen, S.; ESMO Guidelines Committee. Updated treatment recommendations for prostate cancer from the ESMO Clinical Practice Guideline considering treatment intensification and use of novel systemic agents. Ann. Oncol. 2023, 34, 557–563.
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102.
- Abida, W.; Campbell, D.; Patnaik, A.; Bryce, A.H.; Shapiro, J.; Bambury, R.M.; Zhang, J.; Burke, J.M.; Castellano, D.; Font, A.; et al. Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study. Eur. Urol. 2023, 84, 321–330.
- Zhou, F. Pamiparib in mCRPC with HRD or BRCA1/2 Mutation. Available online: https://clinicaltrials.gov/study/NCT05327621 (accessed on 10 February 2024).
- McKay, R.R. Study of Neoadjuvant PARP Inhibition Followed by Radical Prostatectomy in Patients with Unfavorable Intermediate-Risk or High-Risk Prostate Cancer with BRCA1/2 Gene Alterations (NePtune). Available online: https://clinicaltrials.gov/study/NCT05498272 (accessed on 10 February 2024).
- Huang, J.-D. Study of CX-5461 in Patients with Solid Tumours and BRCA1/2, PALB2 or Homologous Recombination Deficiency (HRD) Mutation. Available online: https://clinicaltrials.gov/study/NCT04890613 (accessed on 10 February 2024).




